Answers
Answer:
1. C+ ---- O-
2. O+ ---- Cl-
3. O+ ----- F-
4. C+ ----- N-
5. Cl- ----- C+
6. S- ----- H+
7. S+ ----- Cl -
Explanation:
Electronegativity determines the polarity . There may be two atoms in a bond with high electronegativity, in such cases the positive charge is given to atom with comparatively lower electronegativity. Electronegativity determines the easiness with which an atom attract electrons in a chemical bond. A polar bond is formed when the difference in the electronegativity of two combining atoms is between 0.4 and 1.7. The correct direction is
1. C+ ---- O-
2. O+ ---- Cl-
3. O+ ----- F-
4. C+ ----- N-
5. Cl- ----- C+
6. S- ----- H+
7. S+ ----- Cl -
Related Questions
An isomer of C3H7O undergoes one step oxidation reaction. Answer the following questions due to this reaction.
a) Write a full symbol equation for this reaction.b) Name the proper reagent and catalyst for this reaction.c) Why do you think there is no need to remove the product from the reaction vessel?
Answers
Answer:
C3H7O + O2 → CO2 + H2O
Explanation:
a) The full symbol equation for the oxidation reaction of an isomer of C3H7O can be represented as:
C3H7O + O2 → CO2 + H2O
b) The proper reagent for this oxidation reaction is O2 (oxygen gas). The catalyst required for this reaction depends on the specific conditions. Common catalysts used for oxidation reactions include transition metals such as platinum (Pt), palladium (Pd), or copper (Cu).
c) There is no need to remove the product (CO2 and H2O) from the reaction vessel because they are typically in the gas or liquid phase and do not significantly interfere with the reaction. The product gases can be easily vented out of the vessel, while the liquid water can be left in the reaction mixture. Additionally, the product CO2 is a stable and inert gas, which does not pose any hazards in most cases. Therefore, it is often not necessary to remove the products after the reaction is complete.
TRUE OR FALSE nitric acid is a key industrial chemical, largely used to make fertilizers and explosives. the first step in its synthesis is the oxidation of ammonia. in this reaction, gaseous ammonia reacts with dioxygen gas to produce nitrogen monoxide gas and water.
Answers
Using 645 L /s of oxygen at 195 ° C and 0.88 atm will result in 0.355kg/s of NO.
First, we go through the equation for the conversion of NH3 to NO: 4NH3(g) + 5O2(g) ⟶4 NO(g) +6 H2O(l). (l)
Next, we compile the data that will be used in our computations.
645 L/s O2 Volume Rate
0.88 atm of pressure
Temperature is equal to 195°C plus 273°K.
NO has a molecular mass of 30.01 g/mol.
Third, using the basic gas equation PV =n RT, we must determine how many moles (n) are contained in 645L of O2 in order to compute the number of NO moles created by this amount of O2.Keep in mind that the constant R in this equation is 0.08205Lxatm/Kxmol.
PV =n RT
n= PV / RT
n= [0.08205 Lxatm/Kxmol] x 468K / [(0.08atm x 645L/s)]
n= 14.781 moles of oxygen per second.
Fourth, now that we know how many moles of O2 will be created, we can use the equation to determine how many moles of NO will also be produced. Finally, with the help of the molecular weight, we will be able to get the total mass per second.
14.781 moles of O2 x 4 moles of NO x 5 moles of O2 x 30.01g of NO x 1 mol of NO x 1 kg of NO /1000g of NO = 0.355 kg/s of NO
Complete question:
Nitric acid is a key industrial chemical, largely used to make fertilizers and explosives. The first step in its synthesis is the oxidation of ammonia. In this reaction, gaseous ammonia reacts with dioxygen gas to produce nitrogen monoxide gas and water. Suppose a chemical engineer studying a new catalyst for the oxidation of ammonia reaction finds that liters per second of dioxygen are consumed when the reaction is run at and . Calculate the rate at which nitrogen monoxide is being produced. Give your answer in kilograms per second. Round your answer to significant digits.
learn more about molecular weight Refer:brainly.com/question/27988184
#SPJ4
(4 pts) Fill in the number of protons and electrons for each product and reactant (two boxes have been filled in for you). (2 pts) Verify that the number of protons on the left side of the chemical equation is equal to the number of protons of the right side. Show your work. (2 pts) Verify that the number of electrons on the left side of the chemical equation is equal to the number of electrons of the right side. Show your work. (3 pts) Which substance is being oxidized
Answers
Answer:
a)
Zn(s) + 2 H⁺(aq) ⇒ Zn²⁺(aq) + H₂(g)
#p⁺ 30 2 30 2
#e⁻ 30 0 28 2
b) 32
c) 30
d) Zn
Explanation:
There is some info missing. I will add the complete question.
Consider the following oxidation/reduction reaction.
Zn(s) + 2 H⁺(aq) ⇒ Zn²⁺(aq) + H₂(g)
#p⁺ 2
#e⁻ 2
a) Fill in the number of protons and electrons for each product and reactant (two boxes have been filled in for you).
b) Verify that the number of protons on the left side of the chemical equation is equal to the number of protons of the right side. Show your work.
c) Verify that the number of electrons on the left side of the chemical equation is equal to the number of electrons of the right side. Show your work.
d) Which substance is being oxidized?
a) The atomic number of Zn is 30 so it will have 30 protons. Since it ts neutral, it will also have 30 electrons. Zn²⁺ will also have 30 protons but it lost 2 electrons, so it has 28 electrons. The atomic number of H⁺ is 1, so each H atom will have 1 proton (2 in total). But since H has 1 electron, and H⁺ lost 1 electron, H⁺ will have 0 electrons. The complete chart is:
Zn(s) + 2 H⁺(aq) ⇒ Zn²⁺(aq) + H₂(g)
#p⁺ 30 2 30 2
#e⁻ 30 0 28 2
b) The total number of protons on the left side is: 30 + 2 = 32.
The total number of protons on the right side is: 30 + 2 = 32.
c) The total number of electrons on the left side is: 30 + 0 = 30.
The total number of electrons on the right side is: 28 + 2 = 30.
d) Zn(s) is oxidized because it loses electrons (from 30 to 28) and its number increases.
Suppose that a person eats a diet of 2398 Calories per day.
Convert this energy into joules.
Convert this energy into kilojoules.
Convert this energy into kilowatt-hours.
Answers
Answer:
10,033,232 joules
10,033.232 kilojoules
2.78837209 kilowatt-hours
Explanation:
2398 * 4184 =
2398 * 4.184 =
2398 / 860 =
Who is the Chief of GTOCP
Answers
Answer:
???
Explanation:
Identify whether each element is a halogen, a noble gas, or nonmetal only.
Astatine (At):
Nitrogen (N):
Krypton (Kr):
Chlorine (Cl):
Sulfur (S)
Answers
In descending order
Halogen
Non metal
Noble Gas
Halogen
Non metal
Which of the following characteristics do Element I and Element I have in common?

Answers
Answer:
Option C. The same number of energy levels.
Explanation:
From the diagram given above, element (i) belong to group 2 while element (ii) belong to group 6.
Also, both element i and ii belong to the same period (i.e period 4). This simply means that both element i and ii have the same number of energy levels.
NOTE: Elements in the same period have the same number of shells of electrons which simply means they have the same energy levels.
4 Some elements have symbols that do not appear to match their names. For
example, the symbol for sodium is Na. Why is this?
Answers
Answer:
It is because some elements have the symbol of their Latin names for example - Latin name of sodium is Natrium and potassium is Kalium .Thatswhy their symbol differs from their modern name .
Drag each tile to the correct box.
Arrange the bonds in order based on bond length. Start with the longest bond, and end with the shortest bond.
C-N
bond energy = 305 kJ/mol
C=N
bond energy = 615 kJ/mol
C=N
bond energy = 887 kJ/mol

Answers
The bond length has been defined by the distance shared by the covalently bonded atoms. C≡N (887 kJ/mol) has the longest bond followed by C=N and the shortest C-N.
What is the bond length?Bond length is a parameter that defines the bond distance and measures the length between the bonded atoms. It is determined by bonded electrons.
The bond energy and bond length are directly related and hence the C≡N (887 kJ/mol) has the longest bond length because of high energy followed by C=N (615 kJ/mol) and the shortest C-N (305 kJ/mol).
Therefore, the order of bond length is C≡N > C=N > C-N.
Learn more about bond length, here:
https://brainly.com/question/14494256
#SPJ1
Is the study of matter and energy complete, or do you think there’s still new information to discover?
Answers
Answer:
Physical science, in turn, can be divided into chemistry and physics. Chemistry is the study of matter and energy at the scale of atoms and molecules. ... Physics is the study of matter and energy at all scales—from the tiniest particles of matter to the entire universe but i dont really know if there is new information to discover
Explanation:
hope it help and if it doesnt sorry ;)
explain how the particles of a substance change as a substance changes from a solid to a liquid to a gas.
Answers
Answer:
Some substances can change from the solid state to the gas state without ever becoming a liquid. During this process, known as sublimation, the sur- face particles of the solid gain enough energy to become a gas.When dry ice becomes a gas, it absorbs thermal energy from water vapor in the air.
Explanation:
A vacuum pump is capable of evacuating a vessel to a pressure of 5.0 x 10⁻³ mmHg. What is the pressure in the vessel in atm?
Answers
The vessel's internal pressure is atm, is \(6.66*10^{-6} atm\)
A vacuum pump is a machine that expels air or gas from a sealed space in order to create a partial vacuum and a difference in pressure. Based on the pressure needs and the application it serves, vacuum pumps are designed using a range of technologies.Fatal accidents have happened in the history of pressure vessel invention and use, proving that pressure vessels can be harmful.A container designed to carry gases or liquids at a pressure much higher than atmospheric pressure is known as a pressure vessel.
The pressure in the vessel in atm is \(6.66 * 10^{-6} atm\).
To calculate this, we first convert \(5.0 * 10^{-3} mmHg\) to atm.
1 mmHg = 0.001315789 atm
Here, \(5.0 * 10^{-3} mmHg = 6.66 * 10^{-6} atm.\)
Therefore, the pressure in the vessel in atm is \(6.66*10^{-6} atm\)
learn more about pressure Refer:brainly.com/question/18431008
#SPJ1
A 115.0-g sample of a metal at 165.0 °C is added to 265.0 g of ethylene glycol (specific heat capacity = 2.43 J/g・ °C) in a calorimeter at 25.8 °C. The temperature of the ethylene glycol rises to 41.5 °C. Calculate the specific heat capacity of the metal, assuming that all the heat lost by the metal is gained by the ethylene glycol.
Answers
The specific heat capacity of the metal can be calculated to be 2.03 J/g・ °C. This result can be explained by the fact that the heat lost by the metal is equal to the heat gained by the ethylene glycol, since no other source of heat is present.
What is specific heat capacity?Specific heat capacity is the amount of heat energy needed to raise the temperature of a unit mass of a substance by 1 degree Celsius. It is measured in joules per kilogram Kelvin (J/kg K).
The specific heat capacity (c) of the metal can be calculated using the following equation:
Q = mc∆T
Where Q is the heat lost by the metal, m is the mass of the metal, and ∆T is the change in temperature of the metal.
Q = (115.0 g)(c)(165.0 °C - 25.8 °C)
On the other hand, the heat gained by the ethylene glycol can be calculated using the following equation:
Q = (265.0 g)(2.43 J/g・ °C)(41.5 °C - 25.8 °C)
By equating the two equations, we can solve for the specific heat capacity of the metal:
(115.0 g)(c)(165.0 °C - 25.8 °C) = (265.0 g)(2.43 J/g・ °C)(41.5 °C - 25.8 °C)
c = 2.03 J/g・ °C
Therefore, the specific heat capacity of the metal can be calculated to be 2.03 J/g・ °C.
This result can be explained by the fact that the heat lost by the metal is equal to the heat gained by the ethylene glycol, since no other source of heat is present.
For more questions related to ethylene glycol
https://brainly.com/question/11994953
#SPJ1
Why is an electron ejected when green light hits the metal?
Answers
Explanation:
If a large photon strikes the surface, that has enough strength to take out an electrode, which will then travel to the positive side since it is negative. Current is flowing at this stage. Since the reduced photons will be unable to distinguish between atoms, no power can pass.
What does Ra Ra Ah Ah Ah Ro Ma Ro Ma Ma Ga Ga O La La mean?
Answers
Answer:
They are symbols of elements.
Ra is the symbol of the element Radium
Ah is the symbol of the element Arrhenium
Ma is the symbol of the element Molybdenum
Ga is the symbol of the element Gallium
La is the symbol of the element Lanthanum
Answer:
Ra is the symbol of the element Radium
Ah is the symbol of the element Arrhenium
Ma is the symbol of the element Molybdenum
Ga is the symbol of the element Gallium
La is the symbol of the element Lanthanum
Explanation:
They are elements
Total anions =
Total cations =
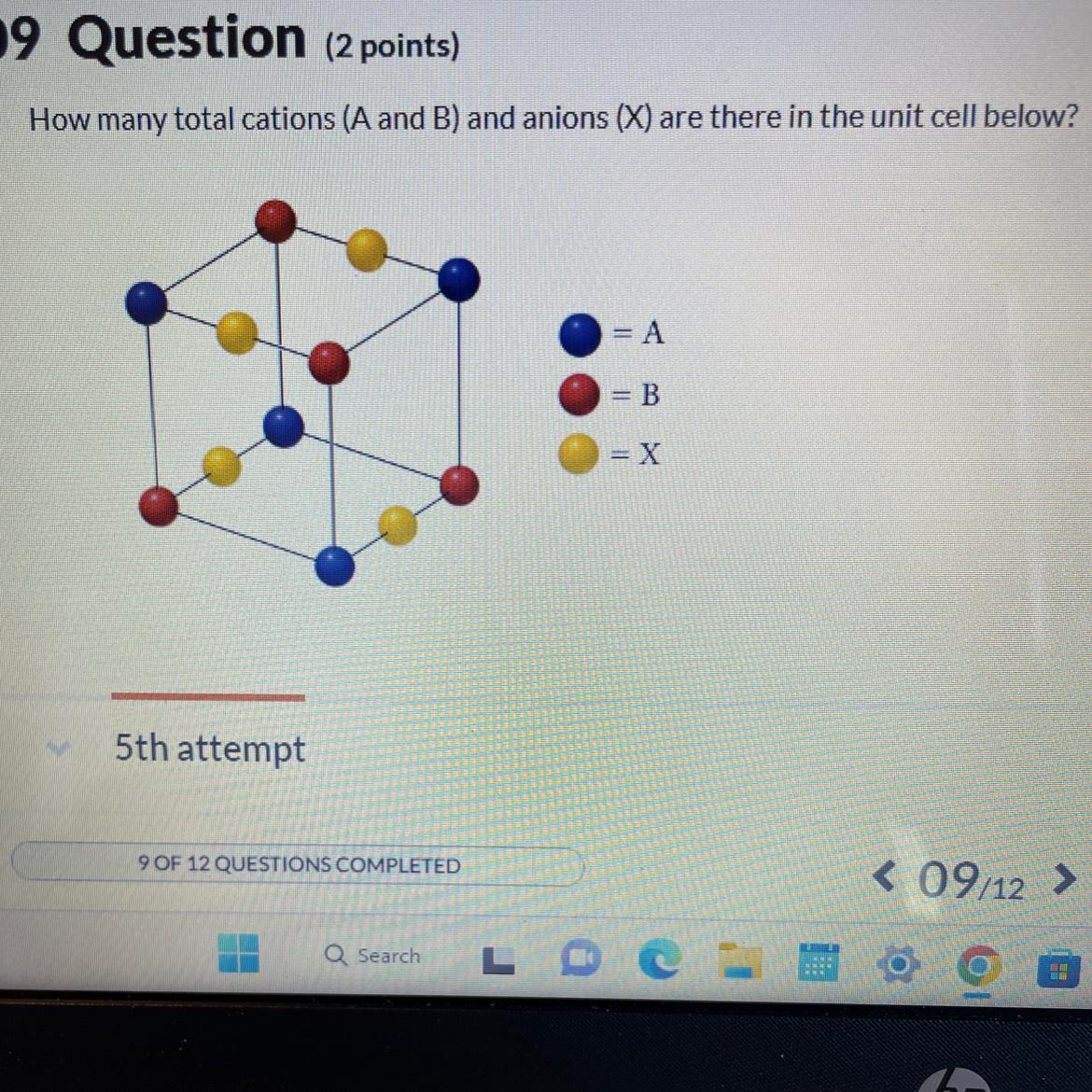
Answers
The total number of cation is 1 and the total number of anion is 1.
What is cation and anion ?The positively charged particles are called as cation and the negatively charged particles are called as anions.
The number of atoms present in unit cell at corner are 4 red ( B cation ) 4 Blue (A cations)
Total number of cations at the corner = 8
1 atom at corner have 1/8th of its portion
8 atom have 1/8th × 8 atom = 1 atom
Number of cation = 1
Atoms at the edge contributes ¼ th portion
There are 4 anions at edges
Contribution from 4 anions
= 1 / 4 × 4
= 1
Total anions = 1
Total cations = 1
Thus, the total number of cation is 1 and the total number of anion is 1.
To learn more about the cation and anion, follow the link;
https://brainly.com/question/4933048
#SPJ1
Which of the following transition is considered the phase
change from water to steam?
Answers
Answer:
the transition from water to steam is call evaporation.
Explanation:
water evaporates or vaporizes and turns into steam this typically happens when heat meets with the water. an example would be if you boil water steam will release from the pot, or when it rains, snows, etc (precipitation) the water evaporates and the ground dries.
Which of the following is/are correct about molar mass?
A. Can be calculated using the elements’ atomic mass in the periodic table
B. It is the mass in grams of one mole of any pure substance
C. Has the unit of mol/g
D. A and B
Answers
The elements atomic mass is the same thing as molar mass. The mass is always in grams per mole.
Choose the one statement that is true of the noble gases.A.They are very reactive.B.Their valence shells are full of electrons.C.They are unstable.D.They are liquids at room temperature.
Answers
B. Their valence shell are full of electrons
That is the main characteristic of noble gases, and it is because of this that they are very stable, very unreactive and they have very low condensation temperature.
during the Electrolysis of a Salt m. a Current OF 0-5A flows for 32m 10. Sec and deposit 0-325-9 what is the Charge • OF Meta Jon (m=65; f=96500]
Answers
Answer:
2
Explanation:
Using m/M=It/nF
0.325/65=0.5×(32×60+10)/96500n
n=62725/31362.5
n=2
under what circumstances do you think credit cards should NOT be used ?
Answers
It's never a good idea to use your credit card when experiencing strong emotions, especially if you tend to steer toward 'retail therapy.
In the combustion of hydrogen gas, hydrogen reacts with oxygen from the air to form water vapor. hydrogen+oxygen⟶water
If you burn 46.2g of hydrogen and produce 413g of water, how much oxygen reacted?
mass of oxygen:
Answers
Answer:
ok, here is your answer
Explanation:
AI-generated answer
To find the mass of oxygen that reacted, we need to use the Law of Conservation of Mass, which states that in a chemical reaction, the mass of the reactants equals the mass of the products.
First, we need to find the number of moles of hydrogen that reacted:
Molar mass of hydrogen (H₂) = 2.016 g/mol
Number of moles of H₂ = mass/molar mass = 46.2 g/2.016 g/mol = 22.92 mol
Next, we need to use the balanced chemical equation to find the number of moles of water produced:
hydrogen + oxygen → water
2H₂ + O₂ → 2H₂O
From the equation, we can see that for every 2 moles of H₂, 1 mole of O₂ is required to produce 2 moles of H₂O. Therefore, the number of moles of O₂ required to produce 22.92 moles of H₂O is:
Number of moles of O₂ = 1/2 x 22.92 mol = 11.46 mol
Finally, we can find the mass of oxygen that reacted by using its molar mass:
Molar mass of oxygen (O₂) = 32.00 g/mol
Mass of oxygen = number of moles x molar mass = 11.46 mol x 32.00 g/mol = 366.72 g
Therefore, the mass of oxygen that reacted is 366.72 g.
mark me as brainliestWhich of the following is the best definition of a physical change?
A. Ice melting into water.
B. A change that occurs without changing the identity of the substance.
C. Something that can be observed or measured while changing the identity of the substance.
D. A nail rusting.
Answers
Help pls
What is the final volume of a gas at 3.6 atm and 95 mL that has expanded until it has pressure of 0.85 atm?
Answers
Answer: 404.71 ML
Explanation:
We can use Boyle's Law to solve this problem, which states that the pressure and volume of a gas are inversely proportional at a constant temperature.
Boyle's Law equation: P1V1 = P2V2
Where:
P1 = initial pressure
V1 = initial volume
P2 = final pressure
V2 = final volume
Given:
P1 = 3.6 atm
V1 = 95 mL
P2 = 0.85 atm
We can rearrange the equation to solve for V2:
V2 = (P1V1)/P2
V2 = (3.6 atm * 95 mL)/(0.85 atm)
V2 = 404.71 mL
Therefore, the final volume of the gas is approximately 404.71 mL. Hopefully this helps gang fasho
We can use Boyle's law to solve this problem, which states that the pressure of a gas is inversely proportional to its volume when temperature and amount are held constant. The formula is:
P1V1 = P2V2
Where:
P1 = 3.6 atm (initial pressure)
V1 = 95 mL (initial volume)
P2 = 0.85 atm (final pressure)
V2 = ? (final volume)
Substituting the given values, we get:
(3.6 atm)(95 mL) = (0.85 atm)(V2)
Simplifying, we get:
V2 = (3.6 atm)(95 mL) / (0.85 atm)
V2 = 404.7 mL
Therefore, the final volume of the gas is 404.7 mL.
Phillip wants to teach his brother that the Sun gives off heat energy. What can Phillip tell his brother to demonstrate that the Sun has heat energy?
Responses
A It easier to see during the day than at night.It easier to see during the day than at night.
B Ice cream melts faster outside on a sunny day.Ice cream melts faster outside on a sunny day.
C It is warmer inside the house than outside.It is warmer inside the house than outside.
D Sunglasses help keep the light out of your eyes.
Answers
Calcium carbonate is reacting with hydrochloric acid and water to form the products calcium chloride hexahydrate and carbonic acid(which decomposes into water and carbon dioxide). Note: water is part of this reaction! if 10.76 g of calcium reacts with 10.51 g of hydrochloric acid, calculate how many grams of the hydrate will be formed
I need the written reaction, the limiting reactant work, and lastly, the stoichiometry work
Answers
Answer:
11.5 g
Explanation:
28.0 g
−
16.5 g
=
11.5 g
How many moles of CaSO4 are required to produce 128 g of SO2? 3CaSO4 + CaS → 4CaO + 4SO2
Answers
2 moles of CaSO4 are required to produce 128 g of SO2 in 3CaSO4 + CaS → 4CaO + 4SO2 in this equation.
What do you mean by mole ?The term mole is defined as a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules, or other specified particles.
1 mole = 6.023 × 10²³ molecules
The balance equation is as follows:
3CaSO4 + CaS → 4CaO + 4SO2
1 mole = 6.023 × 10²³ molecules
1 mole SO₂ = 6.023 × 10²³ molecules
128 mole = ?
Molar mass of SO₂ = 64 gram
The numbers of moles in 128 gram SO₂ = 1 / 64 ×128
= 2 moles
Thus, 2 moles of CaSO4 are required to produce 128 g of SO2.
To learn more about the mole, follow the link;
https://brainly.com/question/26416088
#SPJ1
A teacher has given a lab student a white chemical sample and asks her to confirm that it contains 33.4 g sodium hydroxide (NaOH). If the teacher provides the amount in formula unitswhich value is correct?
A. 40.0 g
B. 46.78
C. 5.03 x 10^23
D. 7.03 x 10^24
Answers
The amount in, formula unit. of 33.4 g sodium hydroxide is 5.03 x \(10^{23\) . Option C.
Formula unitsIn order to calculate the mass of NaOH from formula units, first, we need to know the molar mass of NaOH, which is 40 g/mol1.
The formula units are related to moles by Avogadro’s number which is 6.022 x 10^23 formula units per mole.
Therefore, we can calculate the mass of NaOH as follows:
33.4 g NaOH = (33.4 g NaOH / 40) x (6.022 x 10^23) = 5.03 x \(10^{23\) formula units.
In other words, there are 5.03 x \(10^{23\) formula units in 33.4 g sodium hydroxide.
More on formula units can be found here: https://brainly.com/question/21494857
#SPJ1
After precipitation falls, the water moves into the lithosphere through
openings in the rock or soil. What is this process called?
O
A. Evaporation
B. Mineralization
C. Percolation
OD. Sublimation
SUBMIT
D
HANA
Answers
After precipitation falls, the water moves into the lithosphere through openings in the rock or soil. This process is called percolation.
Therefore, Option C is the correct option.
What is Evaporation?Evaporation is defined as the process of conversion of liquid into vapours.
For example: Conversation of water into water vapours.
But int he question, there is no conversion. So this option is incorrect option.
What is Sublimation?Sublimation is the process of conversion of solid directly into the vapour phase
Since, there is no conversion of of solid into vapours. So, this option also incorrect option.
What is Mineralization?Mineralization is defined as the process through which chemicals which is present in organic matter are decomposed or oxidized easily in the available forms to plants.
As there is no decomposition of minerals. So, this is also incorrect option.
What is Percolation?Percolation is defined as the process in which liquid slowly allow to pass through a filter.
So, this is correct option.
Thus, we concluded that the water moves into the lithosphere through openings in the rock or soil. This process is called percolation.
learn more about Sublimation:
https://brainly.com/question/11044849
#SPJ9
Muối sunfua với hidro sunfua có giống nhau về tính chất vật lý không ạ. Ứng dụng và điều chế muối sufua như thế nào ạ
Answers
the answer is is is is is is is is is is is is is is