Iodine is the only halogen that occurs in a positive oxidation state, in NaIO₃ impurities within Chile saltpeter, NaNO₃.(b) In the production of I₂, IO₃ ⁻ reacts with HSO₃ ⁻:IO₃ ⁻ (aq) 1 HSO₃ (aq) →HSO₄ ⁻(aq)+ SO₄²⁻(aq) +H₂O(l) + I₂(s) [unbalanced]Identify the oxidizing and reducing agents.
Answers
HSO3 acts as the reducing agent.
IO3 acts as the oxidizing agent.
The skeleton equation representing the formation of I2 from I03 and HSO3 is as follows:
IO3(aq) + HSO3 (aq)-------> HSO4(aq) + SO4 (aq) + H₂O(l) + I2 (s)
Assign the oxidation number to each and every species involved in the above reaction to identify the oxidizing agent and reducing agent.
IO3(aq) + HSO3 (aq)-------> HSO4(aq) + SO4 (aq) + H₂O(l) + I2 (s)
(+5)( -2) (+1)(+4)(-2) (+1 )(6) (-2 ) (+6)(-2) (+1)(-2) (0)
Oxidation process involves the increase in the oxidation number whereas the reduction process involves the decrease in the oxidation number. Since the oxidation number of sulfur increases from +4 to +6, sulfur metal is oxidized from HSO3 to SO4.
Thus, HSO3 acts as the reducing agent.
Since the oxidation number of iodine is decreased from +5 to 0, iodine reduced from IO3 to I2.
Thus, IO3 acts as the oxidizing agent.
Learn more about oxidizing agent here:
https://brainly.com/question/10547418
#SPJ4
Related Questions
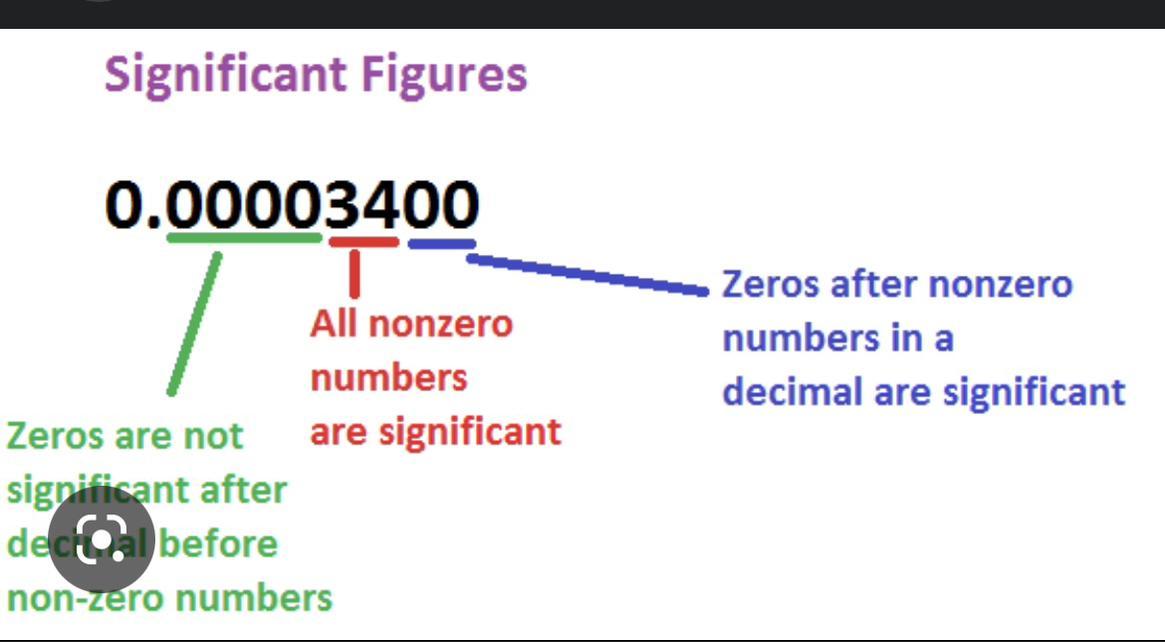
how would you round 34.9279 if there was 3 sig figs and 4 sig figs
Answers
26.How many milliliters of a lotion base must be added to 30 mL of oxiconazole nitrate (OXISTAT) lotion 1% w/v to reduce its concentration to 6 mg/mL
Answers
We need to add 50 mL of lotion base to the 30 mL of oxiconazole nitrate lotion to reduce its concentration to 6 mg/mL.
To solve this problem, we need to find the volume of lotion base that needs to be added to achieve a concentration of 6 mg/mL.
First, we need to convert the concentration of the oxiconazole nitrate lotion from 1% w/v to mg/mL.
1% w/v means that 1 gram of oxiconazole nitrate is present in 100 mL of solution. Therefore, the concentration is 1 gram/100 mL = 10 mg/mL.
We want to reduce the concentration to 6 mg/mL. This means that for every 1 mL of solution, we need 6 mg of oxiconazole nitrate.
Now, let's calculate the volume of lotion base needed. Since we have 30 mL of the original lotion with a concentration of 10 mg/mL, the total amount of oxiconazole nitrate in this lotion is 30 mL * 10 mg/mL = 300 mg.
To achieve a concentration of 6 mg/mL, we divide the total amount of oxiconazole nitrate (300 mg) by the desired concentration (6 mg/mL):
300 mg / 6 mg/mL = 50 mL.
Therefore, to achieve a concentration of 6 mg/mL, 50 mL of lotion base should be added to the existing 30 mL of oxiconazole nitrate lotion.
You can learn more about concentration at
https://brainly.com/question/17206790
#SPJ11
what is the refrigerants state when entering the metering device
Answers
When refrigerant is in a high-pressure, high-temperature state, it enters the metering device. The refrigerant is then changed to a low-pressure, low-temperature state as a result of the metering device. As a result, the refrigerant will expand and the heat will be absorbed as the temperature decreases.
The metering device is a component in a refrigeration or air conditioning system that ensures that the correct amount of refrigerant is delivered to the evaporator. The device functions as a flow control, reducing the refrigerant's pressure before it reaches the evaporator. The metering device may be a simple capillary tube, an orifice, an automatic expansion valve (AEV), or a thermostatic expansion valve (TXV). The capillary tube is the simplest and least expensive metering device. It's just a small copper tube that's thin and long. The AEV is a constant pressure valve that maintains a consistent pressure drop across it, allowing it to regulate the refrigerant flow. A thermostatic expansion valve is the most sophisticated metering device, as it can sense the temperature at the evaporator outlet and adjust the refrigerant flow accordingly.
To know more about capillary tube visit :
brainly.com/question/30097496
#SPJ11
Which of the following is a way that groundwater depletion affects streams? a. Lower water level b. Loss of vegetation c. Wildlife disruption d. All of the above.
Answers
The affect of ground water depletion is Lower water level, Loss of vegetation and Wildlife disruption.
What is ground water?The amount of water that is present below the upper surface area of the land, which are not easily accessible is known as ground water.
And this ground water keeps the soil hydrated and responsible for good vegetation by providing many minerals through water, wildlife is also depends on the ground water as they provide survival for many species. If the ground water is deplected then it also affects the flow of streams and lower water level.
Hence option (d) is correct i.e. All of the above.
To know more about ground water, visit the below link:
https://brainly.com/question/9617
Answer
D. All of the above
Explanation
Answer for number 9 i need it asap thank you

Answers
Answer:
a) Trichloromethyl
b) Trinitrogen Pentoxide
c) Carbogen
Explanation:
I WILL MARK BRAINLYEST!
What are two things that the DDT and ozone story have in common?
there's no answer options I just need sentences please!
Answers
Answer:
DDT (dichloro-diphenyl-trichloroethane) was developed as the first of the modern synthetic insecticides in the 1940s. It was initially used with great effect to combat malaria, typhus, and the other insect-borne human diseases among both military and civilian populations.
Ozone is a naturally occurring molecule. An ozone molecule is made up of three oxygen atoms. It has the chemical formula O3.
Explanation:
It is commonly used as a fire extinguishing agent. The reason it has so much ozone depleting potential is because it contains bromine, which has many times the ozone depleting potential of chlorine.
what is the concentration in molarity of an aqueous solution which contains 5.06% by mass ethylene glycol (mm
Answers
Concentration in molarity of an aqueous solution which contains 5.06% by mass ethylene glycol IS 0.847 mol/L
Molarity [=] mol / L
wt% = (g C₂H₆O₂ / g s)×100
⇒ 0.0506 = g C₂H₆O₂ / g s
∴ mm C2H6O2 = 62.07 g/mol
∴ δ s = 1.04 g/mL
assuming: g s = 1 g
⇒ g C₂H₆O₂ = 0.0506 g
⇒mol C₂H₆O₂ = (0.0506 g)(mol/62.07 g) = 8.154 E-4 mol C₂H₆O₂
⇒ V s = 1g s / 1.04 g/mL = 0.9615 mL = 9.615 E-4 L s
⇒ C₂H₆O₂ = (8.154 E-4 )/(9.615 E-4 L s)
⇒ C₂H₆O₂= 0.847 mol/L
In terms of the amount of material per unit volume of solution, molar concentration is a measurement of the concentration of a chemical species, specifically a solute in a solution. The most frequent measure of molarity in chemistry is the number of moles per liter, denoted by the unit symbol mol/L or mol/dm3 in SI units.
Learn more about molarity here:
https://brainly.com/question/12127540
#SPJ4
Match up the characteristics below with the type of molecular bond they describe. Bonds found in Halite (between Na+ and Cl-) Bonds found between Si and O in the Si-O tetrahedron Bonds inside the water molecule (between the H and O ) Bonds that exist between two water molecules Strongest bond type Weakest bond type Bonds that are used by water to dissolve sal
Answers
The characteristics and the type of molecular bond they describe:
1. Bonds found in Halite (between Na⁺ and Cl⁻): Ionic bond
2. Bonds found between Si and O in the Si-O tetrahedron: Covalent bond
3. Bonds inside the water molecule (between the H and O): Covalent bond
4. Bonds that exist between two water molecules: Hydrogen bond
5. Strongest bond type: Covalent bond
6. Weakest bond type: Van der Waals bond
7. Bonds that are used by water to dissolve salt: Ionic bond
The ionic bond is a type of molecular bond found in halite (between Na⁺ and Cl⁻). The Si-O tetrahedron is held together by a covalent bond. The bond inside the water molecule (between the H and O) is also a covalent bond. The hydrogen bond is the type of bond that exists between two water molecules. The covalent bond is the strongest bond type, while the van der Waals bond is the weakest bond type. Water uses the ionic bond to dissolve the salt.
Learn more about bonds: https://brainly.com/question/32306693
#SPJ11
A 100ml sample of hydrobromic acid is titrated with potassium hydroxide. It took 24.61ml of 0.627m potassium hydroxide to neutralize the acid.
How many moles of acid are present in the solution?
What is the concentration of the acid?
Answers
The concentration of a solution in a titration procedure can be calculated using the following formula;
CaVa = CbVb
Where;
Ca and Va = acid concentration and volume respectivelyCb and Vb = base concentration and volume respectivelyAccording to this question, It took 24.61ml of 0.627m potassium hydroxide to neutralize 100ml of hydrobromic acid.
100 × Ca = 24.61 × 0.627
100Ca = 15.43
Ca = 0.154 M.
Molarity = no of moles ÷ volume
no of moles = 0.1 L × 0.154 M = 0.0154 moles
Learn more about concentration at: https://brainly.com/question/13387755
#SPJ1
The aximuthal quantum number of an orbital is 3. What are the possible values of m?
Answers
For a given valye of l(l shell), values of m are from -l to 0 to l, so m could be; -3, -2, -1, 0, +1, +2, +3.
Easyy!
the value of m is equal to 2l+1 so there must beseven values for m.
m can have values from -l through zero to +l so the values are -3, -2, -1, 0, +1, +2 and +3.
4) Demonstration: Your instructor will demonstrate the reaction t between lithium metal and water. The demonstration will include a test of the resulting solution with universal indicator. Evidence of a chemical reaction: Balanced chemical equation:
Answers
Lithium metal and water form a mixture that reacts violently. This is a chemical reaction that produces lithium hydroxide and hydrogen gas. The reaction equation is as follows: 2Li(s) + 2H2O(l) → 2LiOH (aq) + H2(g). The lithium metal is oxidized by water to produce hydrogen gas and lithium hydroxide.
This reaction is exothermic, producing heat as a result. The demonstration will include a test of the resulting solution with universal indicator. Universal indicator is a pH indicator that is used to determine the acidity or alkalinity of a solution. If the solution is acidic, the universal indicator will turn red. If the solution is alkaline, the universal indicator will turn blue. The test will determine if the solution produced in the reaction is acidic, alkaline, or neutral. If the solution is acidic, the reaction can be used to produce hydrogen gas. If the solution is alkaline, the reaction can be used to produce lithium hydroxide.
To know more about demonstration visit:
https://brainly.com/question/29360620
#SPJ11
What mass of Barium Nitride can be theoretically produced?
Answers
Answer:
151.33 g/mol
Explanation:
TRUST ME -_-
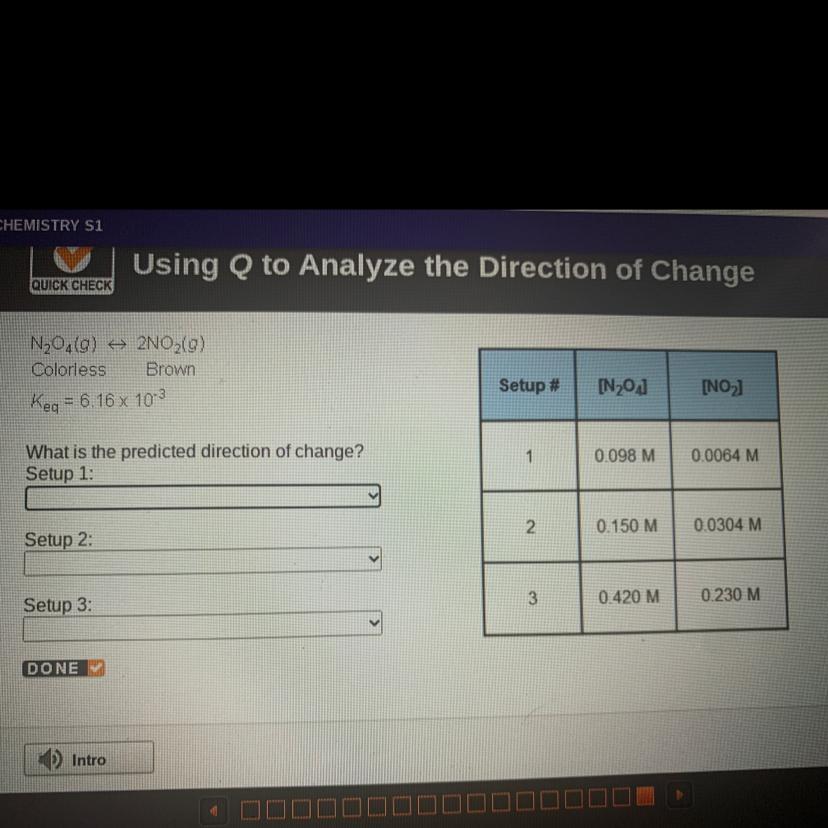
N204(0) + 2NO2(g)
Colorless Brown
Keq = 6.16 x 103
What is the predicted direction of change?
Answers
setup 1 : to the right
setup 2 : equilibrium
setup 3 : to the left
Further explanationThe reaction quotient (Q) : determine a reaction has reached equilibrium
For reaction :
aA+bB⇔cC+dD
\(\tt Q=\dfrac{C]^c[D]^d}{[A]^a[B]^b}\)
Comparing Q with K( the equilibrium constant) :
K is the product of ions in an equilibrium saturated state
Q is the product of the ion ions from the reacting substance
Q <K = solution has not occurred precipitation, the ratio of the products to reactants is less than the ratio at equilibrium. The reaction moved to the right (products)
Q = Ksp = saturated solution, exactly the precipitate will occur, the system at equilibrium
Q> K = sediment solution, the ratio of the products to reactants is greater than the ratio at equilibrium. The reaction moved to the left (reactants)
Keq = 6.16 x 10⁻³
Q for reaction N₂O₄(0) ⇒ 2NO₂(g)
\(\tt Q=\dfrac{[NO_2]^2}{[N_2O_4]}\)
Setup 1 :
\(\tt Q=\dfrac{0.0064^2}{0.098}=0.000418=4.18\times 10^{-4}\)
Q<K⇒The reaction moved to the right (products)
Setup 2 :
\(\tt Q=\dfrac{0.0304^2}{0.15}=0.00616=6.16\times 10^{-3}\)
Q=K⇒the system at equilibrium
Setup 3 :
\(\tt Q=\dfrac{0.230^2}{0.420}=0.126\)
Q>K⇒The reaction moved to the left (reactants)
Answer:
The system will shift toward the products
The system is at equilibrium
The system will shift toward the reactants
Explanation:
This is correct on edg... Good Luck!!!!
An experiment requires 43.7 g of isopropyl alcohol. Instead of measuring out the sample on a balance, a chemist dispenses the liquid into a graduated cylinder. The density of isopropyl alcohol is 0.785 g/mL. What volume, in milliliters, of isopropyl alcohol should be dispensed?
Answers
The density of isopropyl alcohol is 0.785 g/mL. 22volume, in milliliters, of isopropyl alcohol should be dispensed.
What is density ?
Mass per unit volume of a solid substance; density. Density is defined as d = M/V, where M stands for mass, and V for volume. The unit of density that is most frequently used is grams per cubic centimeter.
What is isopropyl alcohol?
Common names for isopropyl alcohol include "isopropanol," "n-propanol," and "dimethylcarbinol." It is a colorless, flammable liquid with the chemical formula C3H8O.
Therefore, density of isopropyl alcohol is 0.785 g/mL. 22volume, in milliliters, of isopropyl alcohol should be dispensed.
Learn more about density from the given link.
https://brainly.com/question/1354972
#SPJ1
8. An element that has strong intermolecular forces is most likely to have:
•a boiling point below room temperature
•a melting point below room temperature
•a boiling point very close to its melting point
•a very high melting point
Answers
Answer:
a very high melting point answer
If 0.092J of heat causes a 0.267 degree C temperature change, what mass of water is present?
Answers
Answer:
0.082g
Explanation:
The following data were obtained from the question:
Heat (Q) = 0.092J
Change in temperature (ΔT) = 0.267°C
Specific heat capacity (C) of water = 4.184J/g°C
Mass (M) =..?
Thus, the mass of present can be obtained as follow:
Q = MCΔT
0.092 = M x 4.184 x 0.267
Divide both side by 4.184 x 0.267
M = 0.092 / (4.184 x 0.267)
M = 0.082g
Therefore, mass of water was present is 0.082.
From the values of Delta H and Delta S predict which of the following reactions would be spontaneous at 25degree C. Calculate the minimum temperature at which each reaction will become spontaneous. Enter "NONE" if the reaction is not spontaneous at any temperature. (a) Delta H = 12.6 kJ/mol, Delta S = 93 J/K middot mol spontaneous at 25degree C not spontaneous at 25degree C (b) Delta H = 9.5 kJ/mol, Delta S = -94.0 J/K middot mol spontaneous at 25degree C not spontaneous at 25degree C
Answers
(a) The reaction with Delta H = 12.6 kJ/mol and Delta S = 93 J/K·mol is spontaneous at 25°C.
(b) The reaction with Delta H = 9.5 kJ/mol and Delta S = -94.0 J/K·mol is not spontaneous at 25°C.
To determine whether a reaction is spontaneous at a given temperature, we can use the Gibbs free energy equation: Delta G = Delta H - T·Delta S, where Delta G is the change in Gibbs free energy, Delta H is the change in enthalpy, Delta S is the change in entropy, and T is the temperature in Kelvin.
For a reaction to be spontaneous, Delta G must be negative. If Delta H is negative (exothermic) and Delta S is positive (increase in disorder), the reaction is more likely to be spontaneous.
(a) For the reaction with Delta H = 12.6 kJ/mol and Delta S = 93 J/K·mol, we have Delta G = 12.6 kJ/mol - (25 + 273) K·(93 J/K·mol/1000 J/kJ) = -5.25 kJ/mol. Since Delta G is negative, the reaction is spontaneous at 25°C.
(b) For the reaction with Delta H = 9.5 kJ/mol and Delta S = -94.0 J/K·mol, we have Delta G = 9.5 kJ/mol - (25 + 273) K·(-94.0 J/K·mol/1000 J/kJ) = 3.57 kJ/mol. Since Delta G is positive, the reaction is not spontaneous at 25°C.
To determine the minimum temperature at which a non-spontaneous reaction becomes spontaneous, we can set Delta G equal to zero and solve for T in the equation Delta G = Delta H - T·Delta S. However, in this case, both reactions are either spontaneous or non-spontaneous at 25°C, so we do not need to calculate the minimum temperature.
To know more about Gibbs free energy refer here:
https://brainly.com/question/29753420#
#SPJ11
How are chemicals both harmful and helpful to humans ?
Answers
Answer:
Chemicals can enter and irritate the nose, air passages and lungs. They can become deposited in the airways or be absorbed by the lungs into the bloodstream. The blood can then carry these substances to the rest of the body. Ingestion (swallowing) of food, drink or other substances is another route of exposure.
Explanation:
hope this helps?
Can some help me please

Answers
8. Plants
Explain crystalization as method of separating mixtures. Imk.
Answers
Crystallization is primarily employed as a separation technique in order to obtain pure crystals of a substance from an impure mixture
What is crystallization in separating mixtures?Crystallization from solution is a separation approach where a solid phase is separated from the mother liquor. In disparity with other separation procedures, however, the dispersed phase consisting of numerous solid particles also forms the final product, which has to meet the needed product specifications.
Crystallization is used to separate a soluble material from a solvent. For example, crystallization can be used to separate salt from a salt solution. We begin with a soluble solid melt in a solvent.
So we can conclude that Crystallization is a powerful and adaptable technique to separate components from a liquid mixture.
Learn more about Crystallization here: https://brainly.com/question/5520192
#SPJ1
please solve k+b2o3 =k2o+b
Answers
Answer:
2k+1k2o=1k2o+2b
Explanation:
In Wittig Reaction, the reaction will be run using _____ as solvent. _______ is added to the residue to leach out your product. Your crude product is recrystallized from _______
Answers
In Wittig reaction, will be run using diethyl ether or THF as solvent. Water is used for leaching the product from the residue. Your crude product is recrystallized from ethanol.
In the Wittig reaction, the reaction is typically run using an inert solvent such as diethyl ether or tetrahydrofuran (THF). Water is added to the residue to leach out the product, as the product is often water-soluble while the residue contains the byproducts and unreacted reagents.
The crude product is then recrystallized from a suitable solvent such as ethanol or methanol to obtain purified crystals. The choice of solvent for recrystallization depends on the solubility of the product and impurities.
Recrystallization is performed using solvents like ethanol or methanol to obtain purified crystals of the desired product. The choice of solvent in each step is crucial for achieving a successful and purified reaction outcome.
Learn more about the Wittig reaction here:
https://brainly.com/question/31385260
#SPJ4
The complete question is:
In Wittig Reaction, the reaction will be run using A. (hexane/methanol/no solvent) as solvent: B (Hexane/methanol/no solvent) is added to the residue to leach out your product: Your crude product is recrystallized from C (hexane/methanol/no solvent)
PLEASE HELP!!!! ANSWER 2 QUESTIONS FOR 50 POINTS AND BRAINLIEST!

Answers
2)the amount of water in each cup caused the food coloring to spread either faster or slower
HELP PLEASE 1pt
Identify the object that has the greatest gravitational pull.
A.
a tennis ball
B.
the moon
C.
the sun
D.
Earth

Answers
The object with the greatest gravitational pull is the Earth (option D).
What is gravitational pull?Gravitational force is a very long-range, but relatively weak fundamental force of attraction that acts between all particles that have mass. It is believed to be mediated by gravitons.
Newton’s Law of Universal Gravitation states that every particle attracts every other particle in the universe with force directly proportional to the product of the masses and inversely proportional to the square of the distance between them.
The force of gravitational attraction is directly dependent upon the masses of both objects and inversely proportional to the square of the distance that separates their centers.
So as the mass of either object increases, the force of gravitational attraction between them also increases. If the mass of one of the objects is doubled, then the force of gravity between them is doubled. If the mass of one of the objects is tripled, then the force of gravity between them is tripled.
According to this question, Earth has the highest mass among the listed objects, hence, in accordance to the law of universal gravitation, Earth will possess the greatest gravitational pull.
Learn more about gravitational pull at: https://brainly.com/question/13467280
#SPJ1
Sulfanilic acid, which is used in making dyes, is made by reacting aniline with sulfuric acid.
(a) Is aniline a Bronsted base, a Lewis base, or both? Explain, using its possible reactions with HCl, BF 33 or other acid.
(b) Sulfanilic acid has a pKa value of 3.23. The sodium salt of the acid, Na(H2NC6H4SO3), is quite soluble in water. If you dissolve 1.25g of the salt in water to give 125mL of solution, what is the pH of the solution?
Answers
(a) Aniline is a Lewis base.
(b) The pH of the solution would be around 3.23.
A Bronsted-Lowry base is a substance that donates a proton (H+) to another substance, called a Bronsted-Lowry acid. A Lewis base is a substance that donates an electron pair to a Lewis acid. Aniline can act as a Lewis base because it has an unshared pair of electrons that can be donated to a Lewis acid.
When aniline reacts with HCl, it acts as a Bronsted-Lowry base, accepting a proton from the HCl to form anilinium ion (C6H5NH3+).
When aniline reacts with BF3, it acts as a Lewis base, donating an electron pair to the Lewis acid BF3 to form a coordination complex.
The pKa value of sulfanilic acid is 3.23, which means that at this pH, half of the sulfanilic acid will be in the protonated form (HSO3-) and half will be in the deprotonated form (SO3^2-).
When the sodium salt of sulfanilic acid is dissolved in water, it splits into sodium ions (Na+) and sulfanilic acid ions (HSO3-).
Since the concentration of sulfanilic acid ions is equal to the concentration of sodium ions (1.25 g / (molecular weight of Na(H2NC6H4SO3))), the pH of the solution can be calculated using the equation:
pH = pKa + log([HSO3-]/[HSO3H])
Since [HSO3-] = [HSO3H], the pH = pKa = 3.23.
To know more about Sulfanilic acid here:
https://brainly.com/question/13195158#
#SPJ11
[11] Write the nuclear symbol for the atom with the following subatomic particles: 53p +
,54n,53e −
.
Answers
There are 53 protons because the atomic number is equal to the number of protons.
The nuclear symbol for the atom with the following subatomic particles: 53p+, 54n, 53e- can be written as follows:
53 is the atomic number because it has 53 protons, and protons' charge is +1.
The mass number of the atom is 107 because it has 53 protons (53 x 1) + 54 neutrons (54 x 1) = 107.
The symbol of the element is X, where X is the symbol of the element as found on the periodic table.
Hence the symbol for this atom is:
X10753
There are 53 protons because the atomic number is equal to the number of protons.
The number of neutrons can be calculated by subtracting the atomic number from the mass number.
To know more about atomic number visit:
https://brainly.com/question/16858932
#SPJ11
classify the following compounds as ionic or covalent: bao, fe₂o₃, zno.
Answers
The compounds BaO, Fe₂O₃, ZnO, they all are ionic compounds.
Ionic compounds are compounds made up of positive and negative ions. Electrostatic forces hold them together. The following elements commonly form ionic compounds: nonmetals and metals, polyatomic ions, and transition metals.
Covalent compounds are compounds made up of two or more non-metallic elements. They join together by sharing electrons to achieve a stable electron configuration
BaO: Ba is a metal, while O is a nonmetal, therefore it's an ionic compound
Fe2O3: Fe is a metal, while O is a nonmetal, therefore it's an ionic compound
ZnO: Zn is a metal, while O is a nonmetal, therefore it's an ionic compound
Thus, all of the above compounds i.e. BaO, Fe₂O₃, ZnO are ionic compounds.
To learn more about ions :
https://brainly.com/question/13692734
#SPJ11
1.0 mol of oxygen gas is added to a container at 25c. the pressure is adjusted to 101.352 kpa. what is the volume?
Answers
The volume of the container is approximately 24.46 liters.
To determine the volume of 1.0 mol of oxygen gas in a container at 25°C and 101.352 kPa, we can use the Ideal Gas Law formula:
PV = nRT
Where:
P = pressure (in atm)
V = volume (in L)
n = number of moles
R = gas constant (0.0821 L atm/mol K)
T = temperature (in K)
First, we need to convert the temperature from Celsius to Kelvin:
T = 25°C + 273.15 = 298.15 K
Next, we need to convert the pressure from kPa to atm:
1 atm = 101.325 kPa
P = 101.352 kPa * (1 atm/101.325 kPa) = 1.000267 atm
Now, we can solve for the volume (V) using the Ideal Gas Law formula:
V = nRT/P
Plugging in the values:
V = (1.0 mol) * (0.0821 L atm/mol K) * (298.15 K) / (1.000267 atm)
V ≈ 24.46 L
The volume of the container is approximately 24.46 liters.
To know more about volume of gas, visit
https://brainly.com/question/27100414
#SPJ11
explain what keeps the electrons confined in the space surrounding the nuleus
Answers
Answer:
Electrons are trapped inside the atom because of the attraction forces with positively charged protons that are found in the nucleus.
When might people use data tables and graphs in their everyday lives? How are these uses similar to and different from the way in which scientists use these same tools?
Do you think scientists need data tables and graphs to do their work?
Answers
Answer:
yes
Explanation:
i think that because when they are conducting an investigation its important to organize the data you have collected. when a scientist organizes the data is much better for them to interpret what has already been observed.