if 600.0 ml of air is heated from 293 k to 333 k what volume will it occupy
Answers
Answer:
Explanation:
V1= 600.0 mL
T1= 293 K
V2 unknown
T2= 333K
Charles law V1*T2=V2*T1
where V1 and T1 are the initial volume and temperature. V2 and T2 are the final temperature and volume.
V2=(V1*T2)/T1 = ( 600.0*333)/293= 682 mL (with significant figures)(681.911 mL before signifcant figures.)
Related Questions
What happens to energy when light is transmitted through or reflected off a material?
Answers
In which cases it’s energy is converted into heat
Answer:
When light hits a material, three different things can happen.
First, the material can absorb the energy.
Second, transmit the energy
Third, reflect the energy cause it to bounce off.
All these things can happen at once.
i need help plsss i need it fast if possible

Answers
The 1000 kg ball would have 62500 J of kinetic energy; The 10 kg ball would have 125 J of kinetic energy; The 100 kg person would also have 1250 J of kinetic energy.
What is kinetic energy?Kinetic energy is the energy an object possesses due to its motion. It is defined as the energy that an object has as a result of its motion, and is dependent on the mass and velocity of the object.
To calculate the kinetic energy of an object, we use the formula:
KE = \(1/2 * m * v^{2}\)
where m is the mass of the object and v is its velocity.
Given that a 100 kg ball is traveling at 5 m/s, we can calculate its kinetic energy:
KE = \(1/2 * 100 kg * (5 m/s)^2\) = 1250 J
Using the same formula, we can calculate the kinetic energy for the following scenarios:
A 1000 kg ball traveling at 5 m/s:
KE =\(1/2 * 1000 kg * (5 m/s)^2\)= 62500 J
The 1000 kg ball would have 62500 J of kinetic energy, which is 50 times greater than the kinetic energy of the 100 kg ball.
A 10 kg ball traveling at 5 m/s:
KE = \(1/2 * 10 kg * (5 m/s)^2\)= 125 J
The 10 kg ball would have 125 J of kinetic energy, which is 10 times smaller than the kinetic energy of the 100 kg ball.
A 100 kg person traveling at 5 m/s:
KE = \(1/2 * 100 kg * (5 m/s)^2\) = 1250 J
The 100 kg person would also have 1250 J of kinetic energy, which is the same as the kinetic energy of the 100 kg ball.
Learn more about energy here:
https://brainly.com/question/1932868
#SPJ1
The molar solubility of Cd(OH)2 is 1.84×10−5M in water. The expected solubility of Cd(OH)2 in a buffer solution of pH=12 is: 1.84×10−9M 2.49×10−M 6.23×10−11M 2.49×10−10M
Answers
The expected molar solubility of Cd(OH)2 in a buffer solution of pH=12 is 4.92 × 10^(-11) M.
The expected molar solubility of Cd(OH)2 in a buffer solution of pH=12 can be determined using the following steps:
1. Calculate the concentration of OH- ions in the buffer solution:
pOH = 14 - pH = 14 - 12 = 2
[OH-] = 10^(-pOH) = 10^(-2) M = 0.01 M
2. Write the solubility equilibrium expression for Cd(OH)2:
Cd\((OH)_{2}\) (s) ⇌\(Cd_{2+}\) (aq) + 2OH- (aq)
Ksp = [\(Cd_{2+}\)] * [\(OH_{-}\)]^2
3. Determine the Ksp value from the given molar solubility in water:
Molar solubility of Cd(OH)2 in water = 1.84 × 10^(-5) M
Ksp = (1.84 × 10^(-5)) * (2 * 1.84 × 10^(-5))^2 = 4.92 × 10^(-15)
4. Calculate the expected solubility of Cd(OH)2 in the buffer solution:
Ksp = [Cd2+] * [OH-]^2
4.92 × 10^(-15) = [Cd2+] * (0.01)^2
[Cd2+] = 4.92 × 10^(-15) / (0.01)^2 = 4.92 × 10^(-11) M
Therefore, the expected molar solubility is 4.92 × 10^(-11) M.
To learn more about "molar solubility", visit: https://brainly.com/question/28202068
#SPJ11
What happens when a bike rusts?
Select all that apply.
Responses
A new substance is formed.A new substance is formed. , ,
A physical change happens.A physical change happens. , ,
The atoms in the reactants rearrange to form the product.The atoms in the reactants rearrange to form the product. , ,
The atoms in the reactants stay the same in the product.The atoms in the reactants stay the same in the product. , ,
The atoms on the surface of the bike react with atoms in the air.

Answers
2.) the atoms in the reactants rearrange to form the product.
3.) the atoms on the surface of the bike react with atoms in the air.
meaning of carbon cycle
Answers
The carbon cycle is nature's way of reusing carbon atoms, which travel from the atmosphere into organisms in the Earth and then back into the atmosphere over and over again. Most carbon is stored in rocks and sediments, while the rest is stored in the ocean, atmosphere, and living organisms
4 steps of Carbon Cycle
Photosynthesis, Decomposition, Respiration and Combustion.

If 3 moles of HgO are reacted, how many kJ of heat will be absorbed
Answers
If the 3 moles of HgO are reacted, the amount of the kJ of the heat will be absorbed is 272.4 kJ.
The chemical equation is as :
2HgO ---> 2Hg + O₂
The heat of the reaction Hrxn = 181.6 kJ
The enthalpy of reaction, ΔHrxn = 181.6 kJ/ 2 mol
The amount of the heat absorbed during the reaction is as :
q = n ΔHrxn
Where,
ΔHrxn = 181.6 kJ/ 2 mol
n = moles = 3 moles
The amount of heat will be absorbed in reaction, q = n ΔHrxn
The amount of heat will be absorbed in reaction, q = 3 mol × 181.6 kJ/ 2 mol
The amount of heat will be absorbed in reaction , q = 272.4 kJ.
To learn more about moles here
https://brainly.com/question/14342636
#SPJ4
This question is incomplete, the complete question is :
If 3 moles of HgO are reacted, how many kJ of heat will be absorbed in reaction.
2HgO ---> 2Hg + O₂ , Hrxn = 181.6 kJ
What is the percent composition of nitrogen in lidocaine c14h22n2o
Answers
11.9% is the percent composition of nitrogen in lidocaine. The mass percent of a solution is represented as the grams of the either by grams of solution.
What is mass percentage?The Mass percent formula provides written to solve for the molar mass as well as the mass of each element in one mole of the compound. With these masses, you'll calculate the mass proportion of each element.
The mass percent of a solution is represented as the grams of the either by grams of solution, and multiplied by hundred to obtain a percentage.
mass percentage of nitrogen =(mass of nitrogen/molar mass of C\(_{14}\)H\(_{22}\)N\(_2\)O)×100
mass percentage of nitrogen =(28/234.34)×100 = 11.9%
Therefore, 11.9% is the percent composition of nitrogen in lidocaine.
To learn more about mass percentage, here:
https://brainly.com/question/28973847
#SPJ9
A spectrophotometric method for the analysis of iron has a linear calibration curve for standards of 0. 00, 5. 00, 10. 00, 15. 00, and 20. 00 ppm. An iron ore sample with an expected iron content of 40–60% w/w is to be analyzed by this method. An approximately 0. 5 g sample is taken, dissolved in a minimum of concentrated HCl, and diluted to 1 L in a volumetric flask using distilled water. A 5. 00-mL aliquot is removed with a pipet. To what volume (10, 25, 50, 100, 250, 500, or 1000 mL) should it be diluted to minimize the uncertainty in the analysis? Explain
Answers
To calculate the concentration of the iron sample by using a spectrophotometric method, it is necessary to dilute the sample. The volume to which the sample should be diluted is a crucial question in achieving the most accurate result.
The process involves diluting the sample, and the concentration must be calculated to determine the precise result of the dilution. This question can be answered by calculating the uncertainty and identifying the value of the uncertainty. The value with the lowest uncertainty will be the best value to choose. The volume with the lowest uncertainty will be the ideal volume to dilute the 5 ml aliquot of the iron sample to achieve a result with the minimum level of uncertainty.
To determine the optimal volume for dilution, the uncertainty should be calculated.
This can be done by using the equation for propagation of uncertainty, which states that the uncertainty of the result is equal to the square root of the sum of the squares of the uncertainties of the individual components. When calculating the uncertainty of the diluted sample, the uncertainty of the initial sample and the uncertainty of the diluent must be considered. The uncertainty of the initial sample can be calculated using the calibration curve. As the expected iron content is 40-60%, the concentration of the sample is expected to be 8-12 ppm. The uncertainty of the calibration curve is given by the standard deviation of the calibration standards.
The diluent has a negligible uncertainty. The uncertainty of the diluted sample will be lower if a larger volume is used for dilution because the relative contribution of the uncertainty of the initial sample will decrease. However, the uncertainty of the measurement will increase if the sample is diluted too much because the concentration of the analyte will be too low to be detected accurately. A 100 mL volume is a good choice because it balances the need for sufficient dilution to reduce the uncertainty of the initial sample with the need for sufficient concentration to allow for accurate detection of the analyte.
The volume of the sample that should be diluted is 5 ml. The minimum level of uncertainty is obtained at a dilution of 100 ml. When the volume of the diluent is greater than 100 ml, the uncertainty of the measurement increases, and when the volume of the diluent is less than 100 ml, the uncertainty of the measurement also increases. Thus, a 100 ml volume of diluent is the ideal volume to minimize the uncertainty in the analysis of iron.
to know more about spectrophotometric visit:
brainly.com/question/31632843
#SPJ11
What do you think? Is our current understanding ofthe Universe backed up with facts? Is it a good theory
Answers
Answer:
Yes
Explanation:
The expansion of the universe is the increase in distance between any two given gravitationally unbound parts of the observable universe with time. It is an intrinsic expansion whereby the scale of space itself changes. The universe does not expand "into" anything and does not require space to exist "outside" it.
How Many Atoms are present in this formula NH4NO3
Answers
3.0 Liters of gas at a pressure of 0.90 atm is compressed to 2.0 atm.
What is the new volume?
Answers
Answer:
5.5 L
Explanation:
an excess of oxygen reacts with 451.4 g of lead, forming 342.3 g of lead(ii) oxide. calculate the percent yield of the reaction.
Answers
The percent yield of the reaction is approximately 83.28%.
To calculate the percent yield of a reaction, we need to compare the actual yield (experimental yield) with the theoretical yield. The actual yield is the amount of product obtained in the experiment, while the theoretical yield is the maximum amount of product that could be formed based on stoichiometry.
Mass of lead (Pb) = 451.4 g
Mass of lead(II) oxide (PbO) = 342.3 g
1. Calculate the molar masses:
Molar mass of Pb = 207.2 g/mol
Molar mass of PbO = 223.2 g/mol
2. Convert the masses to moles:
Moles of Pb = mass of Pb / molar mass of Pb
Moles of PbO = mass of PbO / molar mass of PbO
3. Determine the stoichiometry of the balanced chemical equation:
2Pb + O₂ → 2PbO
According to the stoichiometry, 2 moles of Pb react with 1 mole of O₂ to form 2 moles of PbO.
4. Calculate the theoretical yield:
Theoretical yield of PbO = Moles of Pb × (2 moles of PbO / 2 moles of Pb)
5. Calculate the percent yield:
Percent yield = (Actual yield / Theoretical yield) × 100%
Using the given masses, the calculations would be as follows:
Moles of Pb = 451.4 g / 207.2 g/mol ≈ 2.18 mol
Moles of PbO = 342.3 g / 223.2 g/mol ≈ 1.53 mol
Theoretical yield of PbO = 2.18 mol × (2 mol PbO / 2 mol Pb) ≈ 2.18 mol
Percent yield = (1.53 mol / 2.18 mol) × 100% ≈ 83.28%
Therefore, the percent yield of the reaction is approximately 83.28%.
To know more about stoichiometry refer here:
https://brainly.com/question/28780091#
#SPJ11
Calculate the density of pentane with a mass of 47 grams and a volume of 75 mL.
Answers
Answer:
The answer is
0.63 g/mLExplanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\\)
From the question
mass = 47 g
volume = 75 mL
The density is
\(density = \frac{47}{75} \\ = 0.626666666...\)
We have the final answer as
0.63 g/mLHope this helps you
PLS HELP ME- How many particles (atoms) are in a single molecule of the following compounds?

Answers
Answer:
Explanation:
MgO has 2 atoms
Rb2S has 3 atoms
Ca(OH)2 has 1 + 2 + 2 = 5 atoms
K2SO4 has 2 Ks 1S + 4Os = 6 atoms
Ga2(CO3)^2 has 2Ga + 2Cs 3*2O = 2 + 2 + 6 = 10 atoms
Give 3 examples of a solution
Answers
Answer:
Comrade, figures, and sad
Explanation
The solution of sugar, salt etc in water.
Sublimation of substances like iodine, camphor etc into the air.
Hydrated salts, mercury in amalgamated zinc,
Alcohol in water, benzene in toluene
How many hydrogen molecules are in 1.2 moles of hydrogen?
Answers
Answer:
7.22 x 10²³molecules
Explanation:
Given parameters:
Number of moles of hydrogen = 1.2moles
Unknown:
Number of molecules of hydrogen = ?
Solution:
From the concept of moles, a mole of a substance contains the Avogadro's number of particles.
1 mole of a substance = 6.02 x 10²³ molecules;
So; 1.2 moles of hydrogen = 1.2 x 6.02 x 10²³ molecules;
= 7.22 x 10²³molecules
students of different ages were given the same puzzle to assemble. The puzzle assembly time was measured.
what is the independent variable?
what is thw dependent variable?
what is the constant?
Answers
Answer:
1. Age
2. Time
3. Same Puzzle
Explanation: Independent variable are what changes in the experiment and allow you to get a result. Dependent variables measure what you are trying to find from an experiment. Constants are what remain the same throughout all trials.
What would the mystery charge labeled "?" have to be for this object to have a net electric charge of +3?
Answers
For the net charge to be +3,the mystery charge should be is -2 and net force is towards left.
In first problem, overall charge should be -2 (as mentioned in question).So charge labelled with ? should be negative charge ( -ve). There will be net force on -1 charge towards +3 charge (left). For box (b)net force towards right For box c) net force towards right.It is often referred to as electric charge, electrical charge, or electrostatic charge, and denoted by the letter q, is a property of a unit of matter that indicates how many more or less electrons than protons it has.When retained in an electric or magnetic field, matter's basic physical feature known as electric charge causes it to experience a force.To learn more about charge visit:
https://brainly.com/question/14713274
#SPJ9
Given 5 grams of H2, how many grams of
CH4 are produced?
Answers
Given 5 grams of H₂, 80 grams of CH₄ are produced by From the equation, we can see that for every 2 moles of CH₄, 1 mole of H2 is consumed.
To determine the amount of CH₄ produced from 5 grams of H₂, we need to consider the balanced chemical equation for the reaction between H₂ and CH₄. From the equation stoichiometry, we can calculate the stoichiometric ratio between H₂ and CH₄ and use it to find the mass of CH₄ produced.
The balanced chemical equation for the reaction between H₂ and CH₄ is:
H₂ + 2CH₄ → 4H₂ + C
To find the mass of CH₄ produced, we need to convert the mass of H2 to moles using its molar mass (2 g/mol) and then use the stoichiometric ratio to calculate the moles of CH₄ produced. The molar mass of CH₄ is 16 g/mol.
First, we convert the mass of H₂ to moles:
5 g H₂ * (1 mol H2 / 2 g H₂) = 2.5 mol H2
Since the stoichiometric ratio is 1:2 for H2 to CH₄, we have:
2.5 mol H₂ * (2 mol CH₄ / 1 mol H₂) = 5 mol CH₄
Finally, we convert the moles of CH₄ to grams:
5 mol CH₄ * (16 g CH₄ / 1 mol CH₄) = 80 g CH₄
Therefore, 5 grams of H₂ will produce 80 grams of CH₄.
Learn more about stoichiometry here
https://brainly.com/question/30676346
#SPJ11
Can u see bubbles in chemical reactions

Answers
What is the mass of the oxygen in a 125 g of copper (II) sulfate?
Answers
125 g CuSO4 • 1 mol CuSO4 / 159.609 g CuSO4 • 4 mol O / 1 mol CuSO4 • 16.0 g O / 1 mol O = 50.1 g oxygen
What is the mass of a 49 cm3 object with a density of 63 g/cm3?
Answers
The mass of an object is a measure of the total amount of matter present in it. Mass is usually measured in grams (g) or kilograms (kg).The mass of the object with a volume of 49 cm³ and density of 63 g/cm³ is 3087 g or 3.087 kg.
The given data is;
volume = 49 cm³,
density = 63 g/cm³.
Now, we have to calculate the mass of the object.
Density = mass / volume
Mass = density × volume
Substitute the given values in the above equation.
Mass = 63 × 49
Mass = 3087 g or 3.087 kg
The mass of the object is 3087 g or 3.087 kg.
The mass of the object with a volume of 49 cm³ and density of 63 g/cm³ is 3087 g or 3.087 kg. It means the mass of the object is 3087 times its volume.
The mass of the object with a volume of 49 cm³ and density of 63 g/cm³ is 3087 g or 3.087 kg.
to know more about Density visit:
brainly.com/question/29775886
#SPJ11
Question 14 PM2.5 is defined as ________
- the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air greater than or equal to 2.5 micrometers in diameter. Question 15 Carbon dioxide (CO2) is a criteria air pollutant. - True - False Question 16 Roughly percent of emissions of carbon monoxide in Santa Clara County come from mobile sources (select the choice closest to the correct answer). - 50 - 75 - 25 Question 17
The term "photochemical smog" is most synonymous with which of the following criteria air pollutants? - lead (Pb) - carbon monoxide (CO) - sulfur dioxide ( SO2) - ozone (O3) Question 18 "Attainment" of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards. - True - False
Answers
: PM2.5 is defined as the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter.Question 15: False, carbon dioxide (CO2) is not considered a criteria air pollutant.
Question 16: The closest answer is 50%, but the exact percentage is not provided in the question.Question 17: The term "photochemical smog" is most synonymous with ozone (O3), which is a criteria air pollutant.Question 18: True, attainment of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards.
Question 14 asks about the definition of PM2.5. PM2.5 refers to particulate matter with a diameter less than or equal to 2.5 micrometers. It represents the mass concentration of particles suspended in the air, which are small enough to be inhaled into the respiratory system and can have adverse health effects.
Question 15 states whether carbon dioxide (CO2) is a criteria air pollutant. Criteria air pollutants are a set of pollutants regulated by environmental agencies due to their detrimental impact on air quality and human health. However, carbon dioxide is not considered a criteria air pollutant because it does not directly cause harm to human health or the environment in the same way as pollutants like ozone or particulate matter.
Question 16 asks about the percentage of carbon monoxide (CO) emissions from mobile sources in Santa Clara County. While the exact percentage is not provided in the question, the closest answer option is 50%. However, it is important to note that the precise percentage may vary depending on specific local conditions and emissions sources.
Question 17 inquires about the criteria air pollutant most synonymous with the term "photochemical smog." Photochemical smog is primarily associated with high levels of ground-level ozone (O3). Ozone is formed when nitrogen oxides (NOx) and volatile organic compounds (VOCs) react in the presence of sunlight, creating a hazy and polluted atmospheric condition.
Question 18 addresses the concept of "attainment" of ambient air quality standards. To achieve attainment, measured concentrations of pollutants at all monitoring stations within an air district must be below the established ambient air quality standards. This ensures that the air quality in the given area meets the required standards for protecting human health and the environment.
Learn more about mass concentration here:- brainly.com/question/23437000
#SPJ11
WILL GIVE BRAINLIEST
How long would it take 308 g of the sample to decay to 4.8125 grams? Show your work or explain your answer.
Answers
The time taken to decay from 308 g to 4.8125 g is approximately 114 days, or 3.8 months.
The radioactive decay formula is given by,N(t) = N0 * e^(-λ*t),
where N(t) is the amount of remaining radioactive sample at time t, N0 is the initial amount of the radioactive sample, λ is the decay constant, and e is the mathematical constant approximately equal to 2.71828.
Given,Initial mass, N0 = 308 g
Mass after decay, N(t) = 4.8125 g
We need to find the time taken to reach this decay.
First, we need to find the decay constant from the given half-life,The half-life of the given radioactive sample is 14.3 days.
We know that,Half-life, t(1/2) = 14.3 days.
Decay constant, λ = ln(2) / t(1/2)λ
= 0.0484 / day
Substitute the given values in the radioactive decay formula,N(t) = N0 * e^(-λ*t)
We get,4.8125 = 308 * e^(-0.0484 * t)
Divide both sides by 308,0.015625 = e^(-0.0484 * t)
Taking natural logarithm on both sides, ln(0.015625) = ln(e^(-0.0484 * t))
ln(0.015625) = -0.0484 * t
ln(0.015625) / -0.0484 = t
Hence, the time taken to decay from 308 g to 4.8125 g is approximately 114 days, or 3.8 months.
To learn more about decay visit;
https://brainly.com/question/32086007
#SPJ11
FILL HTE BLANK. with their low ies and small eas, the members of group 1a(1) and 2a(2) are strong _____ agents. with their high ies and large eas, the members of group 6a(16) and 7a(17) are strong _____ agents.
Answers
With their low ionization energies and small effective atomic sizes, the members of Group 1A (1) and 2A (2) are strong reducing agents. With their high ionization energies and large effective atomic sizes, the members of Group 6A (16) and 7A (17) are strong oxidizing agents.
Group 1A (1) and 2A (2) elements (alkali metals and alkaline earth metals, respectively) have low ionization energies, meaning it requires relatively less energy to remove an electron from their outermost shell. Additionally, they have small effective atomic sizes, which means their outermost electrons are closer to the nucleus and experience less shielding from inner electron shells. These characteristics make them more likely to lose electrons and act as reducing agents in chemical reactions. They readily donate electrons to other substances, facilitating reduction reactions.
Group 6A (16) and 7A (17) elements (chalcogens and halogens, respectively) have high ionization energies, indicating it requires a significant amount of energy to remove an electron from their outermost shell. Moreover, they have large effective atomic sizes, causing their outermost electrons to be farther from the nucleus and experience more electron-electron repulsion. These factors make them highly electronegative and efficient at accepting electrons, making them strong oxidizing agents. They readily gain electrons from other substances, promoting oxidation reactions.
To know more about ionization energies here
https://brainly.com/question/28385102
#SPJ4
--The given question is incorrect, the correct question is
"With their low ionization energies (IEs) and small effective atomic sizes (EAs), the members of Group 1A (1) and 2A (2) are strong _____agents. With their high ionization energies (IEs) and large effective atomic sizes (EAs), the members of Group 6A (16) and 7A (17) are strong _____ agents."--
Ayúdenme por favor
Please help me
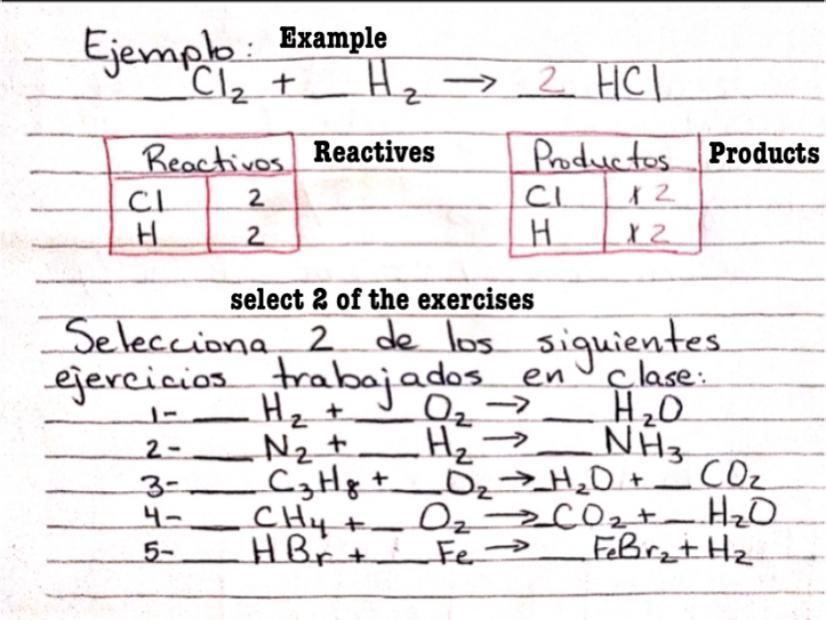
Answers
2H2+O2===>2H2O
N2+3H2==>2NH3
C3H8+5O2==>4H20+3CO2
CH4+2O2===>CO2+2H2O
2HBr +Fe==>FeBr2 +H2
I hope it helped
Spring and autumn both have a certain day where the amount of daytime hours
and nighttime hours are the same. What is this day called?
a. Equator
b. Equilibrium
c. Equality
d. Equinox
Answers
Answer:
d. Equinox
Explanation:
A certain day in spring and autumn where the amount of daytime hours and nighttime hours are the same is called equinox.
Equinox occurs twice in a year on March 20 and September 23rd.
During an equinox, the south and north hemisphere of the earth are receiving equal amount of sunlight. The opposite of equinox is solstice in which the duration of light is unequal. The equator is the only great circle that is a line of latitude.El fluoruro de hidrógeno HF que se utiliza en
la obtención de los freones (los cuales
destruyen la capa de ozono de la
estratosfera), se prepara mediante la
siguiente reacción: CaF2 + H2SO4
CaSO4 + 2HF Si se hacen reaccionar 50gr
de CaF2 con 100 gr de H2SO4 (masas
atómicas Ca=40,F=19, H=1, S=32, O=16)
Answers
Answer:
25.6g de HF son producidos
Explanation:
...¿Cuánto HF es producido?
Para resolver este problema debemos convertir la masa de cada reactivo a moles usando su masa molar. Como la reacción es 1:1, el reactivo con menor número de moles es el reactivo limitante. Con las moles del reactivo limitante podemos obtener las moles de HF y su masa así:
Moles CaF2:
Masa molar:
1Ca = 40g/mol
2F = 19*2 = 38g/mol
40+38 = 78g/mol
50g CaF2 * (1mol/78g) = 0.641 moles CaF2
Moles H2SO4:
Masa molar:
2H = 2g/mol
1S = 32g/mol
4O = 64g/mol
98g/mol
100g H2SO4 * (1mol / 98g) = 1.02 moles H2SO4
Como las moles de CaF2 < Moles H2SO4: CaF2 es reactivo limitante.
Moles HF usando la reacción:
0.641 moles CaF2 * (2mol HF / 1mol CaF2) = 1.282 moles HF
Masa HF:
Masa molar:
1g/mol + 19g/mol = 20g/mol
1.282 moles HF * (20g/mol) =
25.6g de HF son producidoscalculate molarity, molality and mole fraction of 28 percent aqueous solution of a solute with molar mass 11.5 g per mole and density is 1.25 g per mole
Answers
Explanation:
Aight, I'mma give tell ya an easy way for this question. look at this Forumla
M=
\( \frac{10ad}{m} \)
a explains the percentage of aqueous solution
d is the density
m is the molar mass
right, let's just hop to it
M=10×28×1.25/11.5====> M=30.43
at the end of the titration, it was noticed that there was a drop of titrant hanging on the tip of the buret, but not added to the beaker. would this cause the calculated concentration of the unknown naoh to be greater than, less than or equal to that determined in part (c)? justify your answer.
Answers
The calculated concentration of the unknown NaOH would be greater than that determined in part (c).
The presence of a drop of titrant hanging on the tip of the buret, but not added to the beaker, would result in an increased volume of titrant dispensed during the titration. As a result, the volume of titrant recorded would be greater than the actual volume that reacted with the unknown NaOH solution. Since the concentration of the titrant is known, this would lead to an erroneously higher calculated concentration of the NaOH solution.
When performing a titration, the volume of titrant added is crucial in determining the concentration of the analyte. Each drop of titrant matters, and any drop remaining in the buret that is not delivered to the beaker will contribute to an overestimation of the titrant volume used. This excess volume would falsely increase the apparent concentration of the NaOH solution.
The buret should be carefully observed at the end of the titration to ensure that no drops are left hanging on the tip. If there is a drop present, it should be carefully delivered into the beaker to ensure accurate results. Failure to do so may introduce an error in the calculation of the unknown NaOH concentration.
Learn more about Titration techniques
brainly.com/question/13845995
#SPJ11