How many NH4+ ions are in 550 mg of (NH4)3PO4?
My professor usually goes over our in class assignment in class and then assigns us a weekly assignment to finish for homework with the problems being similar to what she has explained. We didn't go over one like this and I don't know how to approach it.
Answers
Answer:
7.53 × 10²¹ NH₄⁺ ions
Explanation:
Step 1: Given data
Mass of (NH₄)₃PO₄: 550 mg
Step 2: Convert 550 mg to grams
We will use the conversion factor 1 g = 1000 mg.
550 mg × (1 g/1000 mg) = 0.550 g
Step 3: Calculate the moles corresponding to 0.550 g of (NH₄)₃PO₄
The molar mass of (NH₄)₃PO₄ is 132.06 g/mol.
0.550 g × (1 mol/132.06 g) = 4.16 × 10⁻³ mol
Step 4: Calculate the moles of NH₄⁺ in 4.16 × 10⁻³ moles of (NH₄)₃PO₄
The molar ratio of (NH₄)₃PO₄ to NH₄⁺ is 1:3. The moles of NH₄⁺ are 3/1 × 4.16 × 10⁻³ mol = 0.0125 mol
Step 5: Calculate the number of ions in 0.0125 moles of NH₄⁺
We will use Avogadro's number: there are 6.02 × 10²³ NH₄⁺ ions in 1 mole of ions of NH₄⁺.
0.0125 mol × (6.02 × 10²³ ions/1 mol) = 7.53 × 10²¹ ions
Related Questions
1. A 0.4 L sample of gas is collected at a temperature of 45°C. At what temperature will the gas fill a 0.725 L container?
Answers
The temperature of sample gas that fills a 0.725L container is 576K which initially is collected in a 0.4L container at a temperature of 318K.
Given the volume of a sample gas (V1) = 0.4L
The temperature of gas (T1) = 45°C = 273 + 45 = 318K
The volume of container that fills gas (V2) = 0.725L
The temperature of gas at volume V2 = T2
We know that from ideal gas laws, the volume of gas and temperature are directly related to each other where its equation is: PV = nRT
If we assume the pressure is constant, then,
From the above equation we can say that:
V1/T1 = V2/T2
0.4/318 = 0.725/T2
T2 = 576.315K
To learn more about temperature click here https://brainly.com/question/29072206
#SPJ1
What is the gas-particle theory?
Answers
Answer:
The kinetic theory of matter (particle theory) says that all matter consists of many, very small particles which are constantly moving or in a continual state of motion.
Explanation:
hopes this helps :D
brainliest pls?
For the reaction
C₂H₂(g)+30₂(g) → 2CO₂(g) +2H₂O(g)
if 5.0 mol of CO₂ are produced, how many moles of O₂ were reacted?
(A) 12.5 mol
(B) 3.3 mol
C)7.5 mol
D)none of these
(E) 6.2 mol
I think it’s C
Answers
Answer: C
Explanation: 3 mol O2 ⇒ 2 mol CO2
O2 reacts to produce 5 mol of CO2 = (3 * 5)/2 = 7.5 mol O2
Use the heat equation to find out how many joules of energy are needed
to raise the temperature of 67 grams of water form 20°C - 45°C. What is the
answer in kilojoules?
(specific heat of water is 4.2 J/gºC.)
Answers
Answer:
About 7.0 × 10³ J or 7.0 kJ
Explanation:
We want to determine the amount of energy needed to raise the temperature of 67 grams of water from 20°C to 45°C.
We can use the heat equation:
\(\displaystyle q = mC\Delta T\)
Where C is the specific heat of water.
Substitute and evaluate:
\(\displaystyle \begin{aligned} q & = (67\text{ g})\left(\frac{4.2\text{ J}}{\text{g-$^\circ$C}}\right)\left(45^\circ \text{ C}- 20.^\circ\text{ C}\right) \\ \\ & = (67\text{ g})\left(\frac{4.2\text{ J}}{\text{g-$^\circ$C}}\right)(25^\circ \text{ C}) \\ \\ & = 7.0\times 10^3 \text{ J}\end{aligned}\)
Recall that there are 1000 J in a kJ. Hence:
\(\displaystyle \begin{aligned} q & = 7.0\times 10^3 \text{ J} \cdot \frac{1\text{ kJ}}{1000\text{ J}} \\ \\ & = 7.0 \text{ kJ}\end{aligned}\)
In conclusion, it will take about 7.0 × 10³ J or 7.0 kJ of energy to raise the temperature of 67 grams of water from 20 °C to 45 °C.
A student carries out a flame test on an unknown solid. a red flame is seen. the student concludes that the solid is lithium carbonate. explain why this conclusion is not justified
Answers

Describe how the thermal energy moves when a can of soda is taken out of the refrigerator and left on a counter for hours.
Answers
PLX HELP ASAP
How many grams of ethanol, C2H6O, are needed to prepare a 0.100 molal solution using 2.20 kg water?
A) 18.3 g
B) 10.1 g
C) 9.5 g
D) 20.6 g
Answers
Answer:
B) 10.1g
Explanation:
Answer:
10.1 g
Explanation:
what is polimation ???????????????????????????????????????????/
Answers
How many grams in 1.61 x 1023 molecules of water (H2O)
Answers
Taking into account the definition of Avogadro's number and molar mass, 4.806 grams of water are present in 1.61×10²³ molecules.
Definition of Avogadro's NumberAvogadro's Number or Avogadro's Constant is called the number of particles that make up a substance (usually atoms or molecules) and that can be found in the amount of one mole of said substance. Its value is 6.023×10²³ particles per mole. Avogadro's number applies to any substance.
Definition of molar massThe molar mass of substance is a property defined as its mass per unit quantity of substance, in other words, molar mass is the amount of mass that a substance contains in one mole.
Mass of waterTaking into account the definition of Avogadro's Number, you can apply the following rule of three: if 6.023×10²³ molecules are contained in 1 mole of water, then 1.61×10²³ molecules are contained in how many moles of water?
amount of moles of water= (1.61×10²³ molecules× 1 mole)÷ 6.023×10²³ molecules
amount of moles of water= 0.267 moles
Now, taking into account the definition of molar mass, and knowing that the molar mass of water is 18 g/mole, you can apply the following rule of three: If by definition of molar mass 1 mole of the compound contains 18 grams, 0.267 moles of the compound contains how much mass?
\(mass= \frac{0.267 molesx 18 grams}{1 mole}\)
mass= 4.806 grams
Finally, 4.806 grams of water are present in 1.61×10²³ molecules.
Learn more about
Avogadro's Number:
brainly.com/question/11907018
brainly.com/question/1445383
brainly.com/question/1528951
molar mass:
brainly.com/question/5216907
brainly.com/question/11209783
brainly.com/question/7132033
brainly.com/question/17249726
#SPJ1
Which of these does not accurated descibe the wright brothers first airplane
Answers
Answer:
it had wings that flapped similat to birds wings
A beaker weighed 53.10g. To the isolated beaker was added 5.348g of iron pellets and 56.1g of hydrochloride acid. What was the total mass of the beaker and the products after reaction?
Answers
114.5 g is the total mass of the beaker .
Total mass of beaker=53.10g+5.348g+ 56.1g
Total mass=114.5 g
Mass is used in physics to specific inertia, a fundamental function of all remember. basically, it's far a mass of rely's resistance to changing its course or pace in response to the software of a force.
The exchange that an applied force produces is smaller the extra mass a body has. The kilogram, the unit of mass within the international machine of gadgets, corresponds to 6.62607015 1034 joule seconds using Planck's consistent (SI). One joule is produced by way of multiplying one kilogram by means of one rectangular meter per 2d.
The kilogram is decided by genuine measurements of Planck's regular on account that the second one and the meter have formerly been described in phrases of other bodily constants.
To know more about mass visit : brainly.com/question/5661976
#SPJ9
What is the theoretical yield of a reaction?

Answers
The maximum amount of product that can be obtained from given amounts of reactants in a chemical reaction. The correct answer is C.
What is a chemical reaction?A chemical reaction is a process that causes one group of chemical components to change chemically into another. Reactants are substances that interact to make new substances, whereas products are those substances that result from the interaction.
Chemical Reaction Types
Synthesis processes.Reactions of decomposition.Responses with only one substitution.Reactions involving two replacements.Learn more about chemical reaction here:
https://brainly.com/question/11231920
#SPJ1
3. During an experiment, 98 g of water is used in the Styrofoam cup. The initial temperature
of water was 23.7°C. A 39.9-g piece of metal with initial temperature of 100.3°C (after
removing from the boiling water) is added to the calorimeter. The final temperature of water
was 28.2°C.
A) calculate the specific heat of the metal
B) identify the metal
C) calculate the percent error
Answers
The percent error is 0.22%. This is a very small error, which suggests that our experimental result is quite accurate.
What is Boiling Point?
Boiling point is the temperature at which a liquid changes state from a liquid to a gas or vapor by the process of boiling. At the boiling point, the vapor pressure of the liquid equals the atmospheric pressure, causing bubbles of vapor to form within the liquid and rise to the surface. The boiling point is a physical property of a substance and can vary depending on the pressure and altitude.
A) To calculate the specific heat of the metal, we can use the equation:
q = m * c * ΔT
q_water = m_water * c_water * ΔT_water
= 98 g * 4.184 J/g°C * (28.2°C - 23.7°C)
= 1903.52 J
where c_water is the specific heat of water, which is 4.184 J/g°C.
Next, we need to calculate the heat released by the metal:
q_metal = -q_water
= -1903.52 J
where the negative sign indicates that the heat is released by the metal and absorbed by the water.
Now, we can calculate the specific heat of the metal:
c_metal = q_metal / (m_metal * ΔT_metal)
= -1903.52 J / (39.9 g * (100.3°C - 23.7°C))
= 0.450 J/g°C
Therefore, the specific heat of the metal is 0.450 J/g°C.
B) To identify the metal, we can compare its specific heat to the known values of specific heats for different metals. The specific heat of the metal we calculated is 0.450 J/g°C. Here are some common metals and their specific heats:
Aluminum: 0.900 J/g°C
Copper: 0.385 J/g°C
Iron: 0.449 J/g°C
Lead: 0.128 J/g°C
Silver: 0.235 J/g°C
Zinc: 0.388 J/g°C
From this, we can see that the specific heat of the metal we calculated is closest to that of iron, which suggests that the metal is likely to be iron.
C) To calculate the percent error, we need to compare the experimental specific heat we calculated (0.450 J/g°C) to the accepted value for iron's specific heat (0.449 J/g°C), using the formula:
percent error = |(0.450 J/g°C - 0.449 J/g°C) / 0.449 J/g°C| * 100%
= 0.22%
Learn more about Boiling Point from the given link
https://brainly.com/question/40140
#SPJ1
Give the name of the ion with 13 protons and 10 electrons
Answers
Answer:
Explanation:
aluminum
Answer: The aluminum ion
Explanation:
Why are fossil fuels considered nonrenewable resources?
Answers
Answer:
They are considered nonrenewable because they are prehistoric animals that has been extinct therefore can't be renew.
An atom with more electrons than protons has an overall positive charge and is called a
positive ion
Answers
That is correct! It is a positive ion!
Besides solubility, state two other physical properties that are different for salt and sand.
Answers
Answer:Electrical Conductivity,soluble
Explanation:
Salt is a non-magnetic solid and is soluble in water. Sand is a non-magnetic solid and is insoluble in water.
Electrical Conductivity: Salt is an electrolyte and conducts electricity when dissolved in water or in a molten state. This is because salt dissociates into ions (Na+ and Cl-) that can carry electric current. In contrast, sand is a covalent compound and does not conduct electricity, as it does not dissociate into ions in the same way as salt. Sand is considered an insulator in terms of electrical conductivity.
C c Why did they work an average brightness for each length of graphite tested?
Answers
An average brightness was calculated for each length of graphite tested to get a better understanding of the relationship between the length of the graphite and the brightness of the line it produced.
What is the use of graphite?Graphite has many uses in various industries. Some of its uses include: Pencils, Lubricants, Refractories, Batteries, Electrodes, Nuclear reactors, Aerospace industry.
By calculating the average, it is possible to see if there is a trend in the data and if longer or shorter lengths of graphite produce brighter or duller lines. This information can be useful in determining the best length of graphite to use for a particular task or project.
Find out more on graphite here: https://brainly.com/question/27860158
#SPJ1
What is the purpose of the arrow in a chemical equation?
O A.
It indicates the direction in which the reaction occurs.
Св.
It separates the elements from the compounds.
O C.
It indicates the direction of increase in the number of molecules.
D.
It indicates the direction of heat movement in a reaction.
Reset
Next
Answers
Answer:
A) It indicates the direction in which the reaction occurs.
Explanation:
The reason we don't use equals signs (=) in chemical reactions is because of the Identity Property of Equality (if a = b then b = a), which would allow us to reverse reactions. Reactions can't just be reversed (since some don't even occur); we need to know what is reacting to make what. Thus, we use the arrow, or yields symbol to denote this.
The substances to the left of the sign are the reactants, the ones that are reacting, and the ones on the right are the products, the ones that are being produced from the reactants. The arrow is an excellent way to represent the direction the reaction occurs in as the way it's written clearly demonstrates direction.
Hope this helps!
How many milliliters of 8.54×10−2 M Ba(OH)2(aq) are required to titrate 54.90 mL of 5.14×10−2 M HNO3
Answers
16.52 milliliters of 8.54×10⁻² M Ba(OH)2(aq) are required to titrate 54.90 mL of 5.14×10⁻² M HNO₃
What is titration?A titration is a procedure that uses a known solution to determine the concentration of an unknown solution. Until the reaction is finished, the titrant (the known solution) is typically supplied from a buret to a known volume of the analyte (the unknown solution).
Titration is an essential technique in analytical chemistry, and it is also known as volumetric analysis.
n-factor for Ba(OH)₂ =2
Thus, dilution equation becomes:
n × M₁V₁= M₂V₂
2 x 8.54 x 10⁻² x V₁ = 5.14 x 10⁻² x (54.90/1000)
V₁= 16.52 × 10⁻³¹
V₁ = 16.52 ml
Thus, 16.52 milliliters of 8.54×10⁻² M Ba(OH)2(aq) are required to titrate 54.90 mL of 5.14×10⁻² M HNO₃
To know more about titration refer to:
https://brainly.com/question/13307013
#SPJ1
In solution, strong acids and bases ionize completely, but weak acids and bases ionize only partially.
Answers
Answer:
A weak acid is one that does not dissociate completely in solution; this means that a weak acid does not donate all of its hydrogen ions (H+) in a solution. ... Therefore, the concentration of H+ ions in a weak acid solution is always less than the concentration of the undissociated species, HA.
Explanation:
Hope it helps
FOLLOW MY ACCOUNT PLS PLS
An atom of oxygen has six valence electrons. In nature, oxygen is a diatomic molecule and is usually found in the form O2. Why would one atom of oxygen want to bond with another one?
A) When the oxygen atoms bond together, they become ionized.
B) The transfer electrons to each other so they both have a full shell of valence electrons.
C) When oxygen atoms share electrons the outer shell of electrons is filled and each atom becomes more stable.
D) A single atom of oxygen is very stable and simply joins with another oxygen in order to destabilize both of them.
Answers
Answer:
I think the answer is B
Explanation:
Answer:
oxygen atom is a unstable atom so it gets attached to other oxygen atom sharing valance electrons to become stable
C is the right ans
Catalysts are substances that increase the rate of reaction but can be recovered unchanged at the end of the reaction. Catalysts can be classified as either homogeneous (same state as reactants) or heterogeneous (different state than reactants).
Platinum is used to catalyze the hydrogenation of ethylene:
H2(g)+CH2CH2(g)−⟶Pt(s)CH3CH3(g)
Chlorofluorocarbons (CFCs) catalyze the conversion of ozone (O3) to oxygen gas (O2):
2O3(g)−⟶CFC(g)3O2(g)
Magnesium catalyzes the disproportionation of hydrogen peroxide to produce water and oxygen:
2H2O2(aq)−⟶Mg(s)2H2O(l)+O2(g)
What type of catalysts are platinum, CFCs, and magnesium under these conditions?
Answers
Answer:
- Platinum acts as a heterogeneous catalyst in the hydrogenation of ethylene.
- CFCs act as homogeneous catalysts in the conversion of ozone to oxygen gas.
- Magnesium acts as a heterogeneous catalyst in the disproportionantion of hydrogen peroxide.
Explanation:
Hello,
For the given reactions, considering the definition of homogeneous and heterogeneous catalyst, we can identify that is each catalyst behave as follows:
- Platinum acts as a heterogeneous catalyst in the hydrogenation of ethylene as all the reactants are gaseous but it remains solid.
- CFCs act as homogeneous catalysts in the conversion of ozone to oxygen gas as it remains gaseous as well as both ozone and oxygen.
- Magnesium acts as a heterogeneous catalyst in the disproportionantion of hydrogen peroxide as it is solid whereas the other species are aqueous, liquid and gaseous
Best regards.
If an atom contains 11 protons and 12 neutrons, its ATOMIC NUMBER is: a 1 b 11 c 12 d 23
Answers
Answer:
B)11
Explanation:
The atomic number is the number of protons and electrons in an atom!!
HOPE THIS HELPS!!!!!!
A molecule H-Y has a bond length of 1.6 Å and a charge of 0.100 electron unit on each atom. Calculate the dipole moment (D) for this molecule. Do not use scientific notation and please show work!! The answer should be between .75 and .80, I am just unsure of how to get there.
Answers
Answer:
1.6/0.100
= 16
16 x 5
= 80
now you need to multiply as you divided before so the answer is
=.80
Your welcome
Predict which of the following reactions has a positive change in entropy.
l. 2N2(g) + O2(g) → 2N2O(g)
II. CaCO3(s) → CaO(s) + CO2(g)
III. Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
Answers
Several factors can dictate entropy in an equation.
These include:
1. Phase changes
⇒ When a solid turns to a liquid, the entropy increases as the particles have more freedom to move around and thus have a greater ability for 'disorder'. Same goes for a liquid turning to a gas. In a gas, the intermolecular forces are much weaker than that of a solid or liquid, allowing the particles more freedom.
So, going from a solid to liquid to gas increases entropy, and going the other way, from gas to liquid to solid, decreases entropy.
Example:
H₂O(l) -> H₂O(g)
This will have a positive entropy change, as the water molecules are becoming gaseous and thus have more freedom.
2. Dissolution
⇒ Similarly, breaking up particles of a solute when dissolving in a solvent will increase entropy as the particles are no longer bound together.
So, dissolving a solute will increase entropy.
Example:
NaCl(s) -> NaCl(aq)
This will have a positive entropy change, as the NaCl particles are more free after being separated.
3. Number of products and reactants
⇒ Generally, if you have more moles of products than reactants, if they are the same phase then entropy will increase. Note this is not necessarily true if you form a gas from two non-gas reactants, as the gas will still have more entropy.
4. Temperature
⇒ Increasing temperature will increase entropy as the particles have more kinetic energy and are then moving faster.
-------------------------------------------
l. 2N2(g) + O2(g) → 2N2O(g)
3 moles of gas are forming 2 moles of gas. The phase of products and reactants are the same, so since we have less moles of product than reactant, entropy will be negative.
II. CaCO3(s) → CaO(s) + CO2(g)
1 mole of solid is forming 1 mole of solid and 1 mole of gas. There is a phase change from solid to gas, and there are more moles of product than reactant, entropy will be positive.
III. Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
While 3 moles of reactant are forming only 2 moles product, we are forming a gas from non-gaseous reactants, so entropy will be positive regardless.
1. What is the primary composition of the objects that make up the Kuiper Belt?
Frozen rock, silicon, and metals
Frozen methane, ammonia, and water
Frozen iron, silicon, and sediment
Frozen methane, rock, and metals
9
Answers
Answer:
Frozen methane, ammonia, and water
Explanation:
The Kuiper Belt is made up of many solar system bodies that orbit around the sun outside Neptune’s orbit. The bodies, including some dwarf planets, are composed primarily of frozen volatile materials such as methane, ammonia, and water.
Kuiper belt is a circular disc in the outer solar system consisting of the remnants of planets. Mostly they are composed of frozen methane, ammonia and water. Thus option B is correct.
What is Kuiper belt?Kuiper belt is circumstellar body existing in the outer space and composed of methane, ammonia and water. It is named after the Dutch chemist Gerard Kuiper.
Kuiper belt is considered as an extension from the solar system from neptune. Around 50 AU from the Sun is where the Kuiper belt's inner, major area stops.
A second region known as the scattered disk overlaps the outer edge of the main Kuiper Belt and extends outward to almost 1,000 AU, with some planets on orbits that extend much beyond.
Unlike other rocks, Kuiper belts are made of frozen methane, ammonia and water. Thus option B is correct.
To find more about Kuiper belt, refer the link below:
https://brainly.com/question/25583240
#SPJ5
which is the graph of the function g(x) = f(-x)
Answers
To graph the function g(x) = f(-x), you can start with the graph of f(x) and then reflect it about the y-axis.
What is a graph of the function g(x) = f(-x)?To find the graph of the function g(x) = f(-x), we can start with the graph of the function f(x) and then reflect it about the y-axis.
If the graph of f(x) is symmetric with respect to the y-axis, meaning it is unchanged when reflected, then g(x) = f(-x) will have the same graph as f(x).
However, if the graph of f(x) is not symmetric with respect to the y-axis, then g(x) = f(-x) will be a reflection of f(x) about the y-axis.
In either case, the resulting graph of g(x) = f(-x) will be symmetric with respect to the y-axis.
Learn more about the graph of functions at: https://brainly.com/question/17089414
#SPJ1

Calculate the heat energy released when 16.2 g
of liquid mercury at 25.00 °C is converted to solid mercury at its melting point.
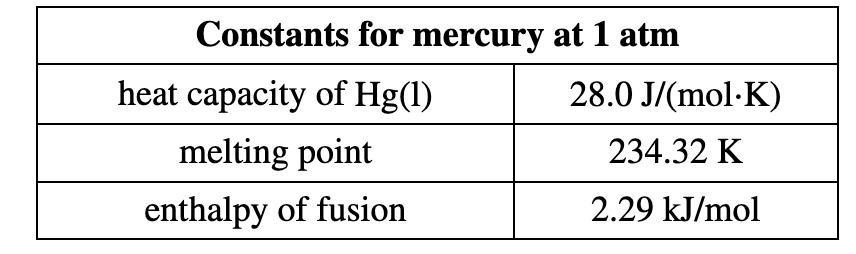
Answers
50.3 J of heat have been released.
How are heat energies released calculated?The equation q = mcT, where m is the mass of the sample, c is the specific heat, and T is the temperature change, can be used to determine how much heat is gained or lost by a sample (q).
Having said that,
Mercury has a mass of 17.7 g and a starting temperature of 25.00 °C.
Mercury's melting point is -39 °C.
Mercury has a specific heat capacity of 0.14 J/g/°C.
Mercury fusion heat equals 11.8 J/g
Obtaining heat from the transformation of liquid mercury into solid mercury;
H = mcθ + mL
H = m(cθ + L)
H = 17.7 g[(0.14 × -64) + 11.8 )
H = 50.3 J
To know more about heat energy visit:-
https://brainly.com/question/7496871
#SPJ1
How many moles of MgCl2 are present in 60.0 mL of 0.100 M MgCl2 solution
Answers
Taking into account the definition of molarity, the number of moles of MgCl₂ present in 60.0 mL of 0.100 M MgCl₂ solution is 0.006 moles.
Definition of molarityMolarity is a measure of the concentration of a solute in a solution and indicates the number of moles of solute that are dissolved in a given volume.
The molarity of a solution is calculated by:
molarity= number of moles of solute÷ volume
Molarity is expressed in units moles/L.
Number of moles of MgCl₂In this case, you have:
Molarity= 0.100 Mnumber of moles of MgCl₂= ?volume= 60 mL= 0.06 L (being 1000 mL= 2 L)Replacing in the definition of molarity:
0.100 M=number of moles of MgCl₂÷ 0.06 L
Solving:
0.100 M × 0.06 L= number of moles of MgCl₂
0.006 moles= number of moles of MgCl₂
Finally, the number of moles of MgCl₂ is 0.006 moles.
Learn more about molarity:
brainly.com/question/9324116
brainly.com/question/10608366
brainly.com/question/7429224
#SPJ1