How are cohesion and surface tension related to the chemical properties of water?.
Answers
Cohesion and surface tension related to the chemical properties of water
Because water molecules can create hydrogen bonds with one another, they've powerful adhesive forces. Cohesive forces are accountable for surface anxiety, the tendency of a liquid's surface to resist rupture when placed under tension or pressure.
The chemical composition and polarity of the water molecule decide concord and surface anxiety. within a water molecule, the bonds among oxygen and hydrogen are covalent, which means that they share valence electron pairs. The negatively charged particles known as valence electrons circle the atomic nucleus of an atom at the very best electricity degree. due to the fact that oxygen has a higher electronegative polarity than hydrogen, it attracts the shared electrons to it. The hydrogen atoms hold their barely tremendous prices while the oxygen profits a little bad fee as a result.The fine charges on close by hydrogen atoms are drawn to the negative prices on oxygen atoms while water molecules are near together. The water molecules adhere to one another and emerge as cohesive due to the vulnerable hydrogen bonds which might be created. because there are fewer molecules with which to establish hydrogen bonds near the surface, the water molecules there are in particular cohesive. that is what offers water its very strong surface tension.
Learn more about Cohesive forces https://brainly.com/question/9007624
#SPJ4
Related Questions
Which of the following indicates if a reaction will proceed forward at any given conditions? ΔG° < 0
ΔG > 0 ΔG° > 0
ΔG < 0
Answers
The indication that a reaction will proceed forward at any given conditions is when the value of ΔG (Gibbs free energy) is less than zero (ΔG < 0).
Gibbs free energy (ΔG) is a thermodynamic quantity that measures the spontaneity and direction of a chemical reaction. When ΔG is negative (ΔG < 0), it indicates that the reaction is thermodynamically favorable and can proceed forward under the given conditions. This means that the reaction has a higher probability of occurring and the products will be more stable than the reactants. A negative ΔG signifies that the reaction releases energy and can occur spontaneously without the need for external input. In contrast, when ΔG is positive (ΔG > 0), the reaction is thermodynamically unfavorable, indicating that it is less likely to proceed forward under the given conditions. ΔG° refers to the standard Gibbs free energy change, which is measured under standard conditions (usually 25°C and 1 atm pressure). A negative value for ΔG° (< 0) suggests that the reaction is spontaneous under standard conditions.
To learn more about Gibbs free energy click here,
brainly.com/question/9179942
#SPJ11
Metal extracted
from
Bloleaching
Pollution
Waste from quarrying
Speed of process Very slow
Produces a solution of toxic
chemicals which may run off
into rivers
Phytom Ining
Contaminated
ground
Compare phytomining and bioleaching.
Use the information in the table.
Slow, made more
efficient using
quick-growing
plants
Takes a long time to stop the
process if river pollution occurs
Involves
combustion of
plants but
decontaminat
polluted ground
Answers
Metals can be removed from ores by reduction – the deletion of oxygen or forming a metal element from a compound.
How is a metal extracted from ore?Metal is extracted from mashed ore by one of two major methods: smelting or electrolysis. Smelting uses heat to discrete the valuable metal from the rest of the ore. Smelting usually needed a reduction agent, or another chemical, to separate metal from its ore.
Isolation of metal from concentrated Ore – Here the ore is changed to its oxide form and then reduced. The steps involved are either scorching or roasting and then heating with a reducing agent.
So we can conclude that trade extraction of metals from minerals is usually practiced by dump leaching, heap leaching, tank leaching, or pushed leaching.
Learn more about metal here: https://brainly.com/question/4701542
#SPJ1
What amino acid would you end up with if you deleted the c from the second reading frame?
Answers
Which of the following statements are true?
1. The number of protons in an element is always the same for all
neutral atoms of that element.
II. The number of electrons in an element is always the same for all
neutral atoms and ions of that element. .
III. The number of neutrons in any element is always equal to the
number of protons of that element.
IV. The number of neutrons in an element can sometimes vary -
resulting in the formation of an unstable isotope that is
slightly
more (or less) massive than the stable atom of the same element.
Answers
Answer:
roman number3
Explanation:
if you have 4.113 g of organic fuel and 4.113 g of oxygen gas, how many grams of carbon monoxide would you produce? (round to 4 significant digits)
Answers
The balanced chemical equation for the combustion of organic fuel in the presence of oxygen is:
CxHy + zO2 → xCO + (y/2)H2O
From the equation, we can see that 1 mole of organic fuel (CxHy) reacts with z moles of oxygen gas (O2) to produce x moles of carbon monoxide (CO) and (y/2) moles of water (H2O).
To find out how many grams of carbon monoxide would be produced, we need to first determine the limiting reactant. This is the reactant that is completely consumed in the reaction, thus limiting the amount of product that can be formed.
The molar mass of the organic fuel and oxygen gas are:
Molar mass of organic fuel = 4.113 g
Molecular weight of organic fuel = Unknown
Molar mass of oxygen gas = 4.113 g
Molecular weight of oxygen gas = 32 g/mol
To find the molecular weight of the organic fuel, we need to know its chemical formula. Let's assume it is C3H8 (propane).
Molecular weight of organic fuel = (3 x atomic weight of carbon) + (8 x atomic weight of hydrogen)
= (3 x 12.01 g/mol) + (8 x 1.01 g/mol)
= 44.11 g/mol
Now we can calculate the number of moles of each reactant:
Number of moles of organic fuel = 4.113 g / 44.11 g/mol = 0.0933 mol
Number of moles of oxygen gas = 4.113 g / 32 g/mol = 0.1287 mol
According to the balanced chemical equation, the stoichiometry of the reaction is 1:z:x:(y/2). We need to find the value of x, which represents the number of moles of carbon monoxide produced.
Since both reactants have the same number of significant digits, we can use either one to determine the limiting reactant. Let's use the organic fuel.
The molar ratio of organic fuel to oxygen gas is:
0.0933 mol organic fuel : 0.1287 mol oxygen gas
The smallest value is 0.0933 mol, which means that the organic fuel is the limiting reactant.
The number of moles of carbon monoxide produced is:
x = 0.0933 mol organic fuel x (1 mol CO / 1 mol organic fuel) = 0.0933 mol CO
The mass of carbon monoxide produced is:
Mass of CO = 0.0933 mol CO x 28.01 g/mol CO = 2.61 g
Rounding to four significant digits gives us:
Mass of CO = 2.61 g
Therefore, if you have 4.113 g of organic fuel and 4.113 g of oxygen gas, you would produce 2.61 g of carbon monoxide.
To learn more about balancing chemical equation :
https://brainly.com/question/28294176
#SPJ11
The balanced equation for the combustion of organic fuel (represented as CH4) with oxygen is:
CH4 + 2O2 → CO2 + 2H2O
From the equation, we can see that for every mole of CH4, we will produce one mole of CO2. To find the number of moles of CH4, we can use its molar mass:
16.04 g/mol CH4 * (4.113 g CH4 / 1) = 0.2561 mol CH4
Similarly, the number of moles of O2 can be found using its molar mass:
32.00 g/mol O2 * (4.113 g O2 / 1) = 0.1282 mol O2
Since we need twice as many moles of O2 as CH4, O2 is the limiting reactant. This means that all of the O2 will be used up in the reaction and any remaining CH4 will not react. The number of moles of CO2 produced will be equal to the number of moles of CH4 used up:
0.2561 mol CH4 * (1 mol CO2 / 1 mol CH4) = 0.2561 mol CO2
Finally, we can use the balanced equation to find the number of moles of CO that will be produced:
CH4 + 2O2 → CO2 + 2H2O
2 mol CO / 2 mol O2 = 1 mol CO / 1 mol O2
0.1282 mol O2 * (1 mol CO / 2 mol O2) = 0.0641 mol CO
The molar mass of CO is 28.01 g/mol, so the mass of CO produced is:
28.01 g/mol CO * (0.0641 mol CO / 1) = 1.795 g CO
Rounding to 4 significant digits, the answer is 1.795 g CO.
Learn more about limiting reactant here:
https://brainly.com/question/14225536
#SPJ11
A 36.04 g sample of water is boiled. When the steam expands to fill the vessel how many atoms of hydrogen would be found in the sample?
Answers
Answer:
The number of hydrogen atoms in 36.04 g sample of steam is 2.408 × 10²⁴ atoms of hydrogen
Explanation:
The given parameters are;
The mass of the sample of water, H₂O = 36.04 g
From the principle of conservation of matter, the mass of the water = The mass of the steam, therefore;
The mass of the steam = 36.04 g
The molar mass of H₂O = 18.01528 g/mol
Therefore, the number of moles, n, of H₂O in the 36.04 g of steam is given as follows;
n = Mass/(Molar Mass) = (36.04 g)/(18.01528 g/mol) = 2.00052399963 ≈ 2 moles
The number of molecules of H₂O per mole of H₂O is geven by the Avogadro's number = 6.02 × 10²³ molecules
Therefore;
The number of H₂O in 2 moles of H₂O = 2 × 6.02 × 10²³ = 1.204 × 10²⁴ molecules
The number of hydrogen atoms per molecule of H₂O = 2 hydrogen atoms
Therefore;
The number of hydrogen atoms in the 1.204 × 10²⁴ molecules of H₂O = 2 × 1.204 × 10²⁴ molecules of H₂O
Which gives;
The number of hydrogen atoms in the 1.204 × 10²⁴ molecules of H₂O = 2.408 × 10²⁴ atoms of hydrogen
Therefore;
The number of hydrogen atoms in 36.04 g sample of steam = 2.408 × 10²⁴ atoms of hydrogen.
describe the chemistry and main ingredients of uv gels
Answers
UV gels are commonly used in the nail industry for artificial nail enhancements. The main ingredients in UV gels are typically oligomers, monomers, photo initiators, and pigments.
Oligomers are long-chain molecules that provide the bulk and strength to the gel. Monomers are smaller molecules that help the gel cure and harden under UV light. Photoinitiators are added to the gel to initiate the polymerization reaction when exposed to UV light. This reaction causes the gel to harden and bond to the natural nail or nail extension. Pigments are added to give the gel its color and opacity.
The chemistry of UV gels involves the process of polymerization, which is the bonding of monomers and oligomers through a chemical reaction. This reaction is triggered by the photoinitiators in the gel when exposed to UV light. As the reaction occurs, the gel becomes solid and adheres to the nail.
Overall, the chemistry and ingredients of UV gels allow for a durable and long-lasting nail enhancement that is popular in the beauty industry.
To know more about pigments refer here :
https://brainly.com/question/14354023
#SPJ11
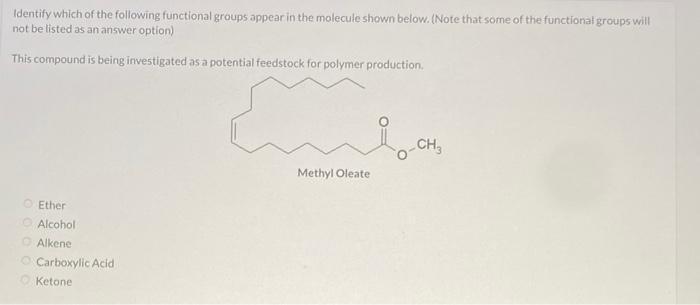
identify which of the following functional groups appear in the molecule shown below. (note that some of the functional groups will not be listed as an answer option)
Answers
The functional groups that we have in the compound are ketone and alkene. Options C and E
What is a functional group?
A functional group is a particular set of atoms in a molecule that are in charge of the molecule's distinctive chemical processes and characteristics. The behavior and functionality of a molecule in chemical processes are determined by a reactive component of the molecule.
The common atoms found in functional groups are carbon, hydrogen, oxygen, nitrogen, sulfur, and phosphorus. They can be recognized by their unique atom arrangement and bonding structure inside a molecule.
Learn more about functional group:https://brainly.com/question/1356508
#SPJ4
.How many unshared electrons and bonding
electrons exist around the central atom in
ozone (O3)?
1. none; two
2. zero; eight
3. one; three
4. one; six
5. two; two
6. four; four
7. four; three
8. three; six
9. two; six
Answers
The answer is option 4: one unshared electron and six bonding electrons exist around the central atom in ozone (O3).
learn more about ozone https://brainly.in/question/19068482?referrer=searchResults
#SPJ11
What is the difference in electrochemical potential between two electrodes of an electrochemical cell called?
Answers
The difference in electrochemical potential between two electrodes of an electrochemical cell is called as the cell potential.
What is the cell potential?The potential difference or voltage that exists between two electrodes in an electrochemical cell when no current is flowing through the cell is called the cell potential. Cell potential, also known as electromotive force (emf), is a measure of the driving force that drives a chemical reaction in an electrochemical cell forward.
The potential difference between the anode and cathode of an electrochemical cell is a quantitative measurement of the cell's capacity to generate electrical energy. The cell potential is usually measured in volts (V), and its sign is determined by the direction in which the electrons flow through the cell. When electrons flow spontaneously from the anode to the cathode, the cell potential is positive, whereas if electrons are forced to flow from the cathode to the anode, the cell potential is negative.
Learn more about Cell potential here:
https://brainly.com/question/1313684
#SPJ11
Given the reaction: o3(g) + no(g) --> o2(g) + no2(g) if you start with 0. 05 m o3 and 0. 01 m no and the reaction reaches completion in 16 seconds, what is the initial rate of this reaction with respect to o3?.
Answers
Since we are basing the rate with respect to O3, therefore the rate would simply be the concentration divided by the total reaction time. That is:
The order with regard to O3 is the rate law power of [O3], which is 2. The total of the powers in the rate law yields the overall order of reaction, which is 2 + (1 = 1).
NO2 is formed when NO(g) and O2(g) react (g). The reaction's rate law is rate=k[NO]2[O2]. Which of the following is the equation for the elementary step if the reaction occurs in a single elementary step that is a three-body molecular collision? 2NO+O2→2NO2.
Time = 16 seconds
[O3] = 0.05 M
[NO] = 0.01 M
The rate of the reaction with respect to O3 is
Rate = Concentration / time
= 0.05 M / 16 s
= 0.00312 M / s
Learn more about initial rate
https://brainly.com/question/1906721
#SPJ4
Ethanol fuel mixtures have "E" numbers that indicate the percentage of ethanol in the mixture by volume. For example, E10 is a mixture of 10% ethanol and 90% gasoline. How much E7 should be mixed with 3000 gal of E10 to make an E9 mixture? Part: 0 / 4 Part 1 of 4 Let x represent the amount of a mixture (in gal) containing 319. ethanol. 3000 gal is the amount of E10 mixture containing 10% ethanol. Therefore, is the amount of the resulting E9 mixture containing 906 ethanol
Answers
To make an E9 mixture 8657.14 gal of E7 should be mixed with 3000 gal of E10
Given to us is the amount of ethanol in the E10 mixture is 10% of 3000 gallons:
Ethanol in E10 = 10% × 3000 gal = 0.10 × 3000 gal = 300 gal
To solve this problem, we can set up an equation based on the amount of ethanol in each mixture.
Let x represent the amount of E7 mixture (in gallons) that needs to be added to the E10 mixture to obtain the desired E9 mixture.
The amount of ethanol in the E7 mixture is 7% of x gallons:
Ethanol in E7 = 7% × gal = 0.07 × gal
The resulting E9 mixture will contain 9% ethanol of the total volume of 3000 + x gallons:
Ethanol in E9 = 9% × (3000 + x) gal = 0.09 × (3000 + x) gal
According to the problem, the resulting E9 mixture contains 906 gallons of ethanol:
Ethanol in E9 = 906 gal
Now we can set up the equation:
Ethanol in E10 + Ethanol in E7 = Ethanol in E9
300 gal + 0.07x gal = 906 gal
Subtracting 300 gal from both sides:
0.07x gal = 606 gal
Dividing both sides by 0.07:
x = 606 gal / 0.07
x = 8657.14
Therefore, approximately 8657.14 gallons of E7 mixture should be mixed with 3000 gallons of E10 to make an E9 mixture.
Learn more about ethanol mixtures here:
https://brainly.com/question/28954299
#SPJ4
Complete question: Ethanol fuel mixtures have "E" numbers that indicate the percentage of ethanol in the mixture by volume. For example, E10 is a mixture of 10% ethanol and 90% gasoline. How much E7 should be mixed with 3000 gal of E10 to make an E9 mixture?
PLEASE ASNWER QUICK!!!! AND RIGHT ANSWERS!! 50 POINTS!!
2C2H2 (g) + 5O2(g) --> 4CO2(g) + 2H2O(g)
How many liters of C2H2 are required to produce 8 L of CO2 assuming the reaction is at STP?
L C2H2
Answers
Answer:
3.95 L
Explanation:
To solve this problem, we need to use stoichiometry and the ideal gas law to determine the amount of C2H2 required to produce 8 L of CO2 at STP.
First, we need to determine the number of moles of CO2 produced from 8 L at STP. The molar volume of an ideal gas at STP is 22.4 L/mol, so:
8 L CO2 * (1 mol CO2 / 22.4 L CO2) = 0.357 mol CO2
Next, we can use the balanced chemical equation to determine the number of moles of C2H2 required to produce 0.357 mol CO2. From the balanced equation, we see that 2 moles of C2H2 produce 4 moles of CO2, so:
2 mol C2H2 / 4 mol CO2 = 0.5 mol C2H2 / mol CO2
0.357 mol CO2 * (0.5 mol C2H2 / mol CO2) = 0.179 mol C2H2
Finally, we can use the ideal gas law to convert the number of moles of C2H2 to volume at STP. The ideal gas law is PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the gas constant, and T is temperature. At STP, the pressure is 1 atm and the temperature is 273 K, so:
V = nRT / P = (0.179 mol) * (0.0821 Latm/(molK)) * (273 K) / (1 atm) = 3.95 L
Therefore, 3.95 L of C2H2 are required to produce 8 L of CO2 at STP.
In a compound, chemical energy is _______________ when bonds break.
Answers
Answer:
In a compound, chemical energy is _____required__________ when bonds break.
On a cool morning, Uyen’s breath can form a cloud when she breathes out. Which changes of state are most responsible for Uyen seeing her breath in this way?
melting
condensation
deposition
sublimation
Answers
What law is “does the dog wag the tail, or the tail wag the dog?“
Answers
how many milliliters of 0.202 m naoh should be added to 25.0 ml of 0.023 3 m salicylic acid (2-hydroxybenzoic acid) to adjust the ph to 3.50 (pka1
Answers
We need to add 2.88 mL of 0.202 M NaOH to 25.0 mL of 0.0233 M salicylic acid to adjust the pH to 3.50.
Salicylic acid has two dissociation constants (pKa values) of 2.97 and 13.02. For this problem, we'll assume that we're interested in the first pKa value of 2.97, which corresponds to the dissociation of the carboxyl group (COOH) of salicylic acid.
The Henderson-Hasselbalch equation for a weak acid is:
pH = pKa + log([A-]/[HA])
where pH is the desired pH, pKa is the dissociation constant, [A-] is the concentration of the conjugate base, and [HA] is the concentration of the weak acid.
Rearranging the equation and solving for [A-]/[HA], we get:
[A-]/[HA] = 10^(pH - pKa)
Substituting the given values, we get:
[A-]/[HA] = 10^(3.50 - 2.97) = 3.98
So the concentration of the conjugate base ([A-]) is 3.98 times higher than the concentration of the weak acid ([HA]) at pH 3.50.
We can use the balanced chemical equation for the reaction of salicylic acid with NaOH:
C7H6O3 (salicylic acid) + NaOH → NaC7H5O3 (sodium salicylate) + H2O
From the equation, we can see that the stoichiometric ratio of salicylic acid to NaOH is 1:1. So we need to add enough NaOH to convert all of the salicylic acid into sodium salicylate.
The amount of salicylic acid in 25.0 mL of a 0.0233 M solution can be calculated as follows:
moles of salicylic acid = M × V = 0.0233 mol/L × 0.0250 L = 5.83 × 10^-4 mol
Since the stoichiometric ratio of salicylic acid to NaOH is 1:1, we need to add 5.83 × 10^-4 mol of NaOH to the solution.
The amount of NaOH required can be calculated as follows:
moles of NaOH = moles of salicylic acid = 5.83 × 10^-4 mol
The concentration of the NaOH solution is 0.202 M, so we can use the following formula to calculate the volume of NaOH required:
V = moles of NaOH / (concentration of NaOH)
V = (5.83 × 10^-4 mol) / (0.202 mol/L) = 2.88 × 10^-3 L
Multiplying by 1000 mL/L, the volume of NaOH required is 2.88 mL.
Therefore, we need to add 2.88 mL of 0.202 M NaOH to 25.0 mL of 0.0233 M salicylic acid to adjust the pH to 3.50.
For more question on salicylic acid click on
https://brainly.com/question/10847219
#SPJ11
4. An object with a mass of 6.0 kg accelerates 4.0 m/s2 when an unknown force is applied to it. What is the amount of the force?
Answers
Answer:
The answer is 24 NExplanation:
The force acting on an object given it's mass and acceleration can be found by using the formula
force = mass × accelerationFrom the question we have
force = 6 × 4
We have the final answer as
24 NHope this helps you
A sample of helium has a volume of 3.20x10^2 mL at STP. What will be its new volume (inL) if the temperature is increased to 425.0 K and its pressure is increased to 3.50 atm?
Answers
Let's see that the STP represents the conditions for the temperature of 0°C (273 K) and for the pressure of 1 atm.
We have this initial data and a volume of 3.20 x 10 ^(2) mL. To solve this problem, we need to use the ideal gas formula:
\(\frac{P_1V_1}{T_1}=\frac{P_2V_2}{T_2},\)where T is temperature, P is pressure, and V volume. Subindex 1 is the initial data and subindex 2 is the final data.
We want to find the final volume, so clearing for V2 in the formula, we're going to obtain:
\(V_2=\frac{P_1V_1T_2}{T_1P_2}\text{.}\)And the final step is replacing the data that we have, where the final data is 425.0 K and 3.50 atm (remember that the volume must be in liters, 1 liter is 1000 mL, so 3.20 x 10^2 mL is 0.32 L):
\(\begin{gathered} V_2=\frac{1\text{ atm }\cdot\text{ 0}.32\text{ L }\cdot425.0K}{273\text{ K }\cdot\text{ 3.50 atm}}, \\ V_2=0.14\text{ L.} \end{gathered}\)The answer is that the new volume of the sample of helium would be 0.14 L.
The EPA sets national air-quality standards for common air pollutants. The
graph shows the change in the concentrations of these pollutants over time.
Which conclusion do the data in the graph support?
A Concentrations of all pollutants have decreased.
B. Monitoring concentrations of pollutants is too expensive.
O C. Concentrations of all pollutants have increased.
O D. Monitoring concentrations of pollutants has been ineffective.

Answers
Answer:
A
Explanation:
Just took the quiz
The irregularity of the chart means that, monitoring concentrations of pollutants has been ineffective.
What is the EPA?The EPA is the environmental protection agency which takes care of the set limits of pollutamts that are released into the environment. The chart shows the monitoring of the concentrations of pollutants over a period of time.
We can see tat the chart is so irregular. The irregularity of the chart means that, monitoring concentrations of pollutants has been ineffective.
Learn more about EPA: https://brainly.com/question/365923
A 30.0-ml sample of 0.165 M propanoic acid is titrated with 0.300 M KOH.
1. Calculate the{\rm pH}at 0{\rm mL}of added base
2. Calculate the{\rm pH}at 5{\rm mL}of added base.
3. Calculate the{\rm pH}at 10{\rm mL}of added base.
4. Calculate the{\rm pH}at the equivalence point.
5. Calculate the{\rm pH}at one-half of the equivalence point.
6. Calculate the{\rm pH}at 20{\rm mL}of added base.
7. Calculate the{\rm pH}at 25{\rm mL}of added base.
Answers
The pH of 0mL of added base is 1.92, the pH of 5 ml added base is 4.39, the pH at 10ml of added base is 4.87, the pH at the equivalence point is, the pH at one-half of the equivalence point is 4.87, the pH at 20 of added base is 9.04, the pH at 25 of added base is 4.76.
At 0 mL of added base, the solution is just propanoic acid, so the pH can be calculated using the expression for the acid dissociation constant, Ka:
Ka = [H+][C3H5O2-]/[C3H5O2H].
Setting up an ICE table and solving for [H+], we get:
Ka = 1.3 x 10^-5
[H+] = sqrt(Ka*[C3H5O2H]) = 0.012 M
pH = -log[H+] = 1.92
At 5 mL of added base, propanoic acid has been partially neutralized, so we need to calculate the concentration of both the acid and its conjugate base to determine the pH. Using the mole-to-mole ratio of propanoic acid to KOH, we can calculate that 0.015 moles of propanoic acid have been neutralized, leaving 0.015 moles of propanoic acid and 0.015 moles of C3H5O2- in the solution. The total volume is 35 mL (30 mL of acid + 5 mL of KOH). Using the Henderson-Hasselbalch equation, we get:
pH = pKa + log([C3H5O2-]/[C3H5O2H])
pKa = -log(Ka) = 4.89
[C3H5O2-]/[C3H5O2H] = 0.015/0.15 = 0.1
pH = 4.89 + log(0.1) = 4.39
At 10 mL of added base, the same process can be repeated with 0.03 moles of propanoic acid neutralized and 0.03 moles of C3H5O2- in the solution. The total volume is 40 mL.
[C3H5O2-]/[C3H5O2H] = 0.03/0.12 = 0.25
pH = 4.89 + log(0.25) = 4.12
At the equivalence point, the moles of KOH added are equal to the moles of PA originally present. This means that all the PA has been converted to KPA. The equation for the reaction between KPA and water (H2O) is:
KPA + H2O ⇌ PA + KOH
The salt KPA is a strong electrolyte and completely dissociates in water, so the solution at the equivalence point contains PA and KOH in equal amounts, and the {\rm pH} is equal to the pKa of propanoic acid, which is 4.87.
At one-half of the equivalence point, half of the moles of PA have been neutralized by KOH. This means that the concentration of PA is equal to the concentration of KPA, and the solution contains a buffer composed of PA and KPA. The pH of a buffer is given by the Henderson-Hasselbalch equation:
pH = pKa + log([A-]/[HA])
where A- is the conjugate base (in this case KPA) and HA is the acid (in this case PA). At one-half of the equivalence point, the concentrations of KPA and PA are equal, so [A-]/[HA] = 1, and the pH is equal to the pKa, which is 4.87.
At 20 mL of added base, we can calculate the moles of PA remaining by multiplying the initial concentration by the volume of acid that has not yet reacted
moles of PA = 0.165 M × (30.0 mL – 20.0 mL)/1000 mL = 0.00165 moles
The moles of KOH added are
moles of KOH = 0.300 M × 20.0 mL/1000 mL = 0.006 moles
Since the moles of KOH are greater than the moles of PA, we are in the region beyond the equivalence point, and the excess KOH will react with water to form OH- ions. The OH- ions will react with the PA that remains to form KPA, and the solution will be a buffer composed of KPA and PA. To calculate the pH, we can use the Henderson-Hasselbalch equation with the concentrations of KPA and PA at this point
pH = pKa + log([A-]/[HA])
At this point, the concentration of KPA is
[KPA] = moles of KOH added/total volume = 0.006 moles/(30.0 mL/1000 mL) = 0.2 M
The concentration of PA is
[PA] = moles of PA remaining/total volume = 0.00165 moles/(30.0 mL/1000 mL) = 0.055 M
Substituting these values into the Henderson-Hasselbalch equation gives
pH = 4.87 + log(0.2/0.055) = 9.04
Propanoic acid is a weak acid, so its reaction with KOH produces a buffer solution. The balanced chemical equation for the reaction is:
CH3CH2COOH + KOH → CH3CH2COOK + H2O
At the equivalence point, all of the propanoic acid is neutralized and converted to the salt, CH3CH2COOK. The volume of KOH needed to reach the equivalence point can be calculated as follows:
moles of propanoic acid = 0.165 M x 0.030 L = 0.00495 mol
moles of KOH needed = 0.00495 mol
volume of KOH needed = 0.00495 mol / 0.300 M = 0.0165 L = 16.5 mL
So, at 25 mL of added base, the reaction is not yet at the equivalence point. To calculate the pH at this point, we need to use the Henderson-Hasselbalch equation for a buffer solution:
pH = pKa + log([A-]/[HA])
The pKa of propanoic acid is 4.87. We can assume that at 25 mL of added base, most of the propanoic acid has been converted to propanoate ion, so we can use its concentration in the calculation:
[HA] = 0.165 - moles of propanoic acid neutralized by KOH
[A-] = moles of KOH added / total volume of solution
moles of propanoic acid neutralized by KOH = 0.300 M x 0.025 L = 0.0075 mol
[HA] = 0.165 - 0.0075 mol / 0.0555 L = 0.137 M
[A-] = 0.0075 mol / 0.0555 L = 0.135 M
pH = 4.87 + log(0.135/0.137) = 4.76
Therefore, the pH at 25 mL of added base is 4.76.
To know more about pH:
https://brainly.com/question/15289741
#SPJ4
an electron in an excited mercury atom is in energy level g. what is the minimum energy required to ionize this atom?
Answers
The minimum energy required to ionize a mercury atom with an electron in the energy level g is 2.48 eV.
The energy of an electron in an energy level can be calculated using the following equation:
E = -2.178 * Z^2 * R_H / n^2
where:
* E is the energy of the electron in electron volts (eV)
* Z is the atomic number of the atom
* R_H is the Rydberg constant, which is equal to 13.606 * 10^-3 eV
* n is the principal quantum number, which represents the energy level of the electron
For mercury, Z = 80. If the electron is in the energy level g, then n = 4. Plugging these values into the equation, we get the following:
E = -2.178 * 80^2 * 13.606 * 10^-3 eV / 4^2
E = -2.48 eV
For such more questions on electron
https://brainly.com/question/860094
#SPJ8
Which of the isoelectronic pairs you determined above has the same electron configuration? Check all that apply. a. CI^- and Ar b. Sc^3+ and Ar c. Fe^2+ and Cr d. Zn^2+ and Ni e. Sn^4+ and Pd
Answers
The isoelectronic pairs that have the same electron configuration among the options given are:
a. CI^- and Ar
c. Fe^2+ and Cr
Isoelectronic species are atoms or ions that have the same number of electrons, despite being different elements or ions. To determine if two species are isoelectronic, we compare their electron configurations.
a. CI^- and Ar: Chlorine ion (CI^-) has gained an extra electron compared to the neutral chlorine atom (Cl). The electron configuration of CI^- is 1s² 2s² 2p⁶ 3s² 3p⁶, which is the same as the electron configuration of argon (Ar). Therefore, CI^- and Ar are isoelectronic.
c. Fe^2+ and Cr: Iron ion (Fe^2+) has lost two electrons compared to the neutral iron atom (Fe). The electron configuration of Fe^2+ is 1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁶, which is the same as the electron configuration of chromium (Cr). Therefore, Fe^2+ and Cr are isoelectronic.
The other options (b. Sc^3+ and Ar, d. Zn^2+ and Ni, e. Sn^4+ and Pd) do not have the same electron configuration, and thus, they are not isoelectronic pairs.
To learn more about isoelectronic pairs visit: brainly.com/question/31873673
#SPJ11
A student claims that if she wanted to make a solution quickly, she should use small pellets instead of powder along with heating and stirring. Do you agree or disagree with the student's claim? I am confused on this so I would greatly appreciate anyone’s help.
Answers
Answer: Yes, the student is right, one should use pellets of the reactant should be heated and stirred for mixing properly.
Explanation:
In case of smaller particles the surface area that is being exposed increases and the due to this the reaction occurs faster.
Increasing the temperature of the temperature, increases the kinetic energy of the particles which helps in easy mixing of the particles.
The collision in between the particles also increase while stirring and thus the rate of reaction increases.
So, the heating and stirring is more preferred over powered reactant for making a solution quickly.
Balance Equation:__H2O + __ F2 > __HF + __O2
Answers
Explanation:
We have to balance the following equation:
__ H₂O + __ F₂ -------> __ HF + __ O₂
First we have to determine the number of atoms of each element that we have on both sides of the equation.
__ H₂O + __ F₂ -------> __ HF + __ O₂
O: 1 O: 2
H: 2 H: 1
F: 2 F: 1
We have 2 atoms of O on the right side and 1 atom of O on the left side. To balance the O atoms we can change the coefficient for H₂O and write a 2 in front of it.
2 H₂O + __ F₂ -------> __ HF + __ O₂
O: 2 O: 2
H: 4 H: 1
F: 2 F: 1
Then we have 4 atoms of H on the left and 1 atom of H on the right side of the equation. We can change the coefficient for HF to balance the H atoms.
2 H₂O + __ F₂ -------> 4 HF + __ O₂
O: 2 O: 2
H: 4 H: 4
F: 2 F: 4
And finally we have 2 atoms of F on the left and 4 atoms of F on the right. We can change the coefficient for F₂ and write a 2 there.
2 H₂O + 2 F₂ -------> 4 HF + __ O₂
O: 2 O: 2
H: 4 H: 4
F: 4 F: 4
The equation is balanced.
Answer: 2 H₂O + 2 F₂ -------> 4 HF + O₂
Radioisotopes of cobalt-60 and carbon-14 have half-lives, , of 5.3 years and 5,730 years, respectively. If you had 1.00 mg samples of each, what mass of each isotope would be remaining after THREE half-lives
Answers
The half-life of a radioisotope can be used to determine how much mass is left over after the radioisotope has decayed. The time it takes for half of a radioactive sample to decay is called its half-life. The residual mass is halved after each half-life.
For example, the half-life of cobalt-60 is 5.3 years and the half-life of carbon-14 is 5,730 years. The remainder mass is calculated by multiplying the original mass by (1/2) to the power of the number of half-lives after three half-lives.
After three half-lives, approximately 0.211 mg of cobalt-60 and approximately 0.937 mg of carbon-14 will remain. These calculations show how radioactive isotopes decay slowly over time, with the residual mass falling rapidly with each half-life.
Learn more about half-life, here:
https://brainly.com/question/31666695
#SPJ4
you are at sea level and have a beaker with no lid that contains 250 ml of distilled water. what is the water potential of the distilled water in the beaker?
Answers
The water potential of the distilled water in the beaker at sea level is equal to the pressure potential, which is approximately 101.3 kPa.
The water potential of the distilled water in the beaker at sea level can be determined by considering the external factors that can affect water potential. In this case, the external factors are atmospheric pressure and gravity. At sea level, the atmospheric pressure is approximately 101.3 kilopascals (kPa). The pressure potential is the contribution of atmospheric pressure to the overall water potential.
Since the beaker is open and exposed to the atmosphere, the pressure potential is equal to the atmospheric pressure. Gravity also affects the water potential. However, at sea level, the gravitational potential is assumed to be zero because the water is already at the lowest possible elevation. Therefore, the water potential of the distilled water in the beaker at sea level is equal to the pressure potential, which is approximately 101.3 kPa.
Learn more about pressure:
https://brainly.com/question/28012687
#SPJ11
determine how many grams of kno3 would dissolve in 100g of water at 50 degrees celcuis to make a saturated solution
Answers
The amount of KNO₃ would dissolve in 100g of water at 50 degrees celcuis to make a saturated solution is 44 g.
To create a saturated solution of KNO₃ in 100g of water at 50 degrees Celsius, the following steps should be taken:
Step 1: Find out the mass of the solvent in the saturated solution.
The solvent is water in this instance. So, using the formula for the mass of a solution, we can calculate the mass of the solvent in the saturated solution as follows:
Mass of solvent = Mass of solution - Mass of solute
= 100 g - Mass of KNO₃
Step 2: Determine the amount of solute that would be dissolved in the solution to make it saturated.
The mass of KNO₃ that would dissolve in 100 g of water at 50°C to create a saturated solution is 56g/100g of water.
Step 3: Calculate the mass of KNO₃ that would dissolve in 100g of water to make a saturated solution.
Mass of KNO₃ = Solubility of KNO₃ × Mass of solvent
Mass of KNO₃ = 56 g/100 g × (100 g - Mass of KNO₃)
Now, let's solve for Mass of KNO₃;
56 = 56g(100-Mass of KNO₃)/100100 - Mass of KNO₃ = 100
Multiply both sides of the equation by 100 to obtain;
5600 - 100 Mass of KNO₃ = 100
Mass of KNO₃ = 5600/10100 - 56
= 44 g
Therefore, 44 g of KNO₃ would dissolve in 100g of water to make a saturated solution.
Learn more about saturated solution: https://brainly.com/question/1851822
#SPJ11
People can obtain groundwater by drilling a well
Answers
Answer:
yes this is I think a true statement
2. How many moles are in 6.55 x 108 grams of H2O?
3.63x10^7 moles
Answers
Answer:
Who helped Hernán Cortés and his soldiers defeat the Aztec?