How could measuring the melting point of a solid help decide whether it was a mixture or a compound?
Answers
Answer: It can't.
Explanation:
In most cases, the melting point alone will not enable you to identify a compound. Millions of solid organic compounds, and their melting points, are known. Perhaps 10,000 of these will have the same melting point as your unknown compound.
Hope this helps!
Related Questions
The dry adiabatic lapse rate is the average temperature decrease with height of 6.5 degrees celsius. group of answer choices true false
Answers
This statement is true.
The dry adiabatic lapse rate is the average temperature dereses with heigh of 6.5 degree celsius.
What is adiabatic lapse?
The rate of decrease of temperature experienced by a parcel of air when it is lifted in the atmosphere under the restriction that it cannot exchange heat with its environment. For parcels that remain unsaturated during lifting, the (dry adiabatic) lapse rate is 9.8°C per kilometer.
it averages about 6.5 °C per kilometre (18.8 °F per mile) in the lower atmosphere (troposphere). It differs from the adiabatic lapse rate, which involves temperature changes due to the rising.
To learn more about adiabatic lapse click the given link
https://brainly.com/question/27349250
#SPJ4
c Shazad's pebble has a mass of 90 g and a volume of 30 cm³. What is its density?
Answers
Answer:
Density = 3 g/cm³
Explanation:
Density is a ratio which compares an object's mass per every 1 unit volume. Grams is a unit of mass and cm³ is a unit of volume. Since you have been given values for both a mass and volume, you can use them to determine the density.
Density = g/cm³ <----- Density units
Density = 90 g / 30 cm³ <----- Insert values
Density = 3 g / 1 cm³ <----- Simplify
Density = 3 g/cm³
If you have 384.3 grams of Al(OH)3 how many moles do you have?
Answers
Explanation:
no of moles = mass / molar mass
mr of Al ( OH)3 = 27+ (16 × 3) + (1 ×3)
= 78
n = 384.3 / 78
n = 4.92692...
n = 4.93
therefore, no of moles = 4.93
(OH) 3 = 27 + (16 x 3) + (1 x 3) which equals 78
348.3 divides by 78 equals 4.92692
Round up and you get 4.93
Explanation:
5. How are the molecular structures of sugars and alcohols similar? How do
they differ?
Answers
The molecular structures of sugars and alcohols are similar due to the presence of the -OH group.
What is Sugar?This is a substance which has a sweet taste and is a form of simple carbohydrate.
They are however not similar due to the different numbers of Carbon atoms attached to the -OH group..
Read more about Sugar here https://brainly.com/question/25936585
#SPJ2
If aluminum is heated from 650°C to 670°C, then it will change from a A.liquid to a gas. B.liquid to a solid. C.solid to a liquid. D.gas to a solid.
Answers
Explanation:
How does the ionosphere help radio transmission?
Answers
I hope this helps !
Explanation:
ionosphere can reflect radio waves directed into the sky back toward the Earth
Hey y’all is these answers correct?

Answers
Answer:
ʏᴇs, ɪ ᴛʜɪɴᴋ
Explanation:
TᕼᗩᑎKՏ ᒪOᗪՏ
source: i’m black
0.2 mol of the transition metal (M) comhine with excess chlorine gas to form 30.3 g of MCl3. What is the electronic configuration of the ion of the metal (M) in this compound?
(Sc=45, Cr=52, Fe=55.8, Co=58.9, Cl=35.5)
A) [Ar], 3d3
B)[Ar], 3d5
C)[Ar], 3d6
D) [Ne], 3s2 3p6
Answers
Given metallic chloride formula =MCl3
Means M has valency 3It belongs to Nitrogen(N) family.
The elements given by Nitrogen,Phosphorus,Arsenic,Stubnum(Tin),Bismuth.But the condition is that its transition element.
The element is vanedium(V)EC given by
\(\\ \sf\longmapsto 1s^22s^22p^63s^23p^64s^23d^3\)
\(\\ \sf\longmapsto [Ar]3d^3\)
Which substance gets broken down in a homogeneous mixture?
colloid
solution
solute
solvent
Answers
Answer:
Should be Solute
Explanation:
I looked it up ;)
Answer:
c. Solute
Explanation:
The blanks and bottom part please!
Thank you in advance

Answers
The complete sentences are:
When all the intermolecular bonds are broken, the transition between phases is complete.The energy of any substance includes the kinetic energy of its particles and the potential energy of the bonds between its particles.What are the complete sentences on matter?Page 3:
The effect of energy in phase transitions of matter is that it is required to break the intermolecular forces that hold the particles of a substance together. When energy is added to a substance, the particles move faster and the intermolecular forces are broken. This can cause the substance to change phase.
The interactive demonstration on the sample of water shows that energy is required to melt ice and boil water. When the ice is heated, the particles start to move faster and the ice melts. The temperature of the water stays constant at 0°C until all of the ice has melted. This is because the energy is being used to break the intermolecular forces in the ice. Once all of the ice has melted, the temperature of the water starts to rise again. When the water is boiled, the particles move so fast that they escape from the liquid state and become a gas. The temperature of the water stays constant at 100°C until all of the water has boiled. This is because the energy is being used to break the intermolecular forces in the water. Once all of the water has boiled, the temperature of the steam starts to rise again.
The complete sentences:
Water stays in a liquid state as the temperature and kinetic energy of the molecules increase from 0°C to 100°C. This consistency indicates that a larger amount of energy is necessary to break the intermolecular forces and change the state of matter. At the melting and boiling points, the temperature does not change because all of the energy is being used to break the intermolecular forces.The energy needed to overcome all the intermolecular forces between molecules must be greater than the potential energy of the bonds between molecules.The transition between phases is a physical change, not a chemical change.Page 4:
Heating curves show the temperature of a substance as it is heated. The curve has a horizontal line at the melting and boiling points, which indicates that the temperature does not change during these phase changes.
Cooling curves show the temperature of a substance as it is cooled. The curve has a horizontal line at the melting and boiling points, which indicates that the temperature does not change during these phase changes.
Both curves show that the temperature of a substance increases as it is heated and decreases as it is cooled.
A heating curve is more choppy than a cooling curve because there are more phase changes during heating than during cooling.
Find out more on matter here: https://brainly.com/question/3998772
#SPJ1
The citric acid cycle is the central reaction sequence in the cellular metabolism of humans and many other organisms. One of the key steps is catalyzed by the enzyme isocitrate dehydrogenase and the oxidizing agent NAD⁺. In yeast, the reaction is eleventh order:Rate = K[ enzyme ] [isocitrate]⁴[AMP]²[NAD⁺]m [Mg²⁺]²What is the order with respect to NAD⁺?
Answers
In yeast, the reaction is eleventh order:Rate = K[ enzyme ] [isocitrate]⁴[AMP]²[NAD⁺]m [Mg²⁺]². The order with respect to NAD⁺ is 2.
What is a citric acid cycle?The citric acid cycle is also called the Krebs cycle. This is the main source of energy that happens in aerobic conditions.
The coefficient that is raised to the active concentration of the reactants is the order in the mass action law. It is determined experimentally and can be fractional, positive, negative, or zero.
Each reactant's order, which is increased to its power in the rate law, is added to determine the order of the entire reaction.
The overall rate = 11
Rate of overall reaction = 1 + 4 + 2 + m + 2 = 11
9 + m = 11
m = 2
Thus, the order with respect to NAD⁺ is 2.
To learn more about citric acid cycle, refer to the below link:
https://brainly.com/question/14438945
#SPJ4
all ionic compounds become electrolytes when thrown into water true or false
Answers
True. When ionic compounds are dissolved or thrown into water, they typically dissociate into ions and become electrolytes.
An electrolyte is a substance that conducts electric current when dissolved in water or molten form due to the presence of freely moving ions. Ionic compounds consist of positively charged cations and negatively charged anions held together by electrostatic forces. When they come into contact with water, the water molecules surround the ions and weaken the forces holding them together, causing the compound to dissociate into its constituent ions. These ions are then free to move in the aqueous solution and carry electric charge, allowing the solution to conduct electricity. Therefore, most ionic compounds can be considered electrolytes when dissolved in water.
To learn more about electrolytes
https://brainly.com/question/17089766
#SPJ11
Why are computer models useful in studying phenomena in the universe?
Answers
Answer:
Computer Models are used to study the complex phenomena in the universe.
Explanation:
The term 'models' can be defined as a representation of something that is real. A model is used to learn the phenomena of the natural world for easy understanding of it.
The computer models are used to study the large and complex data in the universe. These models are used to perform various calculations that, in real, would take years to do. It helps in studying the changes in variables, which in turn helps the scientist to know of any climatic change, or other changes in the nature.
19. 50 cc of N/2 HCl and 10 cc of 2N H₂SO4 solutions are mixed with 0.4 g of NaOH. Calculate the normality of resulting mixture. [0.583N]
Answers
The normality of the resulting mixture is 0.583N.
What is the Normality of a mixture?The normality is described as the number of gram or mole equivalents of solute present in one liter of a solution.
How to calculate the normality:
First, let's calculate the number of moles of NaOH in 0.4 g of the compound:
Molar mass of NaOH = 23 + 16 + 1 = 40 g/mol
Number of moles of NaOH = 0.4 g / 40 g/mol = 0.01 mol
Next, we need to calculate the total number of acid equivalents in the mixture of HCl and H₂SO4:
Number of acid equivalents of HCl = (50 cc / 1000) L x (N/2) = 0.025 N LNumber of acid equivalents of H₂SO4 = (10 cc / 1000) L x (2N) = 0.02 N LTotal number of acid equivalents = 0.025 N L + 0.02 N L = 0.045 N LSince NaOH is a strong base and reacts completely with the acid, the number of moles of NaOH is equal to the number of acid equivalents in the mixture.
Therefore, the normality of the resulting mixture is:
Normality = number of acid equivalents / volume of the mixture in liters
Normality = 0.045 N L / ((50 + 10) cc / 1000) L = 0.583 N
So the normality of the resulting mixture is 0.583N.
Read more about chemical mixtures here:
https://brainly.com/question/24647756
#SPJ1
A mixture contains 5.00 g each of O2, N2, CO2, and Ne gas. Calculate the volume of
this mixture at STP
Answers
The volume of this mixture at STP is mathematically given as
v=15.59L
What is the volume of this mixture at STP?Question Parameters:
A mixture contains 5.00 g
Generally, the equation for the number of moles is mathematically given as
n=mass/molar mass
For O2
nO=5/32
nO=0.156mol
For N2
nN=5/28.02
nN=0.178
For CO2
nCO2=0.114mol
For Ne
nNe=0.248
In conclusion, total moles present
nT=0.156mol+0.178+0.114mol+0.248
nT=0.696
Hence, volume at stp
V=0.696*22.4
v=15.59L
Read more about Molecules
https://brainly.com/question/19922822
Need help with science

Answers
Answer:
See explanation
Explanation:
1 - Physical change
2 - Mixture
3 - Liquid
4 - Physical Properties
5 - Physical change
6 - Chemical change
7 - Melting
8 - Equals
9 - Conservation of mass
10 - Physical Property
11 - Distillation
Potassium is a silvery-white, solid metal. Chlorine is a greenish-yellow gas. When these two elements react chemically, they yield potassium chloride which is an odorless salt with a crystal structure. Potassium chloride is used in the production of fertilizer.
Required:
Which part of atomic structure is responsible for the reactivity of atoms?
Answers
The electrons present in the valence shell of an atom are responsible for the reactivity of atoms.
What is an atom?An atom is defined as the smallest unit of matter which forms an element. Every form of matter whether solid,liquid , gas consists of atoms . Each atom has a nucleus which is composed of protons and neutrons and shells in which the electrons revolve.
The protons are positively charged and neutrons are neutral and hence the nucleus is positively charged. The electrons which revolve around the nucleus are negatively charged and hence the atom as a whole is neutral and stable due to presence of oppositely charged particles.
Atoms of the same element are similar as they have number of sub- atomic particles which on combination do not alter the chemical properties of the substances.
Learn more about atom,here:
https://brainly.com/question/13654549
#SPJ2
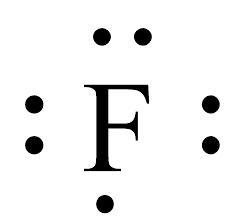
Draw the correct Lewis dot structure from the given shorthand notation below: PLS HELP

Answers
The Lewis structure of the element have been shown in the image attached.
Lewis dot structure of an element:The valence electrons of an atom or molecule are depicted in a simplified manner by the Lewis structure, commonly referred to as the Lewis dot structure or electron dot structure. Gilbert N. Lewis, an American scientist, created it.
The valence electrons of an atom are shown in a Lewis structure as dots surrounding the element's symbol. These dots' placement reveals details about the connectivity and atom-atom bonding in a molecule.
Learn more about Lewis structure:https://brainly.com/question/29756546
#SPJ1
What kind of reaction does this make?2 C₅H₅ + Fe ⟶ Fe(C₅H₅)₂A. Synthesis (S)B. Decompostion (D)C. Single Displacement (SD)D. Double Displacement (DD)E. Combustion (C)
Answers
The answer is option
The reaction:
\(2C_{5_{}}H_5+Fe\rightarrow Fe(C_5H_5)_2\)is a Synthesis reaction, because from 2 different substances it is produced
evaluate the translational partition function for h2 confined to a volume of 126 cm3 at 298 k . (note: the avogadro's constant na=6.022×1023
Answers
The translational partition function for H2 confined to a volume of 126 cm³ at 298 K is 1.06 × 10⁴⁴.
To evaluate the translational partition function for H2 confined to a volume of 126 cm³ at 298 K, we can use the following formula:
Qtrans = (V/(λ³)) * ((2πmkT)/\((h^{2})^{\frac{3}{2} }\)
Where V is the volume of the container, λ is the thermal de Broglie wavelength of the molecule, m is the mass of the molecule, k is the Boltzmann constant, T is the temperature, and h is the Planck constant.
For H2, the mass is 2.016 g/mol or 0.002016 kg/mol. The thermal de Broglie wavelength can be calculated using the formula:
λ = h / √(2πmkT)
Plugging in the values, we get:
λ = (6.626 ×10⁻³⁴ J s) / √(2π(0.002016 kg/mol)(1.38 × 10⁻²³ J/K)(298 K))
λ ≈ 2.47 × 10⁻¹⁰ m
Converting the volume of the container from cm³ to m³, we get:
V = 126 cm³ = 1.26 × 10⁻⁴ m³
Now we can calculate the translational partition function using the formula:
Qtrans = (V/(λ³)) × ((2πmkT)/\((h^{2})^{\frac{3}{2} }\)
Qtrans = ((1.26 × 10⁻⁴ m³)/(2.47 × 10⁻¹⁰ m)³) × ((2π(0.002016 kg/mol)(1.38 × 10⁻²³ J/K)(298 K))/(6. 626 × 10⁻³⁴ J s)²)^(3/2)
Qtrans ≈ 1.06 × 10⁴⁴
Therefore, the translational partition function for H2 confined to a volume of 126 cm³ at 298 K is approximately 1.06 × 10⁴⁴.
Learn more about partition function: https://brainly.com/question/31440337
#SPJ11
Given the following equation, cu +2 agno3 -> cu(no3)2 + 2ag if 89.5 g of silver is produced, how many grams of cu reacted? conversion factors: 1 mole ag=107.87 g ag 1 mole cu= 2 mole ag 1 mole cu = 63.55g cu
Answers
If 89.5 grams of silver is produced, 105.45 grams of Cu reacted.
The Chemical equation is
Cu + 2AgNO₃ → Cu(NO₃)₂ + 2Ag
The equation is balanced,
89.5 grams of Silver is produced in the reaction.
It is asked that how much Cu will be produced.
The amount of Copper can be calculated by using mole concept,
From the reaction,
When 1 moles of Copper Reacts, 2 moles of Ag are formed.
So, we can say,
Mole of Cu (n) = 2 x Moles of Ag
Mole of a compound can be found by using the formula,
Moles = Given mass of compound/Molar mass of the compound
So,
n = 2 x 89.5/107.87 moles
n = 2 x 0.82 moles
n = 1.64 moles.
Moles of Copper are 1.64.
So, the mass of Cu reacted will be
1 mole Cu = 63.55 grams
1.64 moles Cu = 1.64 x 63.55 grams
1.64 moles of Cu = 105.45 grams.
To produce 89.5 grams of silver, 105.45 grams of Copper would have reacted.
To know more about Mole Concept, visit,
https://brainly.com/question/16488605
#SPJ9
which aqueous solution has the highest boiling point? (a) 1.0 M NaOH (b) 1.0MNa2SO4(C)1.0MNH4NO3d) 1.0MKNO3
Answers
1.0 M NaOH has the highest concentration of dissolved solutes and thus the highest boiling point.
The boiling point of an aqueous solution is determined by the concentration of dissolved solutes in the solution. The higher the concentration of solutes, the higher the boiling point of the solution. The boiling point of an aqueous solution of 1.0 M NaOH is approximately 101.5°C. This is due to the fact that, when an ionic compound is dissolved in water, the ions become surrounded by water molecules and the solution becomes more dense. As a result, the boiling point of the water is increased as the number of molecules per unit volume increases.
leqarn more about boiling point Refer:brainly.com/question/2153588
#SPJ4
Put in order:
(a) produce carbon dioxide and molten iron
(b) pour off molten iron
(c) mix with limestone and coal
(d) place in blast furnace
Answers
In order to produce molten iron and carbon dioxide in a blast furnace, the following steps are typically taken:
Place limestone, coal, and iron ore in a blast furnace. (c)
Heat the blast furnace to high temperatures, causing the limestone to break down and release carbon dioxide. (d)
The carbon dioxide reacts with the coal to produce carbon monoxide, which then reacts with the iron ore to reduce it to molten iron. (a)
Pour off the molten iron from the bottom of the blast furnace. (b)
These steps are part of the process of producing iron in a blast furnace, which is a common method used in the production of steel and other iron-based products. It is important to follow proper procedures and safety protocols when working with blast furnaces, as they involve high temperatures and potentially hazardous materials.
learn more about Blast Furnace here:
https://brainly.com/question/22227821
#SPJ11
The percent ionization of a 0.331M solution of HCN is found to be 0.00337%. What is the pH of this solution?
a. 1.992 b. 2.953 c. 3.371 d. 3.992 e. 4.953
Answers
The percent ionization of a 0.331M solution of HCN is found to be 0.00337%. The pH of the solution is 4.953. The correct option is E. 4.953.
To solve this problem, we need to use the equation for percent ionization:
% ionization = (concentration of ionized acid / initial concentration of acid) x 100%
We can rearrange this equation to solve for the concentration of ionized acid:
concentration of ionized acid = % ionization / 100% x initial concentration of acid
Plugging in the given values, we get:
concentration of ionized acid = 0.00337 / 100 x 0.331 = 0.000011187 M
Now, we can use the equation for the ionization of HCN to set up an expression for the equilibrium constant (Ka):
HCN + H2O ↔ H3O+ + CN-
Ka = [H3O+][CN-] / [HCN]
We can assume that the concentration of H3O+ is equal to the concentration of ionized acid, since the ionization of HCN produces one H3O+ ion for every HCN molecule that ionizes. We can also assume that the concentration of CN- is equal to the concentration of H3O+.
Therefore:
Ka = (concentration of ionized acid)^2 / (initial concentration of acid - concentration of ionized acid)
Plugging in the values we calculated, we get:
Ka = (0.000011187)^2 / (0.331 - 0.000011187) = 6.2 x 10^-10
Now, we can use the equation for the pH of a weak acid:
pH = pKa + log([A-] / [HA])
Since we assumed that the concentration of CN- is equal to the concentration of ionized acid, we can substitute [CN-] for [A-] and [HCN] - [CN-] for [HA]. We also know that pKa = -log(Ka).
Therefore:
pH = -log(6.2 x 10^-10) + log(0.000011187 / (0.331 - 0.000011187)) = 4.953
Therefore, the pH of the solution is e. 4.953.
To know more about percent ionization click here:
https://brainly.com/question/31358773
#SPJ11
A solution is prepared at 25C that is initially 0.075M in chlorous acid (HClO2), a weak acid with Ka= 1.1 x 10^-2, and 0.34M in potassium chloride (KClO2). Calc the pH of the solution. Round your answer to 2 decimal places.
Answers
The - pH is 2.6 .You must know the hydronium ion concentration in moles per liter in order to calculate the pH of an aqueous solution (molarity).
How do you determine an acid-base solution's pH?The pH of Acids and Bases
For an acidic solution, [H3O+] > [OH-]
[H3O+] = [OH-] is the formula for a neutral solution.
For the fundamental answer: [H3O+] [OH-]
You must know the hydronium ion concentration in moles per liter in order to calculate the pH of an aqueous solution (molarity).
The formula pH = - log [H3O+] is then used to determine the pH.
You must know the hydronium ion concentration in moles per liter in order to calculate the pH of an aqueous solution (molarity). The formula pH = - log [H3O+] is then used to determine the pH.
ie, 0.075 - 0.34 = - 0.265.
-pH = 2.6.
To learn more about pH refer to:
https://brainly.com/question/172153
#SPJ4
When does the emergency eyewash need to be used? For how long?
Answers
Answer:
When you're eyes or body has been exposed to chemicals. For 15 minutes straight
Explanation:
A hot air balloon is an example of what type of system? *
Answers
Answer:
The balloon transport system or Hot Air Balloon is one of several transportation methods in RuneScape. During the Enlightened Journey quest it can be used for travel between two locations. After the quest is complete, four more locations are available to be unlocked by completing respective journeys to those locations.
Explanation:
transportation
the gas stream from a sulfur burner is composed of 15-mol% so2, 20-mol% o2, and 65-mol% n2. this gas stream at 1 bar and 480c enters a catalytic converter, where the so2 is further oxidized to so3. find the equilibrium conversion of so2. the equilibrium constant for the reaction is 87.824 at 480c. what would be the equilibrium conversion of the reactor if pure oxygen were used under the same conditions?
Answers
The equilibrium conversion of SO2 can be determined using the equilibrium constant and the initial mole fractions of SO2 and O2. The equilibrium conversion (Xeq) is given by the equation: Xeq = (nSO3eq / nSO2o) = K * (nSO2o / (nO2o * nN2o))^0.5.
Given that the mole fractions of SO2, O2, and N2 are 0.15, 0.20, and 0.65 respectively, we can calculate the initial mole fractions of SO2 (nSO2o), O2 (nO2o), and N2 (nN2o).
Using these values and the equilibrium constant (K = 87.824), we can calculate the equilibrium conversion of SO2 in the presence of N2.
However, if pure oxygen is used instead of air, the initial mole fraction of O2 (nO2o) would be 1. Therefore, using the same calculations, we can determine the equilibrium conversion of SO2 when pure oxygen is used.
To know more about equilibrium constant visit:-
https://brainly.com/question/28559466
#SPJ11
To find the equilibrium conversion of SO2 in a sulfur burner gas stream, we need to use the equilibrium constant expression and the given initial gas composition. The equilibrium conversion of SO2 can be calculated using the equilibrium constant and the percentages of SO2, O2, and N2 in the gas stream.
Explanation:To find the equilibrium conversion of SO2, we need to use the equilibrium constant expression. The equilibrium constant (Kc) is given as 87.824 at 480°C. The conversion of SO2 can be calculated using the equation:
Equilibrium constant (Kc) = [SO3] / [SO2] * [O2]0.5
The given gas stream consists of 15% SO2, 20% O2, and 65% N2. Using these percentages, we can calculate the initial concentrations of SO2, O2, and N2. Then, we can substitute these values into the equilibrium constant expression to find the equilibrium conversion of SO2.
Learn more about Equilibrium Conversion of SO2 here:https://brainly.com/question/34963499
#SPJ12
Enter your answer in the provided box. Consider the reaction: N
2
(g)+3H
2
(g)→2NH
3
(g) Suppose that a particular moment during the reaction, molecular hydrogen is reacting with a magnitude of 0.0177M/s. At what rate is molecular nitrogen reacting? M/s
Answers
The balanced chemical equation for the reaction between molecular nitrogen and hydrogen is:N2(g) + 3H2(g) 2NH3(g)
According to the given infromation:Let the rate of reaction of hydrogen be x mol/L/sec and that of nitrogen be y mol/L/sec. By examining the balanced equation, we see that 3 moles of hydrogen react with 1 mole of nitrogen to form 2 moles of ammonia.
That is:y/(-3) = x/1 = -0.0177 M/s
The negative sign is there because the reaction of nitrogen is occurring at a slower rate than the hydrogen reaction. Using the above equation, we can find the rate of nitrogen:
y = (-3) (0.0177 M/s) = -0.0531 M/s
Thus, the rate of nitrogen reacting is -0.0531 M/s.
To know more about molecular nitrogen, visit:
https://brainly.com/question/30459909
#SPJ11
pls answer both! i ran out of
questions! thank you!
Use the References to access important values if needed for this question. The equilibrium constant, Kp, for the following reaction is 1.80 x 10-2 at 698 K. 2HI(g) → H₂(g) + I₂ (g) If an equilib
Answers
The equilibrium concentration of HI is 1.56 x 10-5 M and the equilibrium concentration of H₂ and I₂ is 7.8 x 10-6 M.
Given: The equilibrium constant, Kp, for the following reaction is 1.80 x 10-2 at 698 K.2HI(g) → H₂(g) + I₂ (g)
When equilibrium is reached, the concentration of H₂ is found to be 2.80 x 10-3 M. Calculate the equilibrium concentration of HI and I2.
Solution: Equilibrium constant, Kp = 1.80 x 10-2 at 698 K Since the equation is 2HI(g) → H₂(g) + I₂ (g),therefore the expression for Kp is given as,
Kp = [H₂] [I₂] / [HI]²
At equilibrium,[H₂] = 2.80 x 10-3 M We are to find the equilibrium concentration of HI and I2. Let the equilibrium concentration of HI be x and the equilibrium concentration of I2 be y. Molar concentration of H₂ = 2.80 x 10-3 M Using the equilibrium constant expression, Kp = [H₂] [I₂] / [HI]²= (2.80 x 10-3) (y) / (x)²= 2.80 x 10-3 (y) / (x²)---------------------eqn1We also know that,2HI(g) → H₂(g) + I₂ (g)Initially (before the reaction begins), concentration of HI = x and concentration of H₂ and I₂ are zero. Thus, initially, H₂ = 0and I₂ = 0At equilibrium, 2HI(g) → H₂(g) + I₂ (g).
Thus, initially the concentration of HI = x-moles. Then, for every 2 moles of HI that is converted, one mole of H₂ and one mole of I₂ are produced. So, the concentration of H₂ and I₂ at equilibrium would be x/2 moles. Because, for every 2 moles of HI that is converted, one mole of H₂ and one mole of I₂ are produced.[HI] = x M[H₂] = [I₂] = x/2 M Substituting the values in the expression derived above in eqn1,Kp = 1.80 x 10-2 = 2.80 x 10-3 (y) / (x²)= 2.80 x 10-3 (y) / x²x² = (2.80 x 10-3 y) / (1.80 x 10-2)= 0.15555y / 1Substituting the value of x² in the equation 1,1.80 x 10-2 = 2.80 x 10-3 (y) / 0.15555y1.80 x 10-2 = 18.00 y / 15555y1.80 x 10-2 = y / 865.3y = 1.56 x 10-5 M[H₂] = [I₂] = x/2 = (1.56 x 10-5 M) / 2= 7.8 x 10-6 M
∴ The equilibrium concentration of HI is 1.56 x 10-5 M and the equilibrium concentration of H₂ and I₂ is 7.8 x 10-6 M.
To know more about concentration visit
https://brainly.com/question/16551813
#SPJ11