In the reaction C5H8 + ________ O2 ➡ 8H2O + HCO2, what coefficient should be placed in front of O2 to balance the reaction?
A. 5
B. 8
C. 9
D. No coefficient is needed
Answers
Equalization of chemical reactions can be done using variables. Steps in equalizing the reaction equation:
• 1. gives a coefficient on substances involved in the equation of reaction such as a, b, or c, etc.
• 2. make an equation based on the similarity of the number of atoms where the number of atoms = coefficient × index (subscript) between reactant and product
• 3. Select the coefficient of the substance with the most complex chemical formula equal to 1
For gas combustion reaction which is a reaction of hydrocarbons with oxygen produces CO₂ and H₂O (water vapor). can use steps:
Balancing C, H, and the last O
1. gives a coefficient
C₅H₈+aO₂ ⇒ 5CO₂ + 8H₂O
O⇒left =2a, right= 5.2+8=18 ⇒ 2a=18⇒a=9
So the coefficient in front of O₂ = 9
But this reaction is not balanced because the number of H is not balanced (8 on the left and 16 on the right, but if we use this equation:
C₅H₈ + 7O₂⇒ 5CO₂ + 4H₂O
then the reaction will be balanced
Related Questions
Which element has the greatest first ionization energy?
B
C
Be
Li
Which statement is true about the ionic size of the elements in a group as one moves from bottom to top in that group?
Ionic size decreases from bottom to top within the group.
Ionic size increases from bottom to top within the group.
Ionic size does not vary in any predictable way within the group.
Ionic size stays the same within the group.
Answers
Answer:
helium
Explanation:
The first ionization energy varies in a predictable way across the periodic table. The ionization energy decreases from top to bottom in groups, and increases from left to right across a period. Thus, helium has the largest first ionization energy, while francium has one of the lowest.
Answer: 2nd answer " C "
Explanation:
First ionization energy is a periodic trend that increases up and to the right on the periodic table. After looking at the position of these elements on the periodic table, carbon is clearly the best answer-choice (B) aka " C "
Which unit is used for measuring atomic mass? O A. atomic mole O B. grams/mole Ос. grams O D. atomic mass unit ОЕ. atomic mass weight
Answers
Mark the statement as true or false.
All of the proposed ideas by Dalton, Thomson, and Rutherford are still used in the Modern Theory of the Atom.
true
false
Answers
Answer:
False
Explanation:
The ideas were not proposed
Answer:
false
Explanation:
do all-stars mak energy through fusions
Answers
Describe the structure and bonding in silicon dioxide and explain why it is a suitable material for making welding blankets.
Answers
The molecular geometry of silicon dioxide is linear and bonding in it is covalent due to which it is a suitable material for making welding blankets.
What is molecular geometry?Molecular geometry is defined as a three -dimensional arrangement of atoms which make up the molecule.It includes parameters such as bond length,bond angle and torsional angles.
It influences many properties of molecule such as reactivity,polarity color,magnetism .The molecular geometry can be determined by various spectroscopic methods and diffraction methods , some of which are infrared,microwave and Raman spectroscopy.
Learn more about molecular geometry,here:
https://brainly.com/question/28557524
#SPJ9
I need help with Molar Ratio
______SO2(g) +_____O2(g) ------> _____SO3(g)
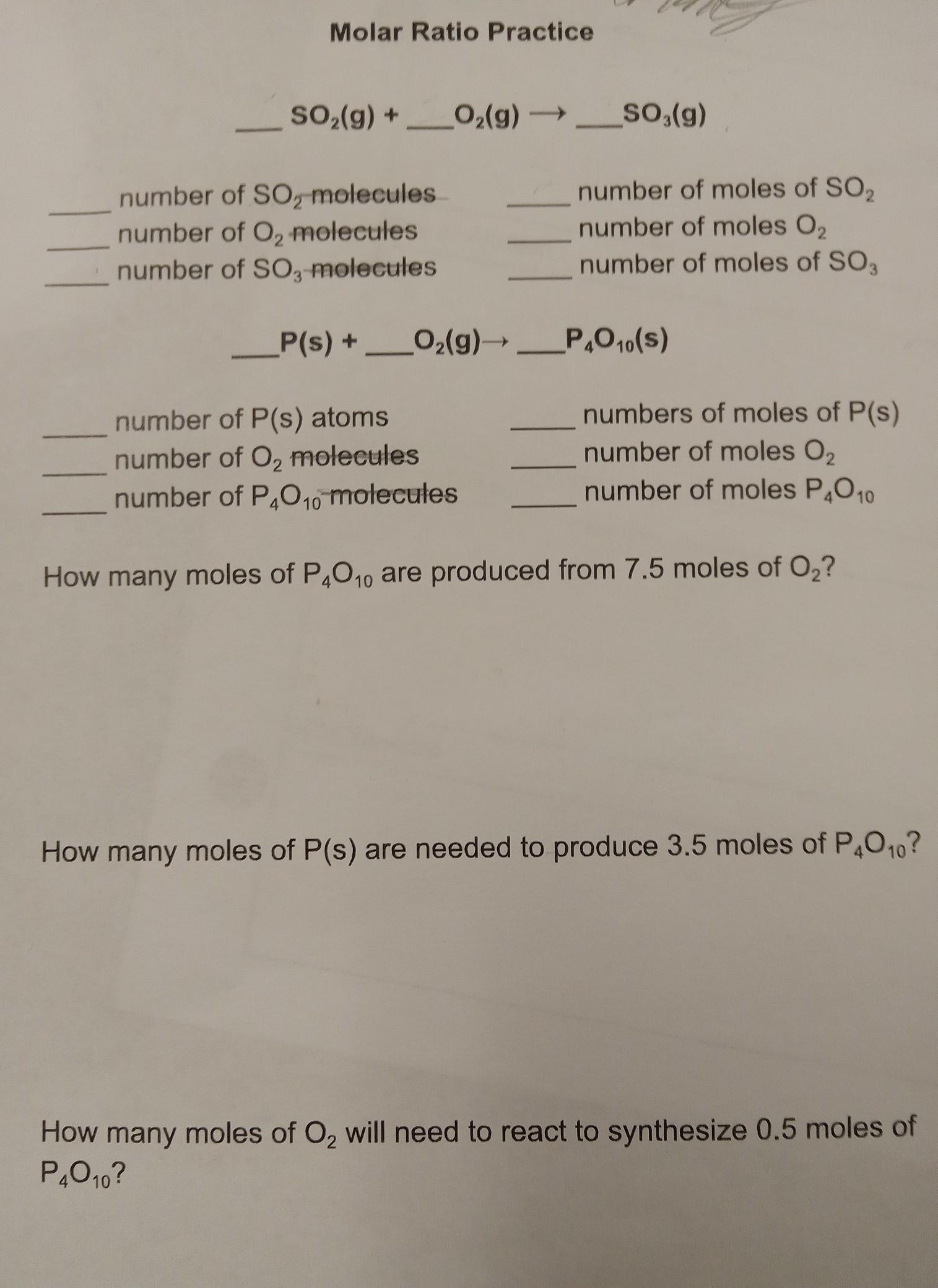
Answers
2 SO₂ (g) + O₂ (g) ---> 2 SO₃ (g)
1.204 * 10²⁴ number of SO₂ molecules = 2 number of moles of SO₂
6.02 * 10²³ number of O₂ molecules = 1 number of moles O₂
1.204 * 10²⁴ number of SO₃ molecules = 2 number of moles of SO,
4 P(s) + 5 O₂ (g) ----> P₄O₁₀ (S)
2.408 * 10²⁴ number of P(s) atoms = 4 numbers of moles of P(s)
3.01 * 10²⁴ number of O₂ molecules = 5 number of moles O₂
6.02 * 10²³ number of moles P₄O₁₀ = number of P₄O₁₀ molecules
1.5 moles of P₄O₁₀ are produced from 7.5 moles of O₂.
14 moles of P(s) are needed to produce 3.5 moles of P₄O₁₀.
2.5 moles of O₂ will need to react to synthesize 0.5 moles of P₄O₁₀.
What is the mole ratio of the given reactions?The mole ratio of the given reactions is obtained from their equations of reaction.
1. 2 SO₂ (g) + O₂ (g) ---> 2 SO₃ (g)
The mole ratio is 2 : 1 : 2
1 mole of atoms or molecules contains 6.02 * 10²³ particles.
Hence, the number of particles is obtained by multiplying the number of moles by 6.02 * 10²³.
4 P(s) + 5 O₂ (g) ----> P₄O₁₀ (S)
7.5 moles of O₂ will produce 7.5/5 moles of P₄O₁₀ = 1.5 moles of P₄O₁₀
3.5 moles of P₄O₁₀ will be produced by 3.5 * 4 moles of + = 14 moles of P(s)
0.5 moles of P₄O₁₀ will be produced by 0.5 * 5 moles of O₂ = 2.5 moles of O₂
Learn more about mole ratio at: https://brainly.com/question/30632038
#SPJ1
Which units express heat capacity? J/°C, J/K, cal/°C, cal/K J/(gi°C), J/(giK), cal/(gi°C), cal/(giK) J, cal °C, K
Answers
Answer:
a
Explanation:
The heat capacity of a substance is the heat energy required to rise its temperature per one degree Celsius. Hence its unit is J/°C.
What is heat capacity ?Heat capacity is the amount of heat energy required to raise the temperature of a substance by 1 degree Celsius or 1 Kelvin. It is expressed in the following units:
Joules per degree Celsius (J/°C)
Joules per Kelvin (J/K)
Calories per degree Celsius (cal/°C)
Calories per Kelvin (cal/K)
Joules per gram per degree Celsius (J/(g·°C))
Joules per gram per Kelvin (J/(g·K)) etc.
If in terms of simply the energy, then, The following units are used.
Joules (J) , Calories (cal) , Degrees Celsius (°C), Kelvin (K)
The choice of unit depends on the specific application and the system of units being used. The SI unit for heat capacity is J/K, while the traditional unit is cal/°C.
The use of per gram units is common in the context of specific heat capacity, which is the amount of heat energy required to raise the temperature of a unit mass of a substance by 1 degree Celsius or 1 Kelvin.
Therefore, here, the unit of heat capacity is J/°C.
Find more on heat capacity :
https://brainly.com/question/28302909
#SPJ7
what is chemical energy
Answers
Answer:
chemical energy is the energy of a chemical substance that is stored in the bonds of chemical compounds and is released when they undergo a chemical reaction and transform into another substance
1. Janet is measuring the amount of matter in a rock. She places the rock on the scale and
obtains its mass in
Answers
Janet obtains the mass of the rock she placed on the scale in kilograms.
In order to obtain the mass of an object, you will have to measure the amount of matter in it.
Matter is defined as substances that are made up of tiny particles (atoms) which occupies space and has mass.
Mass is defined as the quantity of matter in a body (rock). This gives the exact value of a substance (which is the quantity of the substance) no matter where it is measured.
The mass of an object (rock) is measured by chemical or beam balance scale which uses the principles of moment.
The unit of mass is in Kilogram (kg).
Learn more here:
https://brainly.com/question/19600926
What is temperature

Answers
Answer:
I just love the killua pfp
I think b
Answer:
c
Explanation:
because it forces and help it
Which two events will happen if more H2 and N2 are added to this reaction after it reaches equilibrium?
3H2 + N2 to 2NH3
Answers
If more \(H_{2}\) and \(N_{2}\) are added to the reaction 3\(H_{2}\) + N2 → 2\(NH_{3}\) after it reaches equilibrium, two events will occur Shift in Equilibrium and Increased Yield of \(NH_{3}\)
1. Shift in Equilibrium: According to Le Chatelier's principle, when additional reactants are added, the equilibrium will shift in the forward direction to consume the added reactants and establish a new equilibrium. In this case, more \(NH_{3}\) will be produced to counteract the increase in \(H_{2}\) and \(N_{2}\).
2. Increased Yield of \(NH_{3}\): The shift in equilibrium towards the forward reaction will result in an increased yield of \(NH_{3}\). As more \(H_{2}\) and \(N_{2}\) are added, the reaction will favor the production of \(NH_{3}\) to maintain equilibrium. This will lead to an increase in the concentration of \(NH_{3}\) compared to the initial equilibrium state.
It is important to note that the equilibrium position will ultimately depend on factors such as the concentrations of \(H_{2}\), \(N_{2}\), and \(NH_{3}\), as well as the temperature and pressure of the system. By adding more reactants, the equilibrium will adjust to achieve a new balance, favoring the formation of more \(NH_{3}\).
Know more about Le Chatelier's principle here:
https://brainly.com/question/2943338
#SPJ8
to a first approximation the ionization constant of h2s is
Answers
The ionization constant of H₂S is approximately 1.0 x 10⁻⁷.
The ionization constant, also known as the acid dissociation constant (Ka), is a measure of the extent to which an acid dissociates in water. It indicates the degree of ionization of an acid and is typically expressed as the equilibrium constant for the reaction between the acid and water.
In the case of H₂S (hydrogen sulfide), it is a weak acid that can partially dissociate in water to produce hydrogen ions (H⁺) and sulfide ions (HS⁻). The ionization reaction can be represented as follows:
H₂S ⇌ H⁺ + HS⁻
The ionization constant (Ka) represents the equilibrium expression for this reaction. The value of Ka determines the relative strength of the acid. For H₂S, the ionization constant is approximately 1.0 x 10⁻⁷, indicating that it is a weak acid.
This value indicates that H₂S only partially ionizes in water, with a small fraction of H₂S molecules dissociating into H⁺ and HS⁻ ions. The majority of H₂S remains in its molecular form.
It is important to note that the ionization constant can vary depending on factors such as temperature and concentration. The given approximation is a typical value at standard conditions.
To know more about "Ionization constant" refer here:
https://brainly.com/question/30639622#
#SPJ11
draw a structure that is optically inactive because it does not have an asymmetric center.
Answers
The structure of ethane can be represented as:
\(\[\text{H} - \text{C} - \text{C} - \text{H}\]\)
Optical inactivity is observed when a molecule lacks an asymmetric center or chiral centre. An asymmetric center is a carbon atom that is bonded to four different substituents. In the absence of an asymmetric center, the molecule will be optically inactive.
A simple example of a molecule without an asymmetric center is \textbf{ethane} (\(C_2H_6\)). Ethane consists of two carbon atoms bonded to each other by a single bond, with three hydrogen atoms attached to each carbon atom.
The structure of ethane can be represented as:
\(\[\text{H} - \text{C} - \text{C} - \text{H}\]\)
The chemical formula for ethane, a paraffin series hydrocarbon (compound of hydrogen and carbon), is C2H6. It is a colourless, odourless, gaseous hydrocarbon. The only hydrocarbon with a single carbon-carbon bond has the simplest structural makeup, and that is ethane.05
To learn more about ethane from the given link
https://brainly.com/question/18486242
#SPJ4
Problem 1 Water flows through 76 mm ID horizontal pipeline which is 4 km long with the following conditions: Flow rate =27 m 3
/hr Outlet pressure =4 bar (1bar=10 5
Pa) Water density =1000 kg/m 3
Water viscosity =0.001 kg/m−s Pipeline roughness =0.015 mm Calculate the inlet pressure of the pipeline in (bar).
Answers
The inlet pressure of the pipeline in (bar) is 6.7 bar. To calculate the inlet pressure of the pipeline, we can use the Darcy-Weisbach equation.
Darcy-Weisbach equation relates pressure drop, flow rate, pipe characteristics, and fluid properties. The equation is given as:
ΔP = (fLρV²) / (2D) where:
ΔP is the pressure drop
f is the Darcy friction factor
L is the length of the pipeline
ρ is the density of water
V is the velocity of water
D is the diameter of the pipeline
First, we need to convert the flow rate from m³/hr to m³/s:
Flow rate = 27 m³/hr = (27/3600) m³/s = 0.0075 m³/s
Next, we need to calculate the velocity of water:
Area of the pipeline =\(\pi \times \frac {(76/1000)^2}{4} = 0.004556 m^2\)
Velocity
= Flow rate / Area of the pipeline
= 0.0075 m³/s / 0.004556 m² = 1.646 m/s
Now, we can calculate the pressure drop using the Darcy-Weisbach equation. Since we need to calculate the inlet pressure, we assume ΔP is the difference between the outlet pressure and the inlet pressure:
ΔP = (fLρV²) / (2D)
\(\triangle P = \frac {(0.015 \times 4000 \times 1000 \times 1.646^2)}{(2 \times 0.076)} = 10.69 \times 10^5 Pa\)
= 10.7 bar (approx)
Rearranging the equation to solve for the inlet pressure:
Inlet pressure = ΔP - outlet pressure = 10.7 bar - 4 bar = 6.7 bar
Learn more about the Darcy-Weisbach formula here:
https://brainly.com/question/30640818
#SPJ11
1. How many formula units are contained in 270.2 g of zinc nitrate, Zn(NO3)2?
50 POINTS
Answers
Zinc nitrate has 1 molecule of zinc (Zn), 2 molecules of nitrogen (N) and 6 molecules of oxygen (O).
Calculate the molecular mass of zinc nitrate by adding the molecular weights for all molecules in the compound.
Here are the molecular weights for each element:
Zn=65.38 g/mol
N=14.01 g/mol
O=16.00 g/mol
The molecular weight of zinc nitrate is then: 1*(65.38)+2*(14.01)+6*(16.00)=189.40 g/mol.
Using a conversion formula to calculate moles of zinc nitrate:
163.97gZn(NO
3
)
2
∗
189.40gZn(NO
3
)
2
1molZn(NO
3
)
2
=0.866molZn(NO
3
)
2
There are 0.866 moles of zinc nitrate.
Note: These numbers may vary slightly due to the source of the molecular weight data and the number of decimal places used.

Why do surface waves break along the shore?
Answers
Answer:
because the global sea level rises while the poles temperature increases
Explanation:
What is the volume of 8.8g of carbon dioxide at STP?
Answers
The volume of 8.8g of carbon dioxide at STP is 4.38 L.
At STP, what is 22.4 L?1 mole of any gas will take up 22.4 L of space at standard temperature and pressure (STP). A balanced chemical equation and the Ideal Gas Law can be used to determine the amount or mass of gas consumed or created in a chemical process.
n = m/M
where m is the molar mass of carbon dioxide and M is its mass in terms of molecules.
Considering that the molar mass of carbon dioxide is 44.01 g/mol:
n = 8.8 g / 44.01 g/mol
n = 0.1998 mol
Next, we can plug in the values of n, R, P, and T into the ideal gas law and solve for V:
V = (nRT)/P
V = (0.1998 mol x 0.0821 L·atm/(mol·K) x 273.15 K) / 1 atm
V = 4.38 L
To know more about volume visit:-
https://brainly.com/question/16434653
#SPJ1
The principal mechanism for sugar dissolving in water is dissociation
Answers
The principal mechanism for sugar dissolving in water is dissociation. Sugar dissolving in water involves a process of solvation or hydration.
The polar water molecules surround the sugar molecules and break down their structure. The principal mechanism for sugar dissolving in water is not dissociation. Sugar dissolves in water due to a process called solvation, which involves the sugar molecules being surrounded by polar water molecules, breaking down the structure of the sugar.
Dissociation refers to the separation of ions or molecules in a substance that was formerly united. It occurs when an ionic compound dissolves in water, breaking down into its constituent ions. When sugar dissolves in water, it does not ionize or break down into separate ions; rather, it dissolves into individual sugar molecules.
Hence, the principal mechanism for sugar dissolving in water is solvation, not dissociation.
For more such questions on Sugar, click on:
https://brainly.com/question/4326694
#SPJ11
in the partially balanced reaction illustrated below, how many additional complete x molecules are required to balance the reaction?
Answers
To balance this reaction it is required to have 2 more complete x molecules, one y molecule, and two z molecules.
What is to balance a reaction?Balancing a reaction implies adding molecules in the reactants or products to make sure the same number of molecules in the reactants are the same number of each kind in the products.
The original reaction presented is:
2 (X3) + 2 (Y2) = 4 (YX2) or6 x molecules + 4 y molecules = 4 y molecules and 8 x moleculesThis shows the reaction is not balanced because there are more x molecules than the ones at the beginning of the reaction.
What molecules need to be added?To make the reaction balanced we need to add:
2 more X molecules.1 more Y molecule.2 more XY molecules.4 (X3) + 3 (Y2) = 6 (YX2)12 x molecules + 6 y molecules = 6 y molecules + 12 x molecules.Note: This question is incomplete because the equation is not given. Here is the missing part:
Learn more about reactions in: https://brainly.com/question/3664113
MARKING BRAINLIEST!!!
image is attached

Answers
Answer:
2, s
Explanation:
The there will be two orbitals in the s subshell because the first orbital can only contain two electrons and there will be need of a second one
A small area with climate conditions that differ from those around it is called a
Answers
Answer:
Microclimates
Explanation:
Microclimates are small areas with climate conditions that differ from those around them. The main factors that influence temperature are latitude, altitude, distance from large bodies of water, and ocean currents. Earth's surface is divided into three temperature zones.
left- and right-handed mirror image molecules are known as
Answers
Left- and right-handed mirror image molecules are known as stereoisomers. Stereoisomers have the same molecular formula and the same connectivity of atoms, but the arrangement of the atoms in space is different. Stereoisomers are formed due to the presence of a chiral center in the molecule
A molecule is said to be chiral if it has a non-superimposable mirror image. Chiral molecules cannot be superimposed on their mirror image. This means that the left- and right-handed mirror images of a chiral molecule are not identical and are not superimposable on each other. Chiral molecules are very important in the field of biology and pharmacology because they interact differently with other chiral molecules in biological systems and can have different biological activities or therapeutic effects.Most biological molecules, such as amino acids, sugars, and DNA, are chiral. Amino acids and sugars are chiral because of the presence of an asymmetric carbon atom in their structures. DNA is chiral because of the helical structure of its double-stranded form. The handedness of chiral molecules can have significant implications for their biological activity, as the interaction between two chiral molecules can depend on their relative handedness.The study of stereoisomers is important in the field of organic chemistry and biochemistry. Understanding the stereochemistry of molecules is essential for understanding their properties and behavior. Stereoisomers can have different physical properties, such as melting point and solubility, and different biological activities, such as receptor binding and enzyme catalysis.
To know more about Chiral molecules visit :
brainly.com/question/29538057
#SPJ11
What is the difference between a molecular formula, structural formula and an electron dot formula? Give an example of each
Answers
Answer:
The molecular formula tell us what elements the atoms are, and how many moles and atoms are attributed toward each element. For example, molecular formula of glucose is . That means one molecule of glucose has 6 molecules of C, 12 molecules of hydrogen and 6 molecules of oxygen.
The structural formula of a chemical compound is a graphic representation of the molecular structure.
Electron dot diagram or a Lewis diagram or a Lewis structure.
Explanation:
A student mixes 30 grams of baking soda with 100 grams of vinegar in a closed container. Based on the Law of Conservation of Matter, what represents the total mass of all products after the experiment.
A. 30 grams
B. 70 grams
C. 100 grams
D. 130 grams
Answers
Answer: D, 130 grams.
Explanation: All reactants added up equal 130, and matter cannot be created or destroyed. Hope this helps!
According to the laws of conservation of matter or mass, matter can neither be created nor be destroyed. Based on this law, there will be 130 grams of product after the reaction.
What is laws of conservation of matter?According to mass conservation law, mass or matter cannot be created not be destroyed. But it can be transferred from one object to another or from medium to medium.
For a chemical reaction, as per the rule of conservation of matter, the total mass in reactant side is equal to the total mass in the product side.
Also, the atoms or molecules are not lost or created in a reaction but they can be regrouped.
The regrouping of atoms or molecules creates the new products which have the exact mass as that of reactants. If there is a total mass of 100 g, in reactant side the, there will be 100 g in product side.
Here the total mass in the reactant side is 30 + 100 = 130 grams. Thus as per the law of conservation of mass, the total mass of all products after the experiment will be 130 g and option D is correct.
To learn more about laws of conservation of matter, find the link below:
https://brainly.com/question/9434062
#SPJ2
this rock above contains many large (2-5cm across) phenocrysts that are salmon pink. which mineral is this?
Answers
Phenocrysts are the kind of feldspar minerals.
A mineral is an detail or chemical compound that is normally crystalline and that has been shaped because of geological techniques. Examples include quartz, feldspar minerals, calcite, sulfur and the clay minerals inclusive of kaolinite and smectite.
A clearly going on inorganic element or compound having an. orderly internal shape and feature chemical composition, crystal form, and physical. properties. Minerals range from rocks, which are certainly happening solids composed of 1 or more minerals.
Minerals are labeled based totally on their crystal shape and chemistry. Minerals are divided into two types particularly steel and non-metal.
Learn more about metallic minerals here:- https://brainly.com/question/89259
#SPJ4
Describe the emission spectrum of hydrogen. Outline how this spectrum is related to the energy levels in the hydrogen atom. (3 marks)
Answers
The emission spectrum of hydrogen is a series of colored lines that are produced when an electron in a hydrogen atom falls from a higher energy level to a lower energy level.
The spectral lines in the hydrogen emission spectrum correspond to different energy transitions within the atom. The energy of a photon is directly proportional to its frequency, so the emission lines correspond to specific frequencies of light. The emission spectrum of hydrogen consists of a series of discrete lines, called the Balmer series, which correspond to specific wavelengths of light emitted when electrons in a hydrogen atom transition from higher energy levels to lower ones.
This emission spectrum is related to the energy levels in the hydrogen atom as follows:
1. When an electron in a hydrogen atom absorbs energy, it jumps to a higher energy level, also known as an excited state.
2. The electron then releases the absorbed energy in the form of a photon when it transitions back to a lower energy level. The energy of the emitted photon corresponds to the difference between the two energy levels involved in the transition.
3. The distinct lines in the emission spectrum represent the specific energy differences between these energy levels, and each line corresponds to a unique transition between two energy levels. In summary, the emission spectrum of hydrogen is a direct result of electrons transitioning between different energy levels in the atom, and the specific wavelengths of light emitted correspond to the energy differences between these levels.
learn more about the emission spectrum of hydrogen here
https://brainly.com/question/29255944
#SPJ11
A basic amino acid has an R group that contains
A) a methyl group
B) a thiol group
C) an amine group
d) a carboxyl group
Answers
A basic amino acid has an R group that contains ( D) a carboxyl group.
What is acid?Acid is a substance that has a pH level of lower than 7.0 and is capable of corroding or dissolving other substances. It is usually found in aqueous solutions and is a highly reactive substance. Examples of acid include sulfuric acid, hydrochloric acid, nitric acid and acetic acid. These are used in a variety of industries such as food production, industrial cleaning and chemical engineering. Acid is also used in the laboratory for titrations, pH testing and other experiments. Acids can be dangerous if mishandled and can cause skin, eye and respiratory irritation and even chemical burns.
To learn more about acid
https://brainly.com/question/26855500
#SPJ1
In a percentage composition investigation a compound was decomposed into its elements: 20.0 g of calcium, 6.0 g of carbon, and 24.0 g of oxygen. Determine the percentage composition of this compound
Answers
The percentage composition of this compound : 40%Ca, 12%C and 48%O
Further explanationGiven
20.0 g of calcium,
6.0 g of carbon,
and 24.0 g of oxygen.
Required
The percentage composition
Solution
Total mass of compound :
=mass calcium + mass carbon + mass oxygen
=20 g + 6 g + 24 g
=50 g
Percentage composition :
Ca-calcium\(\tt \dfrac{20}{50}\times 100\%=40\%\)
C-carbon\(\tt \dfrac{6}{50}\times 100\%=12\%\)
O-oxygen\(\tt \dfrac{24}{50}\times 100\%=48\%\)
If the statement is true, select True. If it is false, select False.
Substances that enter into a chemical reaction are called products.
True
False
Answers
Answer:
False
Explanation:
They are called products
Carbon monoxide (CO), carbon dioxide (CO2) and nitrogen dioxide (NO2) are low molecular weight covalent compounds. Which of the following best describes the state in which these compounds exist at Standard Temperature and Pressure (STP)?
solid
liquid
gas
plasma
Answers
Answer:
gas
Explanation:
at Standard Temperature and Pressure Carbon monoxide (CO), carbon dioxide (CO2) and nitrogen dioxide (NO2) exist as gases
Option C is correct
Low molecular weight covalent compoundsCovalent compounds are compounds that are characterized by strong Intra-molecular bonds and they are solids as they have higher molecular weights
But when compounds like Carbon monoxide (CO), carbon dioxide (CO2), and nitrogen dioxide (NO2) are formed with low molecular weight covalent bonds They exist as gases
More on Compound
https://brainly.com/question/704297