Answers
Approximately 2.829 kilojoules (kJ) of energy is required to heat 0.24 kg of lutetium from 296.2 K to 373.5 K, assuming a specific heat of 0.154 J/g-K for lutetium.
What is Energy?
Energy is a fundamental concept in physics and refers to the ability of a system to do work. It is a scalar quantity and has various forms, such as kinetic energy, potential energy, thermal energy, electromagnetic energy, and nuclear energy.
To calculate the energy required to heat 0.24 kg of lutetium from 296.2 K to 373.5 K, we can use the formula:
Q = m * c * ΔT
where Q is the energy required, m is the mass of the lutetium, c is the specific heat of lutetium, and ΔT is the change in temperature.
First, we need to convert the mass of lutetium from kg to g:
m = 0.24 kg * 1000 g/kg = 240 g
Next, we can calculate the change in temperature:
ΔT = 373.5 K - 296.2 K = 77.3 K
Finally, we can use the formula to calculate the energy required:
Q = 240 g * 0.154 J/g-K * 77.3 K
Q = 2828.992 J or 2.829 kJ (to 3 significant figures)
Learn more about Energy from given link
https://brainly.com/question/13881533
#SPJ1
Related Questions
what mass of glucose c6h12o6 would be required to prepare 5000 mL of a 0.215 M solution
Answers
Approximately 194.0 grams of glucose (C6H12O6) would be required to prepare a 5000 mL solution with a concentration of 0.215 M.
To determine the mass of glucose (C6H12O6) required to prepare a 0.215 M solution in 5000 mL, we need to use the formula:
Molarity (M) = moles of solute / volume of solution (in liters)
First, let's convert the volume of the solution from milliliters (mL) to liters (L):
5000 mL = 5000/1000 = 5 L
Now, we can rearrange the formula to solve for moles of solute:
moles of solute = Molarity (M) x volume of solution (L)
moles of solute = 0.215 M x 5 Lmoles of solute = 1.075 mol
Since glucose (C6H12O6) has a molar mass of approximately 180.16 g/mol, we can calculate the mass of glucose using the equation:
mass of solute = moles of solute x molar mass of solute
mass of glucose = 1.075 mol x 180.16 g/mol
mass of glucose = 194.0 g (rounded to three significant figures)
Therefore, approximately 194.0 grams of glucose (C6H12O6) would be required to prepare a 5000 mL solution with a concentration of 0.215 M. It's important to note that the molar mass of glucose used in this calculation may vary slightly depending on the level of precision required.
For more such questions on glucose visit:
https://brainly.com/question/397060
#SPJ8
Help me! Who ever gets to answer and has the best answer gets brainliest (very easy!)
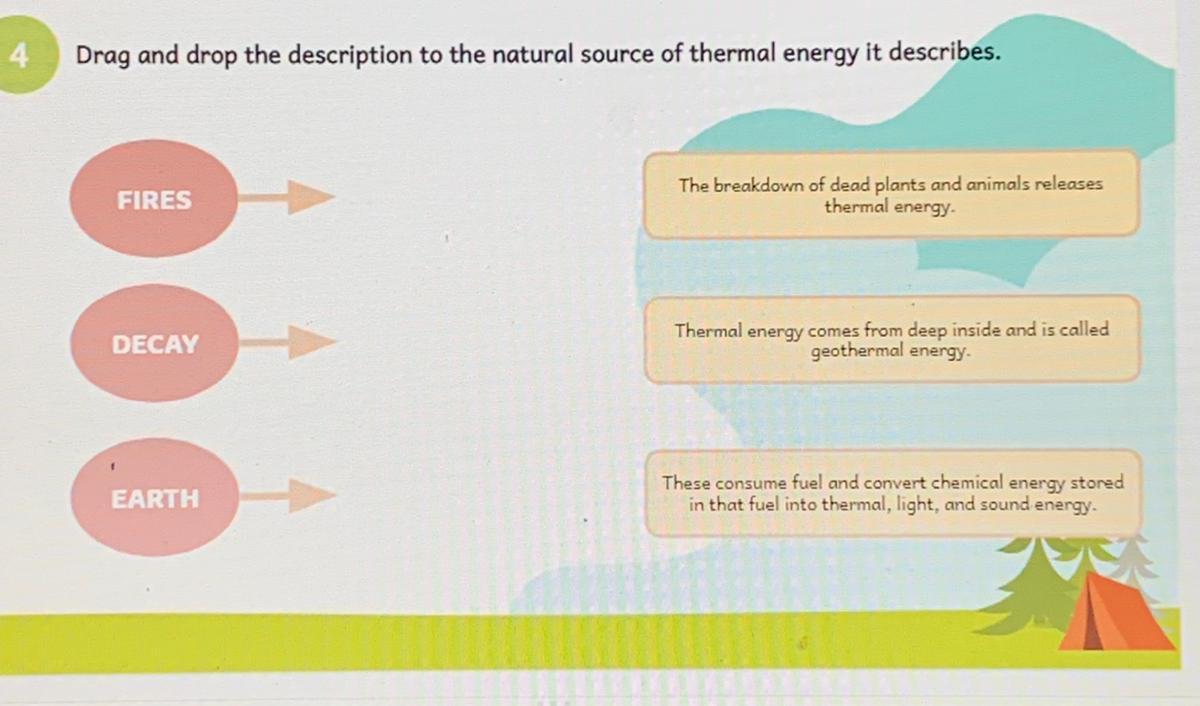
Answers
Decay-1
Earth-2
Answer:
Fires --> These consume fuel and convert chemical energy stored in that fuel into thermal light and sound energy.
Decay -->The breakdown of dead plants and animals releases thermal energy.
Earth --> Thermal energy comes from deep inside and it is called geothermal energy.
Given the following equation: Mg + 2HCI → MgCl₂ + H₂
How many moles of H₂ can be produced by reacting 2 moles
of HCI?
Answers
Taking into account the reaction stoichiometry, 1 mole of H₂ can be produced by reacting 2 moles of HCI.
Reaction stoichiometryIn first place, the balanced reaction is:
Mg + 2 HCl → MgCl₂ + H₂
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
Mg: 1 moleHCl: 2 molesMgCl₂: 1 moleH₂: 1 moleMoles of H₂ producedBy reaction stoichiometry 2 moles of HCl form 1 mole of H₂.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
brainly.com/question/24653699
#SPJ1
Which best describes the motion of pentane molecules in the liquid phase?
A. The pentane molecules move randomly in all directions to fill their
container.
O
B. The pentane molecules move past one another but are held close
together.
O C. The pentane molecules are locked in one place and do not move.
O D. The pentane molecules vibrate and are fixed in one position.
Answers
In the liquid state, the pentane molecules move past one another but are held close together.
What is the liquid phase?The liquid phase is a phase of matter that is made of molecules that in motion but do not posses as much kinetic energy as the molecules of gases. Thus implies that the molecules are able to translate.
Hence, the pentane molecules move past one another but are held close together.
Learn ore about liquid molecules:https://brainly.com/question/9437743
#SPJ1
Name the following hydrocarbon:
CH3CH2CH2CH2F
A. 4-fluorobutane
B. 2-fluorobutane
C. 1-fluorobutane
D. 1-fluoropentane
Answers
Answer:
The correct answer is (C)
Which is "1-fluorobutane"
Explanation:
Hope this helped if so please leave a "Rating" and "Like" and MARK me Brainliest if it was the BEST answer! THANKS! :)
The name of the hydrocarbon CH3CH2CH2CH2F is 1-fluorobutane.
In naming organic hydrocarbons, we count the number of the parent chain family. Here, we have an alkane with 4 carbons, which means that the parent chain is a family of alkane. The fourth compound in the family of an alkane is butane.
Afterwards, we will determine if there is any substituent attached to any of the carbon atoms in the chain and rank them in a way such that we have the lowest possible number.
Here, the fluorine is attached to the first carbon atom. So, it becomes 1-fluorobutane.
Learn more about the naming of hydrocarbons here:
https://brainly.com/question/16043495?referrer=searchResults
Imagine you are sitting on a surfboard in the ocean, waiting to catch a big wave. When the ocean is still, you are sitting at a point that would be a midpoint (the undisturbed position) of any waves that come your way. You look out to see and see a set of waves coming your way. What you see is actually a pattern of troughs followed be crest of the waves moving forward you and the shore. The distance between the crest is 5 meters. A crest passes you every 20 seconds.
Answer these questions:
A. What is the wavelength?
B. What is the frequency?
C. What is the wave velocity?
Plzzz help with is for a Science assignment so plz put a good answer the person who answers this good gets brainly!
Answers
Answer:
a. What is the wavelength?
The wavelength is 5 meters.
b. What is the frequency?
The frequency is 0.05Hz
c. What is the wave velocity?
The wave velocity is 0.25 meters per second
Explanation:
the wavelength is the distance between two troughs or two crests, which in this case is 5 meters.
the frequency=cycles/time. For this equation the equation would be 1/20, which is 0.05
the wave velocity=wavelength x frequency. Plug in the numbers, 5 x 0.05, and you will get 0.25.
Differentate Between Physical and chemical change with 5 example each
Answers
A physical change in matter or substances differs from a chemical change in various ways. A substance's physical state has no bearing on its inherent properties.
A new material is created through a chemical transformation, which involves a chemical reaction, and energy is either released or absorbed.
What are instances of physical and chemical changes?While a physical change occurs when matter changes forms without changing its chemical identity, a chemical change is the outcome of a chemical reaction. The physical transformations of boiling, melting, freezing, and shredding are a few examples.
Chemical Transformation: A permanent change in which new chemicals are created is referred to as a chemical change. Its physical, chemical, and chemical makeup differ from those of the original material.
There is no creation of new substances during physical changes. New compounds are created as a result of chemical transformation. Examples include melting wax, boiling water, shredding paper, etc.
Learn more about physical and chemical changes refer
https://brainly.com/question/17166994
#SPJ9
Which of these substances is closest to a neutral pH?
A) Apple juice
B) An egg
C) A lemon
D) Water

Answers
Answer: water (d)
Explanation:
the nutral value is 7. 7.1 is closest
A class observes two demonstrations water changing into steam and a piece of wood burning and producing smoke. A student concludes
that both demonstrations must be examples of a chemical change because a gas is produced in each.
is the student's conclusion accurate? Explain your answer, referring to both demonstrations.
WILL MARK BRIANLEST
Answers
Answer: The student's conclusion is inaccurate, smoke is a chemical change, but steam is a physical change.
Explanation:
When boiling water changes into steam, the matter composition of water is still the same (it remains H₂O). In this case, only the physical state of the water is changed from a liquid to a vapor (gas). When collected, the water vapor (gas) returns to its original liquid form proving that NO chemical reaction has taken place.
In contrast, a fire is a combustion chemical reaction that converts a fuel material (such as wood, fuel, or paper) and oxygen into carbon dioxide (CO₂) and water (H₂O). As an exothermic chemical reaction, the fire gives off heat in addition to the gaseous smoke. Unlike boiling, the fire permanently alters the fuel material as a result of the chemical reaction process.
Remember to vote for this as Brainliest if I earned it! :)
PLEASE HELP ASAP 20 POINTS!! As the pH of ocean water decreases, it becomes more _____.
Question 2 options:
acidic
basic
Answers
Answer:
acidic
Explanation:
A sample of gas at 2815 torr is cooled from 150.0 C to 100.0 C. Assuming the volume is constant what is the pressure in atm of the gas at 100.0 C
Answers
A sample of gas at 2815 torr is cooled from 150.0 C to 100.0 C. Assuming the volume is constant, 2482.2torr is the pressure in atm of the gas at 100.0 C.
The force delivered perpendicularly to an object's surface per unit area across how that force is dispersed is known as pressure (symbol: p / P). The pressure in relation to the surrounding air pressure is known as gauge pressure, also spelt gauge pressure.
Pressure is expressed using a variety of units. Some of these are calculated by dividing a unit of force by a unit of area; for instance, the metric system's unit of pressure, a pascal (Pa), is equal to one newton / square metre (N/m2).
P₁/T₁=P₂/T₂
2815 ×373/423=2482.2torr
To know more about pressure, here:
https://brainly.com/question/12971272
#SPJ1
How do meteorologists study climate?
a)They study vegetation changes over a long period of time.
b)They study weather changes over a long period of time.
c)They study wind currents over a long period of time.
d)They study ocean currents over a long period of time
Answers
Based on weather data, climate is measured. Since patterns over a length of time must be noted, a general term of 30 years is selected to anticipate the climate of an area.
What is the purpose of studying climate change throughout time?The study of climatic evolution is known as climatology. This branch of science aids with people's comprehension of the atmospheric factors that influence weather patterns and temperature variations throughout time.
What do you name the study of weather, meteorology?Meteorology is the study of weather, and a meteorologist is someone who conducts meteorological research. The area of atmospheric sciences known as meteorology places a strong emphasis on forecasting the weather. Moreover, it incorporates atmospheric physics and chemistry.
To know more about climate visit:-
https://brainly.com/question/29617595
#SPJ1
explain the relationship (linear or exponential) between rate and concentration including what order the iodate ion would be in.
CONCENTRATIONS
EXP. 1: 0.020
EXP 2: 0.019
EXP 3: 0.017
EXP 4: 0.016
EXP 5: 0.014
EXP 6: 0.013
EXP 7: 0.011
EXP 8: 0.01
EXP 9: 8.6x10^-3
EXP 10: 7.1x10^-3
EXP 11: 5.7x10^-3
EXP 12: 4.3x10^-3
RATE (s^-1):
EXP 1: 0.283
EXP 2: 0.1972
EXP 3: 0.2353
EXP 4: 0.2033
EXP 5: 0.1701
EXP 6: 0.133
EXP 7: 0.10
EXP 8: 0.1234
EXP 9: 0.077
EXP 10: 0.07380
EXP 11: 0.05102
EXP 12: 0.03883
By looking at the reaction mechanism, propose a Rate Law (WITHOUT the value of K). Explain the exponents for each reactant. Also, how does the rate law proposed compared to the relationship between rate and iodate concentration observed in the Rate law question?
Discuss, with respect to collision theory, the changes in the rates result from the changing concentrations of the iodate ion. What would you predict if we repeated these reactions at higher temperatures? Explain using collision theory.

Answers
Based on the given data, the relationship between rate and concentration is exponential.
A proposed rate law for the reaction based on the given data is:
Rate = k[IO3⁻]²[H+]What is the collision theory?Collision theory suggests that the rate of a chemical reaction is proportional to the frequency and energy of collisions between the reactant molecules.
As the concentration of iodate ions decreases, the frequency of collisions between reactant molecules decreases, which leads to a decrease in the rate of the reaction.
At higher temperatures, the kinetic energy of the reactant molecules increases, which increases the frequency and energy of collisions between reactant molecules.
Learn more about collision theory at: https://brainly.com/question/20628781
#SPJ1
What is the oxidation state of N in NaNOz?

Answers
The oxidation state of nitrogen (N) in NaNO3 is +5. option B
To determine the oxidation state of nitrogen (N) in sodium nitrate (NaNO3), we need to assign oxidation numbers to each element in the compound.
In NaNO3, we know that the sodium ion (Na+) has a +1 oxidation state because it is an alkali metal. Oxygen (O) typically has an oxidation state of -2 in compounds, and there are three oxygen atoms in NaNO3. Since the compound is neutral, the sum of the oxidation states must be zero.
Let's assume that the oxidation state of nitrogen is x. Therefore, we can set up the equation:
(+1) + x + (-2) * 3 = 0
Simplifying the equation:
+1 + x - 6 = 0
x - 5 = 0
x = +5
Therefore, the oxidation state of nitrogen (N) in NaNO3 is +5.
The oxidation state of an element indicates the number of electrons it has gained or lost in a compound. In this case, the nitrogen atom in NaNO3 has gained five electrons to achieve a stable oxidation state of +5.
It is important to note that oxidation states are formal charges and do not necessarily represent the actual distribution of electrons in a compound. They are assigned based on a set of rules and can be useful in understanding the reactivity and behavior of elements in chemical reactions.
Option B
For more such questions on oxidation state visit:
https://brainly.com/question/25551544
#SPJ8
What kind of intermolecular forces act between a xenon atom and a bromine molecule
Answers
Answer:
intermolecular forces of a tractione are a factor in how given molecules or compour will into act or be attracted to one onother
Explanation:
pa follow po
Which statement describes how the binary ionic compound CaCl2 is named? The nonmetal is named first, and the ending is replaced with -ide. The metal is named first, and the ending is replaced with -ide. The nonmetal is named second, and the ending is replaced with -ide. The metal is named second, and the ending is replaced with -ide.
Answers
Answer:
Option C: The nonmetal is named second, and the ending is replaced with -ide.
Explanation:
Just took test on Edge
The nonmetal is named second, and the ending is replaced with -ide. Hence, option C is correct.
What is a binary ionic compound?A binary ionic compound contains an ion that is a metal (cation) and an ion that is a nonmetal (anion).
Binary ionic compounds (ionic compounds that contain only two types of elements), the compounds are named by writing the name of the cation first followed by the name of the anion.
For example, KCl, an ionic compound that contains \(K^+\)and \(Cl^-\)ions, is named potassium chloride.
Some rules are as follow:
Name the metal by its elemental name.
Name the nonmetal by its elemental name and an -ide ending.
Name metals that can have different oxidation states using roman numerals to indicate positive charge.
Example \(Fe^{2+}\) is Iron(II).
Name polyatomic ions by their names.
The name for \(CaCl_2\) is calcium chloride. This is a binary ionic compound, a metal and a non-metal.
Hence, option C is correct.
Learn more about the binary ionic compound here:
https://brainly.com/question/1669915
#SPJ2
How is the periodic table generally arranged?
Answers
Answer:
It is generally arranged by the atomic number
Explanation:
which of the following lewis diagrams best represents the bonding in the N2O molecule, considering formal charges? justify your answer.

Answers
Considering the formal charges, the lewis structure in which the electronegative atom (O) contains a negative charge and the electropositive (N) contains a positive charge represents bonding in the N₂O. Therefore, option (B) is correct.
What are the formal charges?A formal charge in chemical bonding can be described as the charge assigned to an atom in a molecule by considering that electrons in all chemical bonds are equally shared between atoms, regardless of relative electronegativity.
The formal charge can be described as the difference between the number of valence electrons of an atom in a neutral state and the number assigned in a Lewis structure to that atom.
When determining the best Lewis structure for a molecule, the structure is selected such that the formal charge is as close to zero on each atom.
Learn more about formal charges, here:
https://brainly.com/question/11723212
#SPJ1

Explain two positive aspects of using methane recapture systems.
Answers
Answer:
Two positive aspects of using methane recapture systems are able to generate significant electricity. Another benefit is that the process of anaerobic digestion creates heat that can be used to warm buildings where animals are kept
Answer: The correct answer is;
Two positive aspects of using methane recapture systems include lowering the impact on greenhouse gasses and the production of energy. Methane is a very potent greenhouse gas that is contributing to global warming. As a result, the recapturing process reduces the methane impacts of global warming by reclaiming and reusing the gas for other purposes. Recaptured methane can be stored and used to generate electricity or used as fuel to power updated vehicles and other engines on the farm. The overall benefits from this combination are reducing impacts causing global warming and lower the cost of electricity or fuel on the farm.
Explanation: This answer has been confirmed correct.
When air pressure rapidly falls, what weather change usually occurs?
Answers
hope this helps
Explanation:
a prolong storm will occur
that's what I found
What is the meaning of reference point?
Answers
Answer:
Reference point, or frame of reference, a system of geometric axes in relation to which measurements of size, position, or motion can be made. Reference point, a geometrical point used to define the location of another point
Explanation:
produces pyruvate. the multienzyme complex catalyzes the oxidative of pyruvate to yield carbon dioxide and acetyl coa. the overall equation for
Answers
Pyruvate is a byproduct of glycolysis.The oxidative deacetylation of pyruvate to produce carbon dioxide and acetyl CoA is catalyzed by the multienzyme complex dopamine dehydrogenase complex (PDH complex).Pyruvate + CoA + NAD+ ----> Acetate CoA + NADH + H++ CO2. Acetyl CoA ———- Citric Acid Cycle is the general equation for the reaction.
How is acetyl CoA produced from pyruvate?Coenzyme A is joined with the oxidized two-carbon acetyl group to generate acetyl CoA.
What enzyme is in charge of turning pyruvate into acetyl CoA?The pyruvate dehydrogenase (PDH) enzyme, which is a component of the multienzyme PDC and is frequently described to as a "gatekeeper" in the oxidation of carbohydrates, catalyzes the physiologically irreversible conversion of pyruvate to acetyl-CoA.
To know more about yield carbon dioxide visit:
https://brainly.com/question/14995953
#SPJ4
URGENT PLZ HELP
Which of the following statements is true?
a. In an endothermic process heat is transferred from the surroundings to the
system.
b. In an exothermic process heat is transferred from the surroundings to the
system.
C. The surroundings will feel cooler in an exothermic process.
d. The surroundings will feel warmer in an endothermic process.
Answers
Answer:
A.
Explanation:
In an endothermic reaction heat is applied.
In an endothermic process, heat is transferred from the surroundings to the system.
What are endothermic and exothermic processes?
An exothermic process is one that gives off heat. This heat is transferred to the surroundings. An endothermic process is one in which heat has to be supplied to the system from the surroundings.
The endothermic process is a term that describes a reaction where the system absorbs the energy from its surrounding in the form of heat. A few examples of the endothermic process are photosynthesis, evaporating liquids, melting ice, etc.
The exothermic reaction is the opposite of an endothermic reaction. It releases energy by light or heat to its surrounding. A few examples are neutralization, burning of a substance, reactions of fuels, etc.
Hence, option A is the correct answer.
Learn more about endothermic and exothermic processes here:
https://brainly.com/question/4345448
#SPJ2
One way the U.S. Environmental Protection Agency (EPA) tests for chloride contaminants in water is by titrating a sample of silver nitrate solution. Any chloride anions in solution will combine with the silver cations to produce bright white silver chloride precipitate. Suppose an EPA chemist tests a sample of groundwater known to be contaminated with nickel(II) chloride, which would react with silver nitrate solution like this:
Answers
Answer:
6.5 mg/L.
Explanation:
Step one: write out and Balance the chemical reaction in the Question above:
NiCl2 + 2AgNO3 =====> 2AgCl + Ni(NO3)2.
Step two: Calculate or determine the number of moles of AgCl.
So, we are given that the mass of AgCl = 3.6 mg = 3.6 × 10^-3 g. Therefore, the number of moles of AgCl can be calculated as below:
Number of moles AgCl = mass/molar mass = 3.6 × 10^-3 g / 143.32. = 2.5118 × 10^-5 moles.
Step three: Calculate or determine the number of moles of NiCl2.
Thus, the number of moles of NiCl2 = 2.5118 × 10^-5/ 2 = 1.2559 × 10^-5 moles.
Step four: detemine the mass of NiCl2.
Therefore, the mass of NiCl2 = number of moles × molar mass = 1.2559 × 10^-5 moles × 129.6 = 1.6 × 10^-3 g.
Step five: finally, determine the concentration of NiCl2.
1000/ 250 × 1.6 × 10^-3 g. = 6.5 mg/L.
If the student’s estimate of the balloon’s volume was incorrect and the actual volume was 620 ml, would the amount of glucose that actually reacted be more than or less than the amount calculated in part (c)? Explain your response.
( C answer ) only 1.9 g of glucose reacted and only .0211 mol of co2 was formed.

Answers
The number of moles of CO2 produced is 0.021 moles
If the estimated volume of the balloon is wrong then the amount of glucose reacted must be more than is stated.
What is respiration equation?The respiration equation represents the chemical process of aerobic cellular respiration, which occurs in the mitochondria of cells and is the primary way in which cells generate energy in the form of ATP (adenosine triphosphate).
The equation of the reaction is;
C6H12O6 + 6O2 → 6CO2 + 6H2O + ATP
We know that;
Number of moles of glucose = 10 g/180 g/mol
= 0.056 moles
PV = nRT
n = PV/RT
n = 1 * 0.55/318 * 0.082
n = 0.021
Learn more about glucose:https://brainly.com/question/2252123
#SPJ1
even one or two crystals of copper sulphate can make its solution in water coloured blue. why
Answers
this also may happen because of the water molecules that get attached
describe the pattern of the Lewis dot structures of the 18 elements (include periods and groups/famalies)
Answers
Lewis dot structures are diagrams that depict the interactions between atoms in molecules as well as any lone pairs of electrons that may be present.
What are Lewis dot structures?A Lewis electron dot diagram, also known as an electron dot diagram, Lewis diagram, or Lewis dot structure, is a diagram that employs dots to represent the valence electrons of an atom. The number of dots corresponds to the atom's valence electron count.
By including lines between atoms to represent shared pairs in a chemical bond, Lewis structures expand on the idea of the electron dot diagram.
Learn more about Lewis dot structures at: https://brainly.com/question/20300458
#SPJ1
Hypothesis II: Write the equation with Iron (III) Chloride and balance it: Iron + Copper (II) chloride --> Iron (III) chloride + Copper
Answers
Answer:
Fe + CuCl2 = FeCl2 + Cu
Explanation:
This is already balanced.
Which environmental factor might positively impact a plant's traits
Answers
Answer:
light, temperature, water, humidity, and nutrition.
Determine the number of moles of a sample at 1.8 atm, 130 mL, and 78°C.