Answers
increasing concentration of dissolved CO₂ raises the acidity of ocean water.
What happens when CO₂ is dissolved in ocean?The pH of the ocean (a measurement of how acidic or basic the ocean is) decreases when carbon dioxide dissolves in the water, making the water more acidic. Despite the ocean's size, too much carbon dioxide may have a significant effect.
As atmospheric CO₂ levels rise, carbon dioxide dissolves in water to generate carbonic acid (H₂CO₃), which contributes to the acidification of the oceans.
As temperatures increase, carbon dioxide pours out of the ocean like a glass of root beer on a hot day. Deeper waters, which are rich in carbonate dissolved from limestone and other rocks, must be up welled in order to replenish carbonate, which depletes and must be replenished.
So, increasing concentration of dissolved CO₂ raises the acidity of ocean water.
To know more about dissolved CO₂ refer to:
https://brainly.com/question/9100700
#SPJ1
Related Questions
Why do we monitor chinstrap penguins instead of krill?
Answers
Answer:Yes
Explanation:
Because Chinstrap penguins eat krills
Based on the bond energies for the reaction below, what is the enthalpy of the reaction? H₂ (g) + N₂ (g) + 2 C (g) → 2 HCN (g)
Answers
Answer:
-1222 kj
Explanation:
You can calculate the bond dissociation energy for each species using the table. Subtract the energies of the bonds made from the energies of the bonds broken. Remember to use the coefficients from the balanced chemical reaction.
BDE = [(H-H) + (N≡ N)] - [2 * [(H-C) + (C≡N)]]
BDE = [(432 kJ) + (942 kJ] - [2 * [(411 kJ) + (887 kJ)]] = -1222 kJ
Based on the bond energies for the given reaction, the enthalpy of the reaction is:
-1222 kj
According to the given question, we are asked to calculate the enthalpy of the given reaction based on the bond energies given in H₂ (g) + N₂ (g) + 2 C (g) → 2 HCN (g).
As a result of this, we can see that bond dissociation energy for each of the species on the table need to be subtracted and then to make use of the coefficient of the balanced chemical reaction.
At the end, we would get 2*(411 kj) + (887 kj) which would give us
-1222 kj
Therefore, the correct answer is -1222 kj
Read more here:
https://brainly.com/question/12902391
Why does a solid have a definite shape and volume?
A. The particles in a solid are not attracted to one another.
B. The particles in a solid vibrate around fixed locations.
C. The particles in a solid are not tightly packed.
D. The particles in a solid travel at a high speed of motion.
Answers
Answer:
B
Explanation:
Particles in a solid have fixed locations in a volume that does not change. Solids have a definite volume and shape because particles in a solid vibrate around fixed locations.
Silver (1) oxide → silver + oxygen gas
Answers
Answer: come on lets link can do what i do
Which process do self-feeders use to get energy?

Answers
Answer:
The person above me is correct
Explanation:
It's D
Prepare one solution that has 0.12 M of FeCl3 and 0.40 M of HCl with the reagents 3 M HCl and Solid FeCL3 * 6H20. Provide the calculations and protocol to make the solution in a lab.
Answers
To prepare a 0.12 M solution of FeCl₃, the amount of solid FeCl₃ to be dissolved in a given volume of solvent will be 9.72 grams.
Given,
Molarity of FeCl₃ (M)= 0.12 M
The molecular weight (m) of FeCl₃ is = 162 gm
The volume of the solution (V) to be prepared is =500 ml
The amount of FeCl₃ to be dissolved to make a 0.12 M solution is= x
So,
MV= x ÷ m × 1000
0.12× 500 = x ÷ 162 × 1000
x = 60 × 162 ÷ 1000
x= 9.72 gm
So 9.72 grams of FeCl₃ is dissolved to make 500 ml of 0.12 M solution.
For preparing 0.4 M HCl from 4M HCL:
If we need to make 500 ml of solution with 0.4M of HCL, then we use the formula:
M₁V₁= M₂V₂
0.4 × 500= 4 × x
x= 50 ml
So 50 ml of 4M HCL is taken to make 0.4 M HCL.
To learn more about FeCl₃, refer to the link:
https://brainly.com/question/32098087
#SPJ1
The analysis of a compound gives the following percent composition by mass:
C: 58.59 percent; H: 10.16 percent; S: 10.43 percent; O: 20.82 percent. What is its molecular formula
given that its molar mass is 307.5 g?
Answers
Answer:
C15 H31 O4 S
Explanation:
molecular formula is also the same because the value of "n" is 1
If we assume that the He nucleus is a sphere, its diameter measures approximately 2.0 fm. What is the density of the nucleus in g/cm3? Please provide your answer in grams per cubic centimeter.
Answers
The density of the He nucleus is approximately 2.8 × 10^17 grams per cubic centimeter (g/cm³).
To calculate the density of the He nucleus, we need to know its mass and volume. Given that the diameter of the nucleus is approximately 2.0 fm, we can calculate its radius as 1.0 fm (since the radius is half the diameter).
The volume of a sphere can be calculated using the formula V = (4/3)πr³, where V is the volume and r is the radius. Substituting the values, we get:
V = (4/3)π(1.0 fm)³
Using the conversion factor that 1 fm = 1 × 10^-13 cm, we can convert the volume from fm³ to cm³:
V = (4/3)π(1.0 × 10^-13 cm)³
Simplifying the equation further, we have:
V = (4/3)π(1.0 × 10^-13)³ cm³
Now, to calculate the density, we need the mass of the He nucleus. The mass of a He nucleus is approximately 4 atomic mass units (4 amu).
Using the conversion factor that 1 amu = 1.66 × 10^-24 g, we can convert the mass from atomic mass units to grams:
Mass = 4 amu × (1.66 × 10^-24 g/amu)
Now we have both the mass and volume. The density (D) of an object is defined as mass divided by volume:
D = Mass/Volume
Substituting the values, we get:
D = (4 amu × 1.66 × 10^-24 g/amu) / [(4/3)π(1.0 × 10^-13)³ cm³]
Simplifying the equation and performing the calculations, the density of the He nucleus is approximately 2.8 × 10^17 g/cm³.
For more such questions on density visit:
https://brainly.com/question/26364788
#SPJ8
balanced equation for beta decay of iodine-138
Answers
Answer:
Explanation:
Please take a look at the attached picture about the general formula of beta decay and the answer.
beta decay is an atom breaking down into a new atom of another element which the atomic number is 1 larger than the original, and one electron (β particle). The mass no. of the new atom remains same as before.
138 is iodine's mass number, the atomic number of iodine is 53, referred from a periodic table. The new atom formed will be Xe, which has an atomic no. of 54.
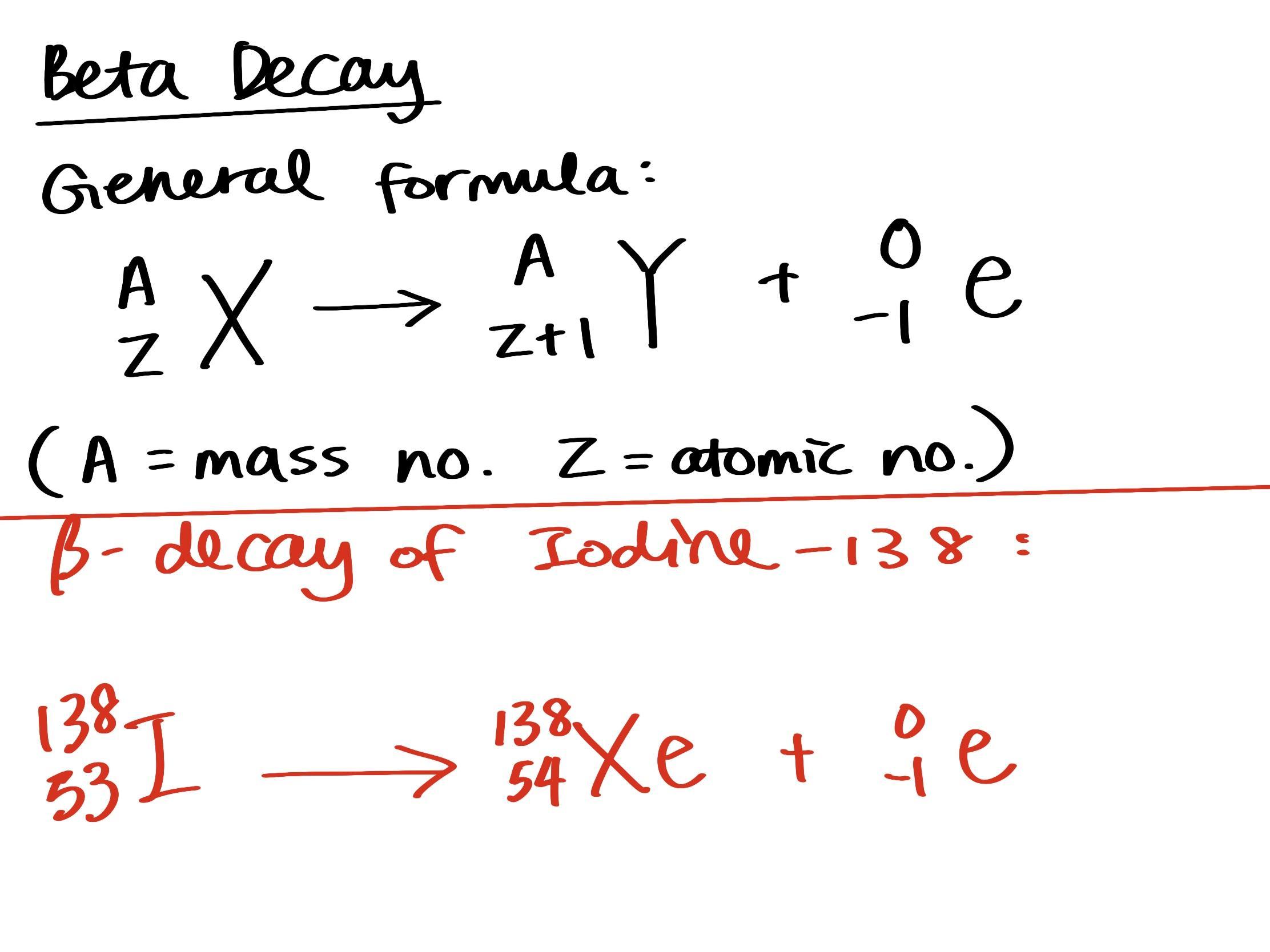
starting with toluene which sequence of reactions below works best to prepare the folowing cyclohexadiene compound
Answers
The toluene which is sequence of reactions below the works best to prepare the following cyclohexadiene compound is given as follows :
NBS , heat NaOCH₃ Na, NH₃
Toluene ------------------> -----------------> ------------>
CCl₄ CH₃OH CH₃OH
The toluene is the substituted aromatic hydrocarbon. the cyclohexadiene is used as the starting material for the synthesis of the natural complex products. the cyclohexadiene can be formed by the toluene . the synthesis which is the best to prepare the cyclohexadiene from the compound that is toluene is given as follows :
NBS , heat NaOCH₃ Na, NH₃
Toluene ------------------> -----------------> ------------>
CCl₄ CH₃OH CH₃OH
To learn more about toluene here
https://brainly.com/question/16243535
#SPJ4
The largest source of power generated in the US is
A)
fossil fuel combustion.
B)
nuclear.
C)
solar.
D)
wind.
Answers
Answer:
A. fossil fuel combustion
Explanation:
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with pH. If the solubility does change, pick the pH at which you'd expect the highest solubility.
a. Ca(OH)2
b. CuBr
c. Ca3(PO4)2
Answers
Answer:
1. Ca(OH)₂ : Yes
Highest solubility = pH of 2
2. Cu Br : Yes
Highest solubility = pH of 4
3. Ca₃(PO₄)₂ : Yes
Highest solubility = pH of 2
Explanation:
From the common ion effect, the solubility of an ionic compound in a solution which already contains one of the ions in that compound will reduce. This is because, when an external stress is placed on a system in equilibrium, the equilibrium position will shift to remove the effect of that stress. Therefore, when more of the same ions are added to a solution already containing that ion, equilibrium will shift towards removal of the ion, thereby reducing solubility.
1. For Ca(OH)₂:
Ca(OH)₂ is a fairly soluble ionic compound whose dissociation equation is given below:
Ca(OH)₂ (s) ----> Ca²+ (aq) + 2 OH- (aq)
Increase in pH will result in addition of more OH- ions, therefore, its solubility will decrease. On the other hand, a decrease in pH will remove OH- ions, thereby increasing solubility. The pH of highest solubility is 2.
2. For CuBr:
CuBr is a slightly acidic salt as it is salt of a strong acid, HBr and a weak base Cu(OH)₂. Hydrolysis of the salt will result in an acidic medium:
2 CuBr (s) + 2 H₂O (l) ----> 2 HBr (aq) + Cu(OH)₂ (aq).
Thus, an increase in pH will result in an increase in the solubility of CuBr due to the removal of H+ ions. The highest solubility will be at pH of 4.
3) For Ca₃(PO₄)₂
Calcium phosphate is a salt of slightly basic salt as it is a salt of a weak acid, H₃PO₄ and stronger base Ca(OH)₂.
Hydrolysis of the salt will result in a basic medium:
Ca₃(PO₄)₂ (s) + 6 H₂O (l) ---> 3 Ca(OH)₂ (aq) + 2 H₃PO₄ (aq)
Therefore, its solubility increases with decrease in pH due to the removal of OH- ions. The highest solubility will be at pH of 2.

URGENT!!! An unknown hydrate of CoCl₂ has been evaporated in a crucible. Given the following data, find the formula and name of the hydrate.
Mass of crucible: 12.090 g
Mass of hydrate before evaporation and crucible: 16.250 g
Mass of hydrate after evaporation and crucible: 12.424 g
Answers
From the given data, the name of the hydrated salt would be \(CoCl_2.83H_2O\).
Formula of hydrateThe formula of the hydrated salt can be determined using the empirical formula approach. That is, we will find the mole equivalent of the anhydrous salt and the water of hydration and then combine them into a single formula after dividing by the smallest mole.
First, we need to determine the mass of the anhydrous salt and the water of hydration.
Mass of crucible (x) = 12.090 g
Mass of hydrated salt + crucible (y) = 16.250 g
Thus, the mass of the hydrated salt can be determined by subtracting x from y.
Mass of hydrated salt = 16.250 - 12.090 = 4.16 g
Mass of hydrate + crucible after evaporating off the water (z) = 12.424 g
Mass of anhydrous salt = z - x
= 12.424 - 12.090
= 0.334 g
Mass of water = 4.16 - 0.334
= 3.826 g
Now, let's find the moles:
Molar mass of \(CoCl_2\) = 129.839 g/mol
Molar mass of water = 18.01 g/mol
Mole of \(CoCl_2\) = 0.334/129.839 = 0.00257 mol
Mole of water = 3.826/18.01 = 0.2124 mol
Dividing through by the smallest mole
\(CoCl_2\) = 0.00257 / 0.00257 = 1
water = 0.2124/ 0.00257 = 83
Thus, the formula of the hydrate would be \(CoCl_2.83H_2O\)
More on hydrate salts can be found here: https://brainly.com/question/16990374
#SPJ1
You want to decaffeinate your coffee by extracting the caffeine outwith dichloromethane. It's too late to extract the caffeinefrom the coffee beans because you've already brewed yourself a 200mL cup of coffee. Your particular brand of coffee contains100 mg of caffeine in that 200mL cup. The partitioncoefficient of caffeine in dichloromethane/water is 9.0.
How much caffeine would still be in your 200 mL if you did:
A. One extraction using 200 mL o fdichloro methane
B. Two extractions using 100 mL of dichloro methane each.
Answers
Solution :
The partition coefficient
\($k_d= \frac{\text{(mass of caffeine in }CH_2Cl_2 / \text{volume of }CH_2Cl_2)}{\text{(mass of caffeine in water/ volume of water)}}$\)
= 9.0
A). 1 x 200 mL extraction
Let m be the mass of caffeine in water
Mass of caffeine in \($CH_2Cl_2$\) = 100 - m
∴ \($\frac{(100-m)/200}{m/200}=9$\)
\($\frac{100-m}{m}=9$\)
\($10 \ m = 100$\)
\($m=\frac{100}{10}$\)
= 10
Therefore, the mass remaining in the coffee is m = 10 mg
B). 2 x 100 mL extraction
First extraction :
Let \($m_1$\) be the mass of the caffeine in water.
Mass of caffeine in \($CH_2Cl_2$\) = 100 - m
∴ \($\frac{(100-m_1)/100}{m_1/200}=9$\)
\($\frac{100-m_1}{m_1}=9$\)
\($5.5 \ m_1 = 100$\)
\($m_1=\frac{100}{5.5}$\)
= 18.18
Mass remaining in the coffee after the 1st extraction \($m_1$\) = 18.18 mg
Second extraction:
Let \($m_2$\) be the mass of the caffeine in water.
Mass of caffeine in \($CH_2Cl_2$\) = 18.18 - \($m_2$\)
∴ \($\frac{(18.18-m_2)/100}{m_2/200}=9$\)
\($\frac{18.18-m_2}{m_2}=9$\)
\($5.5 \ m_2 = 18.18$\)
\($m_1=\frac{18.18}{5.5}$\)
= 3.3
Mass remaining in the coffee after the 1st extraction \($m_2$\) = 3.3 mg
Using the reading "Fossil Fuels" from lesson 12 describe the environmental and economic benefits and drawbacks of fossil fuels. Second, looking over the benefits and drawbacks, in your opinion, what do you think will happen to mining of fossil fuels in the next 50 years?
Answers
Fossil fuels are essential part of the power generation in the world. They are easily combustible and more reliable and cheaper. However, the burning of fossil fuels releases toxic gases to the environment.
What are fossil fuels ?Fossil fuels are fuel generated from the decomposition materials. Petroleum, coal, natural gas etc. are fossil fuels which are excavating from the earth.
Fossil fuels are non-renewable sources of energy. Hence, as the existing fossil sources are exhausted no more fossil fuel can be made. It is cheaper, reliable and easy to use.
However, the toxic hydrocarbon gases released from the burning of fossil fuels make the environment polluted. Therefore, overuse of fossil fuel definitely rise the atmospheric pollution.
Its use over the next 50 years, will increase the global warming and more of it will be exhausted.
Find more on fossil fuels :
https://brainly.com/question/3371055
#SPJ1
Which of the following describes how further studies supported the work done by Ernest Rutherford?
A. Max Planck formulated the Planck's constant.
B. Albert Einstein formulated the energy equation.
C. James Chadwick discovered neutral particles in the nucleus.
D. Joseph Proust stated that each compound has a fixed ratio of elements.
Answers
Answer:
c) James Chadwick discovered neutral particles in the nucleus
Explanation:
James Chadwick discovery of neutral particles in the nucleus best describes the further studies supported the work done by Ernest Rutherford. Hence, option (c) is the correct answer.
30 example of redox reaction
Answers
1. Combustion of gasoline in a car engine
2. Rusting of iron
3. Photosynthesis in plants
4. Respiration in animals
5. Corrosion of metals
6. Bleaching of hair with hydrogen peroxide
7. Formation of ozone in the atmosphere
8. Electroplating of metals
9. Burning of wood
10. Reaction between bleach and ammonia
11. Reaction between copper and nitric acid
12. Reaction between iron and hydrochloric acid
13. Reaction between zinc and sulfuric acid
14. Reaction between magnesium and hydrochloric acid
15. Reaction between aluminum and hydrochloric acid
16. Reaction between sodium and water
17. Reaction between potassium and water
18. Reaction between lithium and water
19. Reaction between calcium and water
20. Reaction between barium and water
21. Reaction between copper and silver nitrate
22. Reaction between lead and silver nitrate
23. Reaction between zinc and copper sulfate
24. Reaction between iron and copper sulfate
25. Reaction between magnesium and copper sulfate
26. Reaction between aluminum and copper sulfate
27. Reaction between sodium and chlorine
28. Reaction between magnesium and chlorine
29. Reaction between aluminum and chlorine
30. Reaction between zinc and hydrochloric acid.
How many molecules are contained in 55.0g of co2??
Answers
Answer: 7.52*10^23 molecules.
Explanation: This is a classic Stoichiometry problem.
In one mole of any substance, there are 6.02*10^23 molecules. This number is called Avogadro's number. We are given 55 grams of Co2 so to convert that to moles, we divided by the molar mass of Co2. We find the molar mass by adding the molar masses of the elements that make up the compound.
There is one molecule of Carbon and two molecules of Oxygen in one molecule of Co2. From the periodic table, the molar mass of Carbon is 12.01 and 16.00 for Oxygen. 1(12.01)+2(16.00) gives us the molar mass. We then divided 55 grams by that mass to find the number of moles. We then multiply the number of moles by Avogadro's number (6.02*10^23) to find the total number of molecules.
You can use this method for solving any problem that asks you to find the number of atoms or molecules of some number of grams of a substance.
PLEASE PLEASE PLEASE HELP ME AS SOON AS POSSIBLE!!!!!!!!!!!!!!!!!!!!!!!

Answers
1) The mechanical advantage of the pulley is the number of pulleys in the system.
2) It is a system of pulleys with ropes between them.
3) The mechanical advantage is equal to the number of rope sections that support the load, minus one.
How do you determine the mechanical advantage of a block and tackle pulley?The mechanical advantage of a block and tackle pulley system is determined by counting the number of sections of rope that support the load. The mechanical advantage is equal to the number of rope sections that support the load, minus one.
It's important to note that the mechanical advantage of a block and tackle pulley system is based on the assumption that the pulleys are frictionless. In reality, friction within the system will decrease the mechanical advantage. Additionally, the mechanical advantage can be further increased by adding more pulleys to the system.
Learn more about pulley:https://brainly.com/question/13752440
#SPJ1
Give the name for this molecule:

Answers
Answer:
\(thank \: you\)

______ is produced anytime current flows in a circuit, due to the collision between the flowing free electrons and the fixed atoms.
Answers
Heat is produced anytime current flows in a circuit, due to the collision between the flowing free electrons and the fixed atoms. When an electric current flows through a conductor, the free electrons move through the lattice of atoms.
As they move, they collide with the fixed atoms, causing the atoms to vibrate and transfer energy to neighboring atoms. This energy transfer increases the temperature of the conductor, resulting in the production of heat.
The amount of heat produced is directly proportional to the amount of current flowing through the conductor, the resistance of the conductor, and the time for which the current flows. This relationship is described by Joule's law, which states that the heat produced is equal to the product of the current, the resistance, and the time.
Mathematically, this can be expressed as H = I^2RT, where H is the heat produced, I is the current, R is the resistance, and T is the time. The collision between the flowing free electrons and the fixed atoms in a conductor leads to the production of heat, which is proportional to the current, resistance, and time for which the current flows.
To learn more about heat
https://brainly.com/question/30603212
#SPJ4
Propane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). If you mix propane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 300 mm hg, what are the partial pressures of.
Answers
Propane gas partial pressure is 300 mm.
Propane gas reaction - C3H8(g)+5O2(g)→3CO2(g)+4H2O(g)
As propane gas is a three-carbon alkali. It has a standard temperature and pressure. It can be compressed into a transport liquid. As per, the gas reacts with the air that is oxygen and gives carbon dioxide. We mix it with the correct ratio then the pressure mix is equal to 300 mm.
The answer is a partial pressure of propane gas 51mmHg and a part of the oxygen gas to be 249 mm. The propane gas is mixed with the oxygen gas and the gas reacts to give out carbon dioxide and thus measuring their correct stoichiometric ratio.
The gas produces a total pressure mix of 300 mg Hg and thus partial pressure is around 51 and that for oxygen is 249 mm. The partial pressure of a gas is 51 mmHg and part of the oxygen gas is 249 mm hg. That of water vapours of 171 mmHg.
To learn more about propane,
brainly.com/question/28358864
#SPJ4
Determine the volume of Hydrogen gas collected over water from the reaction of
25.6g of Magnesium reacting with 45.0g of Hydrochloric acid to make magnesium
chloride and hydrogen gas. The gas is collected at 785.4 torr and 36.5 C. The vapor
pressure of water at 36.5C is 47.1 mmHg.
Answers
The volume of hydrogen gas collected over water is approximately 25.10 liters. We need to consider the stoichiometry of the reaction, the ideal gas law, and the partial pressure of hydrogen gas.
First, let's balance the equation for the reaction between magnesium (Mg) and hydrochloric acid (HCl):
Mg + 2HCl -> MgCl₂ + H₂
From the balanced equation, we can see that 1 mole of magnesium reacts to produce 1 mole of hydrogen gas.
1. Calculate the number of moles of magnesium (Mg):
Molar mass of Mg = 24.31 g/mol
Number of moles of Mg = Mass of Mg / Molar mass of Mg = 25.6 g / 24.31 g/mol = 1.054 mol
2. Calculate the number of moles of hydrogen gas (H₂):
Number of moles of H₂ = Number of moles of Mg = 1.054 mol
3. Apply the ideal gas law to calculate the volume of hydrogen gas (V₂):
PV = nRT
Given:
Pressure (P) = 785.4 torr
Temperature (T) = 36.5 °C = 36.5 + 273.15 K = 309.65 K
R = 0.0821 L·atm/(mol·K) (gas constant)
Number of moles (n) = 1.054 mol
Convert the pressure to atm:
785.4 torr = 785.4 torr * (1 atm / 760 torr) = 1.032 atm
Substituting the values into the ideal gas law equation:
V₂ = (nRT) / P = (1.054 mol * 0.0821 L·atm/(mol·K) * 309.65 K) / 1.032 atm ≈ 25.10 L
For more such questions on stoichiometry.
https://brainly.com/question/16060223
#SPJ8
9. Hydrogen peroxide decomposes to form water and oxygen gas according to the following equation:
2H2O2(aq) to 2H2O(l) + O2(g)
If 315 g of hydrogen peroxide, H2O2, decomposes and all the O2 gas is collected in a balloon at 0.792 atm and 23 degrees C, what is the volume of the O2 gas collected?
Answers
Answer:
\(V=142L\)
Explanation:
Hello,
In this case, for the given reaction:
\(2H_2O_2(aq) \rightarrow 2H_2O(l) + O_2(g)\)
Starting with 315 g of hydrogen peroxide, we can compute the yielded moles of oxygen by using the following stoichiometric factor whereas the hydrogen peroxide to oxygen mole ratio is 2:1:
\(n_{O_2}=315gH_2O_2*\frac{1molH_2O_2}{34gH_2O_2}*\frac{1molO_2}{2molH_2O_2} \\\\n_{O_2}=4.63molO_2\)
Then, by using the ideal gas equation we can compute the resulting volume if the 4.63 moles of oxygen are collected at 0.792 atm and 23 °C as shown below:
\(PV=nRT\\\\V=\frac{nRT}{P}=\frac{4.63mol*0.082\frac{atm*L}{mol*K}*(23+273.15)K}{0.792 atm}\\ \\V=142L\)
Best regards.
An error during which cellular process would create a gene mutation?
Answers
An error during DNA replication would create a gene mutation.
During DNA replication, the genetic information in a cell is copied to make new DNA molecules. However, mistakes can occur during this process, leading to changes in the DNA sequence, which can result in a mutation. Mutations can also be caused by exposure to environmental factors, such as radiation or chemicals, which can damage the DNA molecule directly or affect the cellular processes involved in DNA replication.
Mutations can have a variety of effects on the organism, ranging from no effect to causing serious health problems or even death. Gene mutations can also be inherited from a parent, which can result in genetic disorders or predisposition to certain diseases. Therefore, it is important to understand the mechanisms of gene mutations and their potential impacts on organisms.
To know more about the Gene mutation, here
https://brainly.com/question/15448555
#SPJ1
I'm making a AD for my special ed class room and I am interviewing people. Make 10 unique questions I can ask my fellow classmates about the things they have learned in this room.
Answers
These are 10 unique questions you can ask your fellow classmates about the things they have learned in your special ed classroom:
What is your favorite thing about our classroom?What is one thing you have learned in our classroom that you will never forget?What is one thing you would like to learn more about in our classroom?How has our classroom helped you to succeed?What is one thing you would like to say to your teacher?What is one thing you would like to say to your classmates?What is one thing you would like to say to your parents?What is one thing you would like to say to the world?What is your dream for the future?What is one thing you are grateful for?What are special ed classroom?A special education classroom is a classroom designed to meet the needs of students with disabilities. These classrooms are staffed by specially trained teachers who are able to provide individualized instruction and support to students with a variety of disabilities.
These questions are designed to get your classmates thinking about the things they have learned in your special ed classroom and how those things have impacted them. The answers to these questions can be used to create a powerful and informative ad for your classroom.
Find out more on special ed classroom here: https://brainly.com/question/30029124
#SPJ1
A balloon with an initial volume of 3.1 L at a temperature of 193 K is warmed to 374 K.
What is its volume at the final temperature? (Assume constant temperature.)
Answers
Answer:
New volume = 6.0L
Explanation:
Make a list of variables given in the question, and what we want to find:
Initial volume (L) = Vi = 3.1
Initial temperature (K) = Ti = 193
New temperature (K) = Tn = 374
New volume (L) = Vn
We have volumes and temperatures given and we're talking about gas, the equation including both these variables that should come to mind is the ideal gas equation:
PV = nRT
P = pressure (Pa)
n = number of moles
R = gas constant = 8.31
Now, we construct an equation we can solve to get the new volume:
P(Vn) = nR(Tn)
And insert the values we know:
P(Vn) = n(8.31)(374)
P(Vn) = n·3107.94
The equation contains 3 unknown variables;
We should first try to see if we can eliminate the 2 we are not interested in, namely P and n;
What we should recognise is that the pressure and number of moles will not change upon warming the balloon;
Firstly, heating the gas inside the balloon doesn't add anything to the balloon, i.e. doesn't increase the moles of gas, it simply raises the energy of the gas particles already within the balloon, so n will not change;
Secondly, I think there is a mistake in the question, it should read "assume constant pressure" in the brackets, since the temperature does change, which we are told;
Also, we can assume normal atmospheric pressure inside and outside the balloon as would be the case ordinarily;
What we want to do with this information is rearrange the equation we constructed to have these 2 constant or unchanging variables on one side and everything else on the other, so:
\(\frac{P}{n} = \frac{3107.94}{V_{n} }\)
Next, we construct an equation for the balloon before warming:
P(Vi) = nR(Ti)
P(3.1) = n(8.31)(193)
P·3.1 = n·1603.83
Once again, rearrange to get P and n on one side of the equation and everything else on the other:
\(\frac{P}{n} = \frac{1603.83}{3.1} \\\\ \frac{P}{n} = 517.364516\)
Now, we have two equations for P/n, we can eliminate P/n since both these values remain the same before and after warming the balloon as previously established:
\(\frac{3107.94}{V_{n} } = \frac{P}{n} = 517.364516 \\\\ \frac{3107.94}{V_{n} } = 517.364516 \\\\\)
Now, rearrange and solve for Vn:
\(\frac{3107.94}{V_{n} } = 517.364516 \\\\ V_{n} = \frac{3107.94}{517.364516} \\\\ V_{n} = 6.00725...\)
Vn = 6.0L
If the balloon had a volume of 3 L at a depth of 50 m, what was the original volume of the balloon it we assume the pressure at the surface of the water is 14.7 psi? Express your answer using one significant figure and include the appropriate units.
Answers
If the balloon had a volume of 3 L at a depth of 50 m, the original volume of the balloon will be
What is Significant figures ?Significant figures are the number of digits in a value, often a measurement, that contribute to the degree of accuracy of the value.
The pressure at a depth of 40 m is the hydrostatic pressure of the water plus the atmospheric pressure.
The hydrostatic pressure P of a liquid is given by the formula ;
P = ρgh
where,
ρ = the density of the liquid (Density of water = 1000 kg m⁻³)g = the acceleration due to gravity (9.81 ms⁻²)h = the depth of the liquid (Given : 50 m)P = 1000 kg m⁻³ x 9.81 ms⁻² x 50 m
= 1000 kg m⁻¹ x 9.81 s⁻² x 50
= 4.9 x 10⁵ Pa
Lets's Convert Pa in to atm
= 4.9 x 10⁵ Pa x 1 / 103. 325 x 10³ Pa
= 4.7 atm
Mow, lets calculate P(atm) ;
P(atm) = 14.7 psi x 1 atm / 14.7 psi
= 1 atm
Total Pressure at 50 m is ;
P(total) = 4.7 atm + 1 atm
= 5.7 atm
Now we can apply Boyle's Law to calculate the volume of the balloon at the surface.
P₁V₁ = P₂V₂
V₂ = P₁V₁ / P₂
According to the question ;
P₁ = 5.7 atm P₂ = 1 atmV₁ = 3 LV₂ = ?V₂ = 3 L x 5.7 atm / 1 atm
= 17.1 L
= 20 L ( 1 significant figure)
Learn more about Boyles law here ;
https://brainly.com/question/1437490
#SPJ1
Which of the following statements is
incorrect about heat and
temperature?
Answers
How many hydrogen atoms are represented by (CH3)2CHOH?
Answers
Answer:
8 Hydrogen Atoms
Explanation:
The subscripts that are next to the coefficient (letter) indicates how much atoms the element/compound has.
So:
(CH3) × 2 ⇒ 6 hydrogen atoms
+ 1 from (CH) and another from (OH) ⇒ 8 atoms