How are electrons arranged around the nucleus of an atom
Answers
Answer: electrons are arranged in shells around an atom's nucleus.
Explanation:
Related Questions
if you weigh out 1.86 g of the fe(nh4)2(so4)2 · 6h2o, how many moles of iron (fe 2) are in the sample?
Answers
There are 0.00968 moles of iron (Fe 2) in the 1.86 g sample of Fe(NH4)2(SO4)2 · 6H2O.
To determine the moles of iron (Fe 2) in the sample, we need to first calculate the molar mass of the compound Fe(NH4)2(SO4)2 · 6H2O.
The molar mass of Fe(NH4)2(SO4)2 · 6H2O is calculated as follows:
Fe: 55.85 g/mol
N: 14.01 g/mol x 2 = 28.02 g/mol
H: 1.01 g/mol x 12 = 12.12 g/mol
S: 32.07 g/mol x 2 = 64.14 g/mol
O: 16.00 g/mol x 14 = 224.00 g/mol
Total molar mass = 55.85 + 28.02 + 12.12 + 64.14 + 224.00 = 384.13 g/mol
Now we can use the formula for moles to calculate the moles of iron (Fe 2) in the sample:
moles = mass / molar mass
moles = 1.86 g / 384.13 g/mol
moles = 0.00484 mol
Since there are two moles of iron (Fe 2) in the compound, we can multiply the moles of the compound by 2 to get the moles of iron:
moles of iron (Fe 2) = 0.00484 mol x 2 = 0.00968 mol
Therefore, there are 0.00968 moles of iron (Fe 2) in the 1.86 g sample of Fe(NH4)2(SO4)2 · 6H2O.
To know more about moles, refer here:
https://brainly.com/question/26416088#
#SPJ11
Calculate the enthalpy change for the reaction: 4Al(s) + 3MnO2(s) --> 2Al2O3 (s) + 3Mn(s)2Al(s) + 3/2 O2 (g) --> Al2O3(s). ∆ H=-1676.0 kJMn(s) + O2 (g) --> MnO2(s). ∆ H = -521.0 kJ

Answers
Explanation:
To solve this question, we need to use the Hess's Law.
Hess's Law states that: in a chemical reaction, the heat released or absorbed is constant and independent of the number of steps the reaction goes through. That is, the law states that the enthalpy change of a chemical reaction depends only on its initial state and its final state.
Hess's Law is also known as the law of the sum of heats of reaction, because the enthalpy change is equal to the sum of the changes in the steps through which the chemical reaction passes (intermediate reactions). The calculation is performed as follows:
- If the chemical reaction is inverted, the sign of the enthalpy change must also be inverted;
- If the equation is multiplied, the enthalpy change must also be multiplied;
- If dividing the equation, the enthalpy change must also be divided.
So we're going to invert, divide, or multiply each given equation in order to get the equation that the question gives us.
The equation the question gives us is:
4Al(s) + 3MnO2(s) --> 2Al2O3 (s) + 3Mn(s)
Let's multiply the first equation by 2:
4 Al + 3 O2 --> 2 Al2O3
ΔH = -1676 * 2 = -3,352 kJ
Let's invert and multiply by 3 the second equation:
3 MnO2 -> 3 Mn + 3O2
ΔH = -521 * 3 * (-1)
ΔH = 1,563 kJ
Answer: -1,789 kJ

which type of membrane transport process uses atp as a source of energy?
Answers
The type of membrane transport process that uses ATP (adenosine triphosphate) as a source of energy is called active transport.
Active transport is a cellular process that enables the movement of ions or molecules across a cell membrane against their concentration gradient (from an area of lower concentration to an area of higher concentration). This movement is energetically unfavorable because it goes against the natural tendency of molecules to move from areas of higher concentration to lower concentration (down the concentration gradient). Therefore, active transport requires the input of energy.
ATP, as the energy currency of cells, is utilized by specific proteins called ATPases or ATP-powered pumps to actively transport molecules or ions across the membrane. These pumps use the energy released by ATP hydrolysis (the breakdown of ATP into ADP and inorganic phosphate) to perform work against the concentration gradient.
ATP-powered pumps are involved in various vital physiological processes, such as the maintenance of ion gradients across cell membranes, nutrient uptake in cells, and removal of waste products. Examples of ATP-powered pumps include the sodium-potassium pump, calcium pump, and proton pump.
The active transport process is highly selective, allowing the cell to control the movement of specific ions or molecules across the membrane and maintain concentration gradients necessary for cellular functions.
Learn more about ATP here:
https://brainly.com/question/32091528
#SPJ11
What is the molarity of 0.25 moles of FeCl3 dissolved in 120 ml of solution?
Answers
Answer:
Molarity = 2.0833 M (Approx)
Explanation:
Given:
Number of moles (n) = 0.25
Volume of solution = 120 ml = 0.12 L
Find:
Molarity
Computation:
Molarity = Number of moles (n) / Volume of solution.
Molarity = 0.25 / 0.12
Molarity = 2.0833 M (Approx)
Metal objects, such as knifes or bullets, that come into contact with bones can leave trace evidence on them.
O True
O False
Answers
Look at the activity series and select which two of the following reactions would happen on their own. (Remember, if the lone element is more active than the metal in the compound, the lone element will react and replace the metal in the compound.) Lithium (Li)
Potassium (K)
Calcium (Ca)
Sodium (Na)
Aluminum (Al)
Zinc (Zn)
Iron (Fe)
Tin (Sn)
Lead (Pb)
(Hydrogen) (H)
Copper (Cu)
Silver (Ag)
Gold (Au)
A.
2Li + ZnBr2 ->2LiBr + Zn
B.
Al + 3LiCl ->AlCl3 + 3Li
C.
Sn + ZnSe ->SnSe + Zn
D.
3Ca + Al2O3 ->2Al + 3CaO
Answers
Answer:
2Li + ZnBr2 ->2LiBr + Zn
3Ca + Al2O3 ->2Al + 3CaO
Explanation:
Spontaneous reactions are reactions that can happen on their own. For a spontaneous reaction to occur, a metal that is higher in the activity series must displace a metal that is lower in the activity series from its solution and not vice versa.
If we look at the two reactions selected in the answer, lithium is above zinc in the activity series and calcium is above aluminum in the activity series hence the two reactions occur spontaneously.
Calculate the maximum
mass of magnesium oxide
that can be made from
2.4g of magnesium and
2.4g of oxygen.
1
1
2Mg + 0, → 2Mgo
Answers
Answer:
Mass = 3 g
Explanation:
Given data:
Mass of magnesium = 2.4 g
Mass of oxygen = 2.4 g
Mass of magnesium oxide formed = ?
Solution:
Chemical equation;
2Mg + O₂ → 2MgO
Number of moles of oxygen:
Number of moles = mass / molar mass
Number of moles = 2.4 g/ 32 g/mol
Number of moles = 0.075 mol
Number of moles of magnesium:
Number of moles = mass / molar mass
Number of moles = 2.4 g/ 24 g/mol
Number of moles = 0.1 mol
now we will compare the moles of magnesium oxide with both reactant.
Mg : MgO
2 : 2
0.075 : 0.075
O₂ : MgO
1 : 2
0.1 : 2/1×0.1 = 0.2
Mass of magnesium oxide:
Mass = number of moles × molar mass
Mass = 0.075 mol × 40 g/mol
Mass = 3 g
which of the following is the conjugate acid of CIO-
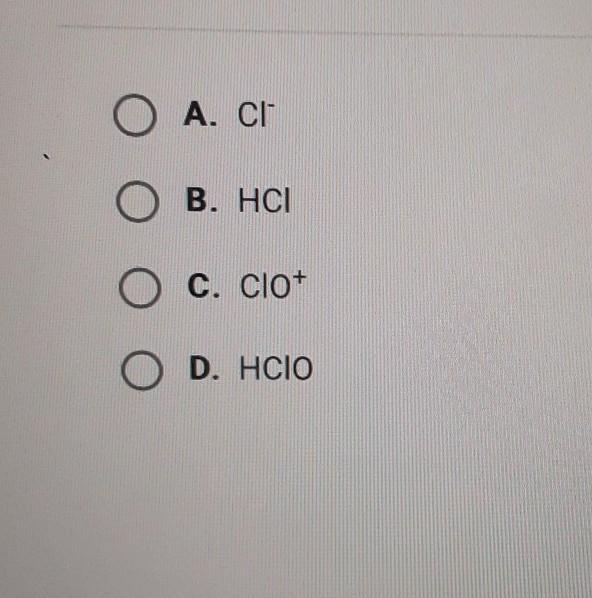
Answers
The conjugate acid of CIO⁻ is HClO. Hence, Option (D) is correct.
What is Conjugate Acid - Base concept ?A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid donates a proton to a base.
In other words, it is a base with a hydrogen ion added to it, as in the reverse reaction it loses a hydrogen ion.
Therefore, The conjugate acid of CIO⁻ is HClO. Hence, Option (D) is correct.
#SPJ2
Chemical Reactions - Problems
Molecule Bank
Cor
Incc
Drag molecules to
the scale to balance
the reaction
O Fe
+
0 02
- 0 Fe2O3
A
Submit your answer

Answers
Answer:
The balanced chemical equation is,
4 Fe + 3 O₂ → 2 Fe₂O₃
Drag four iron atoms and 3 oxygen molecules and place it on the left plate.
And drag 2 molecules of ferric oxide and place it on the right plate.
When 8.0 g H₂ react with 8.0 g O₂ in the reaction 2H₂ + O₂ → 2H₂O, what are the theoretical yield and the limiting reactant?
Answers
Answer:
Now, we have to determine the limiting reagent.
Now, we have to determine the limiting reagent.4 g of H₂ reacts with 32 g of O₂ 1 g of H₂ reacts with 32/4 g of O₂ 3 g of H₂ reacts with 32/4 x 3 = 24 g of
Now, we have to determine the limiting reagent.4 g of H₂ reacts with 32 g of O₂ 1 g of H₂ reacts with 32/4 g of O₂ 3 g of H₂ reacts with 32/4 x 3 = 24 g ofBut according to the question, 29 g of O₂ is present. 2
Now, we have to determine the limiting reagent.4 g of H₂ reacts with 32 g of O₂ 1 g of H₂ reacts with 32/4 g of O₂ 3 g of H₂ reacts with 32/4 x 3 = 24 g ofBut according to the question, 29 g of O₂ is present. 2So, the limiting reactant is hydrogen.
Now, we have to determine the limiting reagent.4 g of H₂ reacts with 32 g of O₂ 1 g of H₂ reacts with 32/4 g of O₂ 3 g of H₂ reacts with 32/4 x 3 = 24 g ofBut according to the question, 29 g of O₂ is present. 2So, the limiting reactant is hydrogen.Now, 4 g of H₂ forms 36 g of H₂O
Now, we have to determine the limiting reagent.4 g of H₂ reacts with 32 g of O₂ 1 g of H₂ reacts with 32/4 g of O₂ 3 g of H₂ reacts with 32/4 x 3 = 24 g ofBut according to the question, 29 g of O₂ is present. 2So, the limiting reactant is hydrogen.Now, 4 g of H₂ forms 36 g of H₂O1 g of H₂ forms 36/4 g of H₂O. 3 g of H₂ forms 36/4 x 3 = 27 g of H₂O
Now, we have to determine the limiting reagent.4 g of H₂ reacts with 32 g of O₂ 1 g of H₂ reacts with 32/4 g of O₂ 3 g of H₂ reacts with 32/4 x 3 = 24 g ofBut according to the question, 29 g of O₂ is present. 2So, the limiting reactant is hydrogen.Now, 4 g of H₂ forms 36 g of H₂O1 g of H₂ forms 36/4 g of H₂O. 3 g of H₂ forms 36/4 x 3 = 27 g of H₂OMaximum amount of water that can be formed is 27 g.
Now, we have to determine the limiting reagent.4 g of H₂ reacts with 32 g of O₂ 1 g of H₂ reacts with 32/4 g of O₂ 3 g of H₂ reacts with 32/4 x 3 = 24 g ofBut according to the question, 29 g of O₂ is present. 2So, the limiting reactant is hydrogen.Now, 4 g of H₂ forms 36 g of H₂O1 g of H₂ forms 36/4 g of H₂O. 3 g of H₂ forms 36/4 x 3 = 27 g of H₂OMaximum amount of water that can be formed is 27 g.For, amount of oxygen left of unreacted, Only 24 g of oxygen will react.
Now, we have to determine the limiting reagent.4 g of H₂ reacts with 32 g of O₂ 1 g of H₂ reacts with 32/4 g of O₂ 3 g of H₂ reacts with 32/4 x 3 = 24 g ofBut according to the question, 29 g of O₂ is present. 2So, the limiting reactant is hydrogen.Now, 4 g of H₂ forms 36 g of H₂O1 g of H₂ forms 36/4 g of H₂O. 3 g of H₂ forms 36/4 x 3 = 27 g of H₂OMaximum amount of water that can be formed is 27 g.For, amount of oxygen left of unreacted, Only 24 g of oxygen will react.But 29 g is the given amount. Amount of oxygen unreacted = 29 - 24 = 5 g
The theoretical yield of the given chemical equation is 64 g and limiting reactant is oxygen.
What is chemical equation?Chemical equation is a symbolic representation of a chemical reaction which is written in the form of symbols and chemical formulas.The reactants are present on the left hand side while the products are present on the right hand side.
A plus sign is present between reactants and products if they are more than one in any case and an arrow is present pointing towards the product side which indicates the direction of the reaction .There are coefficients present next to the chemical symbols and formulas .
As per the equation 4 g hydrogen reacts with 32 g oxygen thus 8 g hydrogen will react with 8×32/4=64 g oxygen.
Thus, the theoretical yield of the given chemical equation is 64 g .
Learn more about chemical equation,here:
https://brainly.com/question/28294176
#SPJ7
What is the molar concentration a a 12 % sodium chloride solution (MW 58.5)
Answers
The molar concentration of a 12% sodium chloride solution is approximately 2.05 M.
To determine the molar concentration of a 12% sodium chloride solution, we need to convert the given percentage concentration into molarity.
First, we need to understand that the percentage concentration refers to the mass of the solute (sodium chloride) relative to the total mass of the solution.
In this case, a 12% sodium chloride solution means that there are 12 grams of sodium chloride in 100 grams of the solution.
To convert this into molar concentration, we need to consider the molar mass of sodium chloride, which is 58.5 g/mol.
We can start by calculating the number of moles of sodium chloride in 12 grams:
Moles of sodium chloride = mass of sodium chloride / molar mass of sodium chloride
Moles of sodium chloride = 12 g / 58.5 g/mol = 0.205 moles
Next, we calculate the volume of the solution in liters using the density of the solution. Since the density is not provided, we assume a density of 1 g/mL for simplicity:
Volume of solution = mass of solution / density
Volume of solution = 100 g / 1 g/mL = 100 mL = 0.1 L
Finally, we calculate the molar concentration (Molarity) by dividing the number of moles by the volume in liters:
Molar concentration = moles of solute / volume of solution
Molar concentration = 0.205 moles / 0.1 L = 2.05 M
Therefore, the molar concentration of a 12% sodium chloride solution is approximately 2.05 M.
To learn more about molarity click here: brainly.com/question/31545539
#SPJ11
If Thomson’s atomic theory was accurate, what would the results of Rutherford’s gold foil experiment have been?
Answers
Answer:
If Thomson's atomic theory was accurate, the positively charged particles would have gone through the foil.
Given the following reactions:
CaCO3 (s) -> CaO (s) + CO2 (g) H = 178.1
C (s, graphite) + O2 (g) -> CO2 (g) H = -393.5 kJ
The enthalpy of the reation CaCO3 (s) -> CaO (s, graphite) + O2 (g) is _______ kJ
Answers
The enthalpy change for the reaction CaCO3 (s) -> CaO (s, graphite) + O2 (g) is 571.6 kJ.
The enthalpy of the reaction CaCO3 (s) -> CaO (s, graphite) + O2 (g) can be calculated by summing the enthalpies of the individual reactions involved. The given information provides the enthalpy change for the decomposition of CaCO3 (s) and the combustion of C (s) to form CO2 (g). By combining these reactions, the enthalpy change for the overall reaction can be determined.
The given reactions are:
CaCO3 (s) -> CaO (s) + CO2 (g) (H = 178.1 kJ)
C (s, graphite) + O2 (g) -> CO2 (g) (H = -393.5 kJ)
To calculate the enthalpy change for the reaction CaCO3 (s) -> CaO (s, graphite) + O2 (g), we need to subtract the enthalpy change of reaction 2 from the enthalpy change of reaction 1. Since the enthalpy change is an extensive property, we can subtract the enthalpies directly:
ΔH = H(reaction 1) - H(reaction 2)
= 178.1 kJ - (-393.5 kJ)
= 178.1 kJ + 393.5 kJ
= 571.6 kJ
Therefore, the enthalpy change for the reaction CaCO3 (s) -> CaO (s, graphite) + O2 (g) is 571.6 kJ.
To learn more about enthalpy click here:
brainly.com/question/32882904
#SPJ11
20.97 during research studies to determine the absolute stereo- chemistry of a bromohydrin, the investigators observed an unexpected skeletal rearrangement.12 provide a plausible mechanism for the forma- tion of epoxide 2 from bromohydrin 1.
Answers
The skeletal rearrangement of the forma- tion of epoxide 2 from bromohydrin 1.
Skeletal rearrangement reactions, which contain a exchange of connectivity of the substrate thru cleavage of carbon-carbon, carbon-heteroatom, and heteroatom-heteroatom bonds, have attracted plenty interest as a synthetic method of highly substituted organic.
Intermediate is a 3-membered ring (halonium ion). Halohydrin formation (specifically chlorohydrin and bromohydrin formation) is the end result of the addition of a halogen (Cl or Br--less substituted side) and a hydroxyl institution (greater substituted aspect) across an alkene.
Learn more about research here:-
brainly.com/question/968894
#SPJ4

0.00002grams of Hg was found dissolved in 1000g water sample. What is the concentration in ppm?
Answers
The mass of Hg in the sample is 17.1g.
One of the fundamental quantities in physics and the most fundamental feature of matter is mass. The quantity of matter in a body is referred to as its mass. The kilogram, the standard international unit of mass (kg). You can write the mass formula as follows:
Mass = Density × Volume
The water weighs 1400 g. And one night later, we grew by one. Therefore, multiplying X 12.2 by 1400 multiplied by a million. We therefore possess 0.01708 grammes of mercury. When converted to milligrams, this amount equals 17.1 milligrams of mercury.
Learn more about mass on:
brainly.com/question/9120030
#SPJ1
What is the percent of Boron in Sr3(BO3)2
Answers
Answer:
5.68%
Explanation:
Percent Composition of Boron = Atomic Mass of Boron / Molar mass of Sr3(BO3)2 * 100%
Atomic mass of B = 10.811 g/mol
Molar mass of Sr3(BO3)2 = 380.4784 g/mol
Percent composition = 2 (10.811) / 380.4784 * 100
Percent composition = 21.622 / 380.4784 * 100
Percent composition = 5.68%
a sample of gas weighs 3.33 g and occupies a volume of 1.365 l at 95 °c and 790 torr. identify the gas sample.A. Cl₂ (molar mass-70.90 g/mol)B. NH (molar mass- 17.03 g/mol)C. N₂0 molar mass-44.02 g/mol)D. CHC, (molar mass-119.4 g/mol)E. SO₂ (molar mass - 64.07 g/mol)
Answers
The gas sample is Cl₂. Answer A.
The ideal gas equation formula PV = nRT
P = the gas pressure (atm)1 atm = 760 torr
P = 790 torr = 790 ÷ 760 = 1.04 atmV = the gas volume (L)
V = 1.365 Ln = the number of moles (mol)R = the gas constant = 0.0821 L atm/K molT = the temperature (K)
T = 95 °C = 95 + 273 = 368 K
Calculating the number of moles of the gas sample.
PV = nRT
1.04 × 1.365 = n × 0.0821 × 368
1.4196 = n × 30.21
n = 1.4196 ÷ 30.21
n = 0.04699 mol
The formula for mass and number of moles m = n × Mr
m = the mass of the gas (grams)m = 3.33 gMr = the molar mass (g/mol)
A. Mr Cl₂ = 70.90 g/mol
B. Mr NH₃ = 17.03 g/mol
C. Mr N₂O = 44.02 g/mol
D. Mr CHCl₃ = 119.4 g/mol
E. Mr SO₂ = 64.07 g/mol
Calculating the molar gas from the sample
Mr = m ÷ n
Mr = 3.33 ÷ 0.04699
Mr = 70.9 g/mol
From the info given, gas Cl₂ has the same molar mass as the sample.
So, the gas sample is Cl₂, chlorine gas.
Learn more about ideal gas equation here: https://brainly.com/question/20348074
#SPJ4
A molecule contains 3 atoms. It has 1 triple bond and 1 single bond. There are no lone pairs on any of the atoms. How many rheds are within the molecule?.
Answers
The central atom of the molecule has three electrons on its valence shell.
What is the valance shell electro pair repulsion theory?
The valance shell electron pair repulsion theory shows us the number of electron pairs that can be found on the valance shell of the central atom of the molecule. Recall that the shape of the molecule is determined by the number of electron pairs that can be found on the valance shell of the central atom of the molecule.
Hence, given the fact that the molecule contains 3 atoms. It has 1 triple bond and 1 single bond, we can be able to conclude that the molecule has three electron pairs on its valance shell.
Learn more about valence shell:https://brainly.com/question/7871741
#SPJ1
66.7 mL of Ethanol was dissolved in 222.2 mL of water. What is the volume % of the ethanol in the solution?
Respond with the correct number of signficant figures in scientific notation. (Use E notation and only 1 digit before decimal, for example, 2.5E5 for 2.5 times 10 to the power of 5)
Answers
66.7 mL of Ethanol was dissolved in 222.2 mL of water. the volume % of the ethanol in the solution is 30.0 %.
Given that :
volume of the solute = 66.7 mL
volume of the solution = 222.2 mL
by using the volume % formula we can directly determine the volume % of ethanol, the percent by volume expression is given as :
volume % = (volume of solute / volume of solution ) × 100 %
volume % = (66.7 / 222.2 ) × 100 %
volume % = 0.300 × 100 %
volume % = 30 .0 %
( v / v ) % = 30.0 %
Thus, the percent by volume is 30.0 %.
To learn more about percent by volume here
https://brainly.com/question/23847585
#SPJ4
4. Given the balanced equation: 2Na + S → Na₂S
How many grams of sulfur are needed to react with 43 grams of Na? Round to the nearest whole number.
a. 119 g
b. 158 g
c. 32 g
d. 30 g

Answers
Answer:
Option D. 30 g
Explanation:
The balanced equation for the reaction is given below:
2Na + S —> Na₂S
Next, we shall determine the masses of Na and S that reacted from the balanced equation. This is can be obtained as:
Molar mass of Na = 23 g/mol
Mass of Na from the balanced equation = 2 × 23 = 46 g
Molar mass of S = 32 g/mol
Mass of S from the balanced equation = 1 × 32 = 32 g
SUMMARY:
From the balanced equation above,
46 g of Na reacted with 32 g of S.
Finally, we shall determine the mass sulphur, S needed to react with 43 g of sodium, Na. This can be obtained as follow:
From the balanced equation above,
46 g of Na reacted with 32 g of S.
Therefore, 43 g of Na will react with = (43 × 32)/46 = 30 g of S.
Thus, 30 g of S is needed for the reaction.
an equation thats shows how an objects acceleration relates to its mass
Answers
Answer:
Newton's second law of motion
F = ma
Molar Mass related question (22b)
how do I solve?

Answers
The molar mass of potassium nitrate is 101.102 g/mol while that of potassium nitrite is 85.103 g/mol.
Molar mass calculationPotassium nitrate goes by the chemical formula \(KNO_3\).
K = 39.098
N = 14.007
O = 15.999
Molar mass of \(KNO_3\) = 39.098 + 14.007 + (3x15.999)
= 101.102 g/mol
Potassium nitrite goes by the chemical formula, \(KNO_2\).
Molar mass of \(KNO_2\) = 39.098 + 14.007 + (2x15.999)
= 85.103 g/mol
In other words, the molar masses of KNO3 and KNO2 are 101.102 g/mol and 85.103 g/mol respectively.
More on molar masses can be found here: https://brainly.com/question/22997914
#SPJ1
1 point
A 5-gram sample of water is heated and the temperature rises from 10°C
to 15°C. The total amount of heat energy absorbed by the water is
1) 104.5 J
2) 83.63
3) 62.7 J
4) 20.9 J
Answers
Answer:
104.5j
Explanation:
on a hot summer day, you make a glass of refreshing sweet iced tea by combining boiling water, tea, sugar, and ice. what type of change occurs and how do you know?
Answers
A physical change will occur when boiling water, tea, sugar, and ice are combined to form sweet iced tea.
What is a physical change?A physical change is a change affecting the form of a chemical substance, but not its chemical composition.
The characteristics of some physical changes are as follows:
textureshapetemperaturechange in the state of matterAccording to this question, one makes a glass of refreshing sweet iced tea by combining boiling water, tea, sugar, and ice on a hot summer day. The resulting solution is an example of a mixture, which contains constituents that retain their individual identity.
This suggests that the change is a physical change because there is no formation of a new substance.
Learn more about physical change at: https://brainly.com/question/17931044
#SPJ1
A double-blind study is one in which neither the researchers nor the subjects know whether the subject is receiving the real treatment or the placebo. What is the value of this kind of study?
Answers
A kind of clinical experiment where neither the research team nor the subjects are aware of the treatment they are getting until the trial is over. As a result, the study's findings are less likely to be skewed.
Describe single-blind research. How do double-blind studies work?Single-blind research typically involves keeping the research subject's treatment assignment a secret. In a double-blind study, the participant in the research, the investigator, the study coordinator or nurse, the study sponsor, and occasionally the data analyst are kept in the dark regarding the treatment allocation.
What is protected by a double-blind study?As doctors assess the results of their patients, bias is avoided by double blind investigations. As a result, clinical trial outcomes have increased reliability. Your doctor may "unblind" you if you experience health issues during a trial, such as a potential drug reaction, to reveal the treatment you are receiving.
To know more about experiment visit-
https://brainly.com/question/30712062
#SPJ1
Put the following elements in order from smallest to largest atomic radius: Carbon, Oxygen, Tin, Strontium
Rank the following elements by increasing atomic radius: Carbon, Aluminum, Oxygen, Potassium
Answers
The order of the atomic radius is;
1) Potassium, Aluminum, Carbon, Oxygen
2) Potassium, Aluminum, Carbon, Oxygen
What is the order of the atomic radius?We know that the atomic radius has to do with the distance between one atom and another in that are not covalently combined. We know that the atomic radius is a function of the group to which the atom belongs.
We know that the atomic radius increases down the group but would tend to decrease across the period as more shells are added to the atom of the element.
Learn more about atomic radius:https://brainly.com/question/14544878
#SPJ1
PLEASE HELP ME ASAP

Answers
The orbital diagram suggest Pauli's exclusion principle.
option C.
What is Pauli's exclusion principle?Pauli's Exclusion Principle is a fundamental principle of quantum mechanics that states that no two identical fermions (particles with half-integer spin, such as electrons, protons, and neutrons) can occupy the same quantum state simultaneously.
In other words, if one fermion is in a particular quantum state, then no other fermion can be in that same quantum state at the same time.
This principle is crucial in understanding the behavior of matter at the atomic and subatomic level. It explains, for example, why electrons in an atom occupy different energy levels and why atoms and molecules have unique chemical and physical properties.
The diagram suggest that the spin is different, so it describes Pauli's exclusion principle.
Learn more about Pauli's exclusion principle here: https://brainly.com/question/16950638
#SPJ1
Why are bacteria necessary for life on Earth to exist? Select three options.
Bacteria help produce the oxygen that plants and animals need.
Bacteria help produce some of the foods we eat.
Bacteria put atoms together to form water that plants and animals need.
Bacteria are required for plants and animals to reproduce.
Bacteria decompose rotting plant and animal material.
Answers
Answer:
Bacteria decompose rotting plant animal material.
Explanation:
Theres good bacteria, and theres bad bacteria. some helps, and some makes things worse. so really, in some sense all of the above.
Hope that helps.
Bacteria are necessary for life because they help produce oxygen for plants and animals. Similarly bacteria decompose rotting plant and animal materials.
What are bacteria?Bacteria are a type of organism which are classified as a kingdom of life. Bacteria are prokaryotic microbes that can spread diseases in animals and plants.
Some of the bacteria are very useful in for our life. Some of them are autotrophs thus, produce energy and oxygen. For example cyanobacteria release oxygen which is used animals and plants to respire.
Bacteria are very essential to degrade wastes and remove them from the surface. They consume the decomposing plants and animals and make them biodegradable. Some bacteria living in plants helps in nitrogen cycle as well.
Find more on significance of bacteria:
https://brainly.com/question/14225790
#SPJ2
Explain why a high-mass star has a much shorter lifespan than a low-mass star despite the fact
that a high-mass star has much more hydrogen (fuel) to burn. Don't forget that an explanation
needs to answer how and whí, something happens. Be sure to include the following in your
explanation:
a. How gravity leads to nuclear fusion
b. How the rate of fusion in a high mass star compares to the rate of fusion in a low mass
star
c. Why a high-mass star has a much shorter lifespan than a low-mass star despite the fact
that a high-mass star has much more hydrogen (fuel) to burn
Answers
Answer:
just trust me the answer is B
Mr.Davies has requested that you wait for 10 more minutes is that informal or formal
Answers
Answer:
Formal
Explanation:
Answer:
I think its formal because the question looks a bit fancy by using all the present perfect tense..
hope this helped you
please mark as the brainliest ( if its correct)(ㆁωㆁ)