high quality charcoal that is light weight and easily broken is made from ______. multiple choice question.
Answers
High quality charcoal that is lightweight and easily broken is made from hardwoods such as oak, maple, and hickory.
Charcoal is a porous, black, carbon-rich material that is produced by heating organic matter (such as wood or coconut shells) in the absence of air. The quality of charcoal can vary depending on the type of organic matter used, as well as the production method.
High quality charcoal that is lightweight and easily broken is typically made from hardwoods such as oak, maple, and hickory. These woods have a high density and a low moisture content, which makes them ideal for producing charcoal with a high carbon content and low ash content. In addition, the heating process used to produce this type of charcoal is typically done at high temperatures for a short period of time, which helps to remove impurities and create a more porous and lightweight material.
Overall, the type of organic matter used and the production method are important factors in determining the quality of charcoal, with hardwoods being a common choice for producing high quality charcoal that is lightweight and easily broken.
To learn more about High quality charcoal, here
https://brainly.com/question/19402908
#SPJ4
Related Questions
¿Cuál es la fórmula química del hidróxido de potasio?
Answers
Answer:
Hidróxido de potasio
Compuesto químico
El hidróxido de potasio es un compuesto químico inorgánico de fórmula KOH. Tanto él como el hidróxido de sodio son bases fuertes de uso común. Tiene muchos usos tanto industriales como comerciales. La mayoría de las aplicaciones explotan su reactividad con ácidos y su corrosividad natural. Wikipedia
Fórmula: KOH
Explanation:
1Write a word equation for the neutralisation reaction between sodium hydroxide and hydrochloric acid.
Answers
The overall equation for the reaction of neutralization is NaOH + HCl → H2O and NaCl.
What is neutralization reaction?A neutralization reaction is a type of reaction n which an acid and a base react to form water and a salt. This involves the combination of Hydrogen ions and hydroxyl ions in order to form water. The neutralization reaction of a strong acid and strong base has a pH equal to value 7.
Neutralization is defined as a type of chemical reaction in which an acid reacts with a base forming salt and water. For example, Reaction between hydrochloric acid and sodium hydroxide to produce salt i.e. sodium chloride and water.
So we can conclude that NaOH + HCl → H2O and NaCl.
Learn more about reaction here: https://brainly.com/question/11231920
#SPJ1
1) Purpose of Acetic Acid?and draw 3 products
Answers
Acetic acid is a clear and colorless liquid that is used in a variety of applications, both industrially and domestically. Its main purpose is as a chemical reagent in the production of various substances, including plastics, textiles, and pharmaceuticals.
Acetic acid is also used as a solvent, meaning that it can dissolve other substances and be used to extract desired chemicals from them.
One of the most common applications of acetic acid is in the food industry. It is used as a preservative in various foods, such as pickles and condiments, to prevent bacterial growth and prolong shelf life. It is also an important ingredient in the production of vinegar, which is widely used as a flavoring and condiment in cooking.
Another important use of acetic acid is in the manufacture of vinyl acetate, which is used in the production of many common household items such as adhesives, paints, and coatings. Acetic acid is also used as a cleaning agent, due to its ability to dissolve dirt and grime.
In conclusion, acetic acid is a versatile chemical that has numerous applications in both industry and everyday life. Its ability to dissolve other substances and act as a preservative makes it a valuable ingredient in a wide range of products, from pharmaceuticals to foodstuffs and household cleaners.
to know more about acetic acid click this link
brainly.in/question/8384
#SPJ11
Someone Help Me Pls
Describe the relationship between Chemistry and PCR test
Answers
Answer:
In Chemistry, proteins are defined as biomolecules that are essential for the human body to maintain homeostasis. In a PCR test, short for Polymerase Chain Reaction test, scientists are tracking for a certain chemical substance in our body that may be present in the process of protein synthesis or DNA replication that could possibly show if a person is positive for the Corona Virus.Therefor, the relation between Chemistry and the PCR test is that, without Chemistry, the PCR test would not be able to stand on its own, which in other words means the PCR test is dependent upon the presence of Chemistry.
ABC common stock is expected to have extraordinary growth in earnings and dividends of 20% per year for 2 years, after which the growth rate will settle into a constant 6%. If the discount rate is 15% and the most recent dividend was $2.50, what should be the approximate current share price
Answers
The approximate current share price of ABC common stock can be calculated using the dividend discount model. The model takes into account the expected growth rate of earnings and dividends, the discount rate, and the most recent dividend.
The dividend discount model is commonly used to estimate the intrinsic value of a stock based on the present value of its expected future dividends. In this case, we have two years of extraordinary growth followed by a constant growth rate. To calculate the current share price, we need to determine the present value of dividends for the extraordinary growth period and the constant growth period.
First, we calculate the present value of dividends during the extraordinary growth period. We use the formula: Present Value = Dividend / (1 + Discount Rate)^t, where t is the number of years. In this case, we have two years of extraordinary growth, so we calculate the present value of dividends for each year.
Next, we calculate the present value of dividends during the constant growth period using the formula: Present Value = Dividend * (1 + Constant Growth Rate) / (Discount Rate - Constant Growth Rate).
Finally, we sum up the present values of dividends for both periods to obtain the approximate current share price.
Please note that the calculation involves several assumptions and approximations, and it's important to consider other factors and conduct a thorough analysis before making investment decisions.
Learn more about ABC common stock here:
https://brainly.com/question/31129940
#SPJ11
Balance the equation by putting the correct number in front of each compound. You can work this out mathematically or by drawing pictures on a piece of paper like the videos.
Al + 02--> Al203 corrosion of aluminum
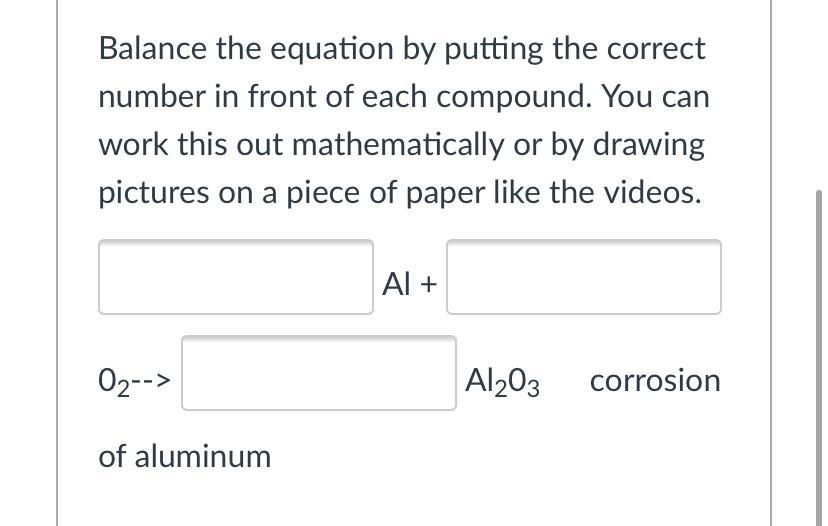
Answers
Answer:
4Al + 3O₂ —> 2Al₂O₃
The coefficients are: 4, 3, 2
Explanation:
__ Al + __ O₂ —> __ Al₂O₃
The above equation can be balance as illustrated below:
Al + O₂ —> Al₂O₃
There are 2 atoms of O on the left side and 3 atoms on the right side. It can be balance by writing 3 before O₂ and 2 before Al₂O₃ as shown below:
Al + 3O₂ —> 2Al₂O₃
There are 4 atoms of Al on the right side and 1 atom on the left side. It can be balance by writing 4 before Al as shown below:
4Al + 3O₂ —> 2Al₂O₃
Now the equation is balanced.
The coefficients are: 4, 3, 2
9. How many grams of zinc are needed to produce 12 grams of zinc chloride according to the following equation? Y-
Zn + Cl2 --> ZnCl2
12 grams of zinc
O 2.9 grams of zinc
O 11.6 grams of zinc
O 5.8 grams of zinc
Answers
Given :
A balanced chemical equation :
\(Zn +Cl_2->ZnCl_2\)
To Find :
How many grams of zinc are needed to produce 12 grams of zinc chloride.
Solution :
Moles of \(ZnCl_2\) ,
\(n=\dfrac{Given \ wt}{Molecular\ Mass}\\\\n =\dfrac{12}{136.30}\ mol\\\\n=0.088\ mol\)
Now, by balanced chemical equation we can say that 1 mol of Zn produce
1 mol of \(ZnCl_2\) .
So, 0.088 mol of Zn is required to produced 0.088 mol of \(ZnCl_2\) .
\(Mass \ required = molecular \ mass \times moles\\\\m = 65.38 \times 0.088\\\\m=5.8 \ gm\)
Therefore, 5.8 grams of zinc is required.
Hence, this is the required solution.
which of these does an open office or microsoft office wizard do?
Answers
Answer:
C. guides you through the steps to complete a complex task.
Explanation:
mark me brainliest!!
You are given the following information about an unknown
substance & you need to determine whether it is an acid;
base, or neutral.
It is corrosive e reacts with carbonates
Answers
The substance can be classified as an acid. The fact that it is corrosive and reacts with carbonates suggests that it has acidic properties.
How do the corrosiveness, reaction with carbonates, low pH value indicating excess hydrogen ions (H+) ?In addition to being corrosive and reacting with carbonates, the substance is likely to have a low pH value, indicating the presence of excess hydrogen ions (H+). This further supports the classification of the substance as an acid.
Furthermore, when the substance reacts with metals, it may produce hydrogen gas. This is a common characteristic of acids, as they can release hydrogen ions when in contact with reactive metals. Therefore, based on its corrosiveness, reactivity with carbonates, and potential hydrogen gas production, it is highly likely that the substance is an acid.
Acids have the ability to donate protons (H+) in aqueous solutions. If the unknown substance exhibits this behavior, it further supports its classification as an acid. Furthermore, acids can turn blue litmus paper red and have a sour taste. These observations can be used as additional evidence to confirm that the substance is an acid.
Learn more about Carbonates
brainly.com/question/22530423
#SPJ11
What is the bond angle that corresponds to the
geometry associated with C1 in the structure?
A. 120° B. 90O
C. 1800
D. 109.5o
7. Between which bonded elements in the above
structure is there the strongest dipole moment?
A. Carbon to Carbon B. Hydrogen to Carbon
C. Oxygen to Carbon D. Oxygen to Hydrogen
8. What is the geometry associated with the C2 molecule in the structure?
A. linear B. trigonal planar C.bent D. tetrahedral
9. Among the choices below, which correctly describes the bonding taking place in the above structure?
A. 6 sigma bonds, 1 pi bond C. 6 sigma bonds, 2 pi bonds
B. 7 sigma bonds, 1 pi bond D. 7 sigma bonds, 2 pi bonds

Answers
What is the frequency of an x-ray wave with an energy of 2×10 to the power of -17 jewels in hertz
Answers
The frequency of an x-ray wave with an energy of 2×10^(-17) J in hertz is 3.01 × 10^(16) hz.
We can find the value of frequency of an x ray wave by using the relation between energy and frequency.
As we know that,
Energy is directly proportional to the frequency of the wave.
Mathematically,
E = h. f
Where,
E is the energy in joule
f is the frequency of the wave in hertz
h is the planck's constant
Given,
E = 2 × 10^(-17)J
h = 6.63 × 10^(-34) kg.m2/s
By substituting all the values, we get
2 × 10^(-17)J = 6.63 × 10^(-34) kg.m2/s × f
f = 2 × 10^(-17)J / 6.63 × 10^(-34) kg.m2/s
f = 3.01 × 10^(16) hz.
Thus, we concluded that the frequency of an x-ray wave with an energy of 2×10^(-17) J in hertz is 3.01 × 10^(16) hz.
learn more about frequency:
https://brainly.com/question/14419982
#SPJ1
The temperature
of a thermometer
increases during a chemical reaction.
What happens to the energy of the
reaction (system)?

Answers
Answer:
The reaction absorbs energy ( + or exothermic)
Explanation:
When the temperature of a thermometer increases during a chemical reaction, then the reaction absorbs energy (+ or endothermic). Therefore, the correct option is B.
A rise in the temperature of a thermometer during a chemical reaction is a sign that the system is absorbing energy. This implies that the reaction is endothermic, meaning that energy must be supplied for it to proceed. Generally, endothermic reactions take heat from the environment and raise the temperature. Chemical bonds are broken and the process is accelerated by the absorbed energy.
Therefore, the correct option is B.
Learn more about endothermic reaction, here:
https://brainly.com/question/28909381
#SPJ2
SUB TO RahimZ for 15 points i want 100 subs
Answers
What is the acceleration of the object’s motion? 0.5 m/s2 -0.5 m/s2 2 m/s2 -2 m/s2

Answers
Answer: -2m/s2
Explanation:
Using the following equation ; acceleration = Change in velocity / time
i.e a = v - u / t
where 'a' = acceleration
v = final velocity
u = initial velocity
t = time
Therefore; from the graph we have acceleration to be, 0 - 6m/s / 3s = -2m/s2
Ca(CO3) + 2HCl --> CaCl2 + H2O + CO2Assume you already found the BCA table for this formula and there should be 4.397g of CO2 at the end.If 1.55g of CO2 were produced, how many moles of Ca(CO3) were consumed?
Answers
If 1.55g of \(CO_2\) were produced, the number of moles of \(Ca(CO_3)\)consumed is 0.03523 mol.
The reaction's balanced chemical equation is:
\(Ca(CO_3)\) + 2\(HCl\) → \(CaCl_2\) +\(H_2O\) + \(CO_2\)
From the equation, we can see that 1 mole of \(Ca(CO_3)\) reacts to produce 1 mole of \(CO_2\) . Therefore, the number of moles of \(Ca(CO_3)\) consumed is equal to the number of moles of \(CO_2\) produced.
The molar mass of \(CO_2\) is:
M\((CO_2)\) = 12.01 + 2(16.00) = 44.01 g/mol
The mass of \(CO_2\) that should be produced according to the balanced equation is:
m\((CO_2)\) = 4.397 g
The total number of moles \(CO_2\) generated is
n\((CO_2)\) = m\((CO_2)\) / M\((CO_2)\) = 4.397 g / 44.01 g/mol = 0.09995 mol
Since 1 mole of \(Ca(CO_3)\) reacts to produce 1 mole of \(CO_2\), the number of moles of \(Ca(CO_3)\) consumed is also 0.09995 mol.
If only 1.55 g of \(CO_2\) was produced, we can find the number of moles of \(Ca(CO_3)\) consumed as follows:
m\((CO_2)\) = n\((CO_2)\) x M\((CO_2)\)
n\((CO_2)\) = m\((CO_2)\)/ M\((CO_2)\) = 1.55 g / 44.01 g/mol = 0.03523 mol
Therefore, 0.03523 mol \(Ca(CO_3)\) is consumed
For more such questions on moles, click on:
https://brainly.com/question/13314627
#SPJ11
Arrange He,Br2 and NaCl in increasing boiling point and explain why
Answers
Answer:
fdsfesd
Explanation:
fesdfes
How many moles of carbon tetrachloride (CCI4) can be produced from reacting 100.0 grams of chlorine (CI2) with excess methane?
CH4 +4CI2 CCI4+ 4HCI
Answers
The number of moles that can be produced from reacting 100.0 grams of chlorine with excess methane are 53.83 g CCl₄
What are moles?The mole is a SI unit of measurement that is used to calculate the quantity of any substance.
The reaction is:
CH₄+ 4Cl₂ → CCl₄ + 4HCl
(100.0 g)/(70.9 grams/mole) = 1.4104 moles Cl₂
Now use the molar ratio of 4 to determine the moles of CCl₄
(1.41 moles Cl₂) x (1 mole CCl₄/4 mole Cl₂) = 0.35 moles CCl₄
Multiply moles CCl₄ by its molar mass to get grams
0.35 moles CCl₄) x (153.8g/mole) = 53.83 g CCl₄
Therefore, the moles of carbon tetrachloride (CCl₄) that can be produced are 53.83 g CCl₄.
To learn more about moles, visit here:
https://brainly.com/question/17217973
#SPJ1
What does the identity of an element depend on the number of?
A. protons in the atom
B. electrons in the atom
C. protons and neutrons in the nucleus
D. neutrons in the atom
Answers
Answer: A. protons in the atom
Explanation: I just took the test and got a 100% so this should be right. Unless the question is worded differently than mine then it should be right. Hope this helps :)
Answer:
A
Explanation:
Which of the following are said to be physical properties of gases?
Select one:
a.
The atoms or molecules of a gas are relatively far apart.
b.
A gas expands to fill the space in a closed container.
c.
A gas is easily compressed.
d.
All of the above.
e.
Answers A and B only.
Answers
Answer:
Gases have three characteristic properties: (1) they are easy to compress, (2) they expand to fill their containers, and (3) they occupy far more space than the liquids or solids from which they form.
SO the answer is- D. All of the above
Explanation:
5. What charge does iron have in iron (111) oxide?
Answers
Answer:
0
Explanation:
The entropy change of the surroundings can be calculated from _________ of the reaction if temperature is known.
Answers
The entropy change of the surroundings can be calculated from the enthalpy change (ΔH) of the reaction if the temperature is known.
This relationship is described by the equation:
ΔS_surroundings = -ΔH / T
where ΔS_surroundings is the entropy change of the surroundings, ΔH is the enthalpy change of the reaction, and T is the temperature in Kelvin. The negative sign indicates that the entropy change of the surroundings is opposite in sign to the enthalpy change of the reaction. Entropy is a concept used in various fields, including physics, information theory, and statistics. The term "entropy" refers to the measure of disorder or uncertainty in a system.
Learn more about entropy here : brainly.com/question/20166134
#SPJ11
As a Formulation chemist, you're required to do a diet (dark) chocolate D optimal (experimental design) Table with variables and response factors ( viscosity, polyphenol content, fat content). How would you do the D optimal design table? (Note!! You can use other literature papers or other online papers to check how it's done. Also you don't have to have the results for the response factors but you need values on how you would set up the variables).
Answers
By following these steps, you can create a D optimal design table for a diet (dark) chocolate formulation, which will help optimize the variables and response factors for your experiment.To create a D optimal design table for a diet (dark) chocolate formulation, follow these steps:
1. Identify the variables: Start by listing the variables that may affect the desired response factors. In this case, the variables could include cocoa percentage, sugar content, emulsifier type, and temperature during processing.
2. Determine the response factors: Identify the response factors that you want to measure and optimize. In this case, the response factors could be viscosity, polyphenol content, and fat content.
3. Use a statistical software or online tool: Utilize statistical software or online tools specifically designed for experimental design, such as Design-Expert or JMP. These tools can help generate a D optimal design table based on the identified variables and response factors.
4. Set up the design table: Enter the identified variables and their corresponding levels in the software/tool. For example, cocoa percentage can be set at levels of 60%, 70%, and 80%, while sugar content can be set at levels of 20%, 30%, and 40%.
5. Specify the number of experimental runs: Decide on the number of experimental runs you want to conduct. A D optimal design table will suggest the most efficient and informative number of runs based on the specified variables and desired level of accuracy.
6. Run the experiments: Follow the experimental plan provided by the D optimal design table and conduct the experiments accordingly. Make sure to record the values of the response factors for each run.
To know more about software, visit at:
https://brainly.com/question/32393976
#SPJ11
Unilt lest
Unit Test Active
TIME REMAINING
01:22:52
Which shower would most likely be the control group in this
study?
Mildew is a fungus that grows in wet areas and can slowly
grow from microscopic to large stains in showers and sinks
Researchers are studying different sprays that could be used
to slow down the growth of mildew. They set up different
showers that are exactly the same and expose them each to
water with the same microscopic amount of mildew. They
then spray some showers with mildew-preventing sprays and
observe how fast mildew grows over time.
O the shower sprayed with many test sprays
the shower not exposed to any mildew at all
O the shower not sprayed with any mildew-preventing spray
O the shower sprayed with a test spray that is already
known to work
Answers
Answer:
Option C, the shower not sprayed with any mildew-preventing spray is most likely be the control group in this study
Explanation:
Complete question
Mildew is a fungus that grows in wet areas and can slowly grow from microscopic to large stains in showers and sinks. Researchers are studying different sprays that could be used to slow down the growth of mildew. They set up different showers that are exactly the same and expose them each to water with the same microscopic amount of mildew. They then spray some of the showers with mildew-preventing sprays and observe how fast mildew grows over time. Which show would most likely be the control group in this study? A- the shower sprayed with many test sprays B- the shower not exposed to any mildew at all C- the shower not sprayed with any mildew-preventing spray D- the shower sprayed with a test spray that is already known to work
Solution
In any experiment, a control group is a group of participants that is not exposed to any treatment so that it can be compared with the experimental group on which the treatment is applied/given.
In this case - experimental group are the stained showers that are subjected to the different types of mildew test sprays while the control group is the the shower not sprayed with any mildew-preventing spray.
Hence, option C is the correct answer
If 0.327 g of an unknown metal completely reacts with 10.00 ml, of 1.00 M HCl according to Eq.2, calculate the molar mass of the unknown metal. Identify the metal from its molar mass. A. Calculate the moles of acid added to the beaker. B. Calculate the moles of metal that reacted with the moles of acid in 1A. C. Determine the molar mass of the metal. D. Identify the unknown metal. ___
Answers
0.0100 moles of acid were added to the beaker. 0.00500 moles of metal reacted with the acid. The molar mass of the metal is 65.4 g/mol. The unknown metal is most likely Zinc (Zn).
A. To calculate the moles of acid added to the beaker, we need to use the equation:
moles of acid = concentration of acid x volume of acid
Here, the concentration of acid is 1.00 M (given in the question) and the volume of acid is 10.00 ml (also given in the question). However, we need to convert the volume to liters to match the unit of concentration. So,
Volume of acid = 10.00 ml = 0.01000 L
Now, we can calculate the moles of acid:
moles of acid = 1.00 M x 0.01000 L = 0.0100 moles
Therefore, 0.0100 moles of acid were added to the beaker.
B. According to the equation given in the question (Eq.2), the reaction between the metal and HCl is:
Metal + 2HCl → MetalCl\(^{2}\) + H\(^{2}\)
From this equation, we can see that one mole of metal reacts with two moles of HCl. Therefore, the moles of metal that reacted with the moles of acid in part A can be calculated as:
moles of metal = 0.0100 moles of acid x (1 mole of metal/2 moles of acid) = 0.00500 moles
Therefore, 0.00500 moles of metal reacted with the acid.
C. To determine the molar mass of the metal, we can use the equation:
molar mass = mass of metal/moles of metal
From the question, we know that the mass of metal that reacted is 0.327 g (given in the question) and the moles of metal are 0.00500 moles (calculated in part B). Substituting these values in the equation, we get:
molar mass = 0.327 g/0.00500 mol = 65.4 g/mol
Therefore, the molar mass of the metal is 65.4 g/mol.
D. To identify the unknown metal, we need to compare its molar mass with the molar masses of known elements. From the periodic table, we see that the molar mass of the closest element to 65.4 g/mol is Zinc (Zn), which has a molar mass of 65.4 g/mol. Therefore, the unknown metal is most likely Zinc (Zn).
More on molar mass and moles: https://brainly.com/question/30319334
#SPJ11
How many moles are there in 7.4 X 1023 molecules of AgNO3?
Answers
PLEASE HELP!
Which of the following is an example of a chemical change?
A. A large nail rusting
B. Folding a piece of paper
C. Filling a balloon with hot air
D. Wax melting from a candle
Answers
Answer: A. a large nail rusting
Explanation:
A) A large nail rusting
Explanation:
A nail rusting is a chemical change because the iron has changed into a new substance. It is the iron (Fe) and oxygen (O) combining.
Chemical change:
When a chemical reaction occurs and a new substance is made.
E.g. Burning wood
Physical change:
When matter change form but not chemical identity.
E.g. Melting ice
Hope this helped you!
Can someone please please help me with the first two !!!! I really need to turn this in !!!!!

Answers
Answer:
Be Electron configuration: 1s2 2s2
Be Orbital Diagram: \//\ \//\ (it would be little arrows going up and down to show the spins)
F Electron Configuration: 1s2 2s2 2p5
F Orbital Diagram: \//\ \//\ \//\ \//\ \/
Which statement best describes the kinetic molecular theory?
A.the kinetic molecular theory relates the properties of a state of matter to the motion of its molecules
B.the kinetic molecular theory relates the properties of a state of matter to the mass of its molecules
C. The kinetic molecular theory relates the properties of a state of matter to the size of its molecules.
D. The kinetic molecular theory relates the properties of a state of matter to the diameter of its molecules
Answers
Answer:A.the kinetic molecular theory relates the properties of a state of matter to the motion of its molecules
When refering to the kinetic molecular theory, it refers to the belief that all things in the Universe are made of smaller particles called molecules, which are made of smaller parts called atoms. When we look at a solid, the particles are packed closely together, which is why the solid holds a shape. The same goes for gas, the particles are spaced out from one another, which is why the don't take a shape. While a liquid takes the shape of the container it is in, because the particles are far, but not far enough to seperate a gas until being boiled. I added a picture to better show the relation.
I hope this helps & Good Luck <3!!!

you have samples of each of the following gases, all at 25 °c and one atmosphere pressure. which sample has the lowest density?
a. Ammonia
b. Argon
c. Carbon dioxide
d. Nitrogen dioxide
e. Oxygen
Answers
Answer:
Yeah. Mhm. So on this question, it asked about finding the highest density for a few gasses all at the same temperature. And since gasses will all have the same volume, if they have the same number of moles, your density would be The mass of each over the volume of 25°C. Now the volume of 0°C is 22.4. In order to find the volume at 25°C, you could do PV Equals NRT and plug in one mole, 25°C Plus 2 73. Right to get to Calvin's. Um The pressure value which I don't believe was given in the question of one atmosphere of pressure. Okay, so one atmosphere of pressure, the R value and atmospheres and then you would find the volume. Each of them. You would take their molar mass over that volume. Or if all you want to do is rank them. The highest molar mass would be the most dense. So if you needed the actual density, okay, you would do the molar mass Over the volume. For one mole, each gas would have the same volume. But if all you have to do is rank them. The one with the highest molar mass is the most ends and the one with the lowest molar mass would be the least ends.
Explanation:
PLEASE MARK AS BRAINLIEST
Electrolytic Cells and the Determination of Avogadro’s Number What are some possible sources of error in this experiment? Would solid sodium chloride conduct electricity? And why. What did you notice about the solution as the experiment proceeded?
Answers
In an experiment involving electrolytic cells and the determination of Avogadro's number, some possible sources of error may include inaccuracies in measurements, impurities in the electrolyte solution, or inconsistencies in the current applied during the experiment.
Solid sodium chloride does not conduct electricity because its ions are locked in a crystal lattice, which prevents the free movement of ions necessary for electrical conduction. However, when sodium chloride dissolves in water, it forms an electrolyte solution with freely moving ions, which can conduct electricity.
As the experiment proceeds, you may observe a change in the solution, such as the formation of gas bubbles at the electrodes due to the redox reactions occurring. These observations are important as they indicate the progress of the electrolysis process, which helps in the determination of Avogadro's number.
Overall, maintaining accurate measurements, using pure solutions, and ensuring consistent current application can help reduce the potential sources of error in such an experiment, leading to a more accurate determination of Avogadro's number.
Know more about Avogadro's number here :
brainly.com/question/1513182
#SPJ11