Answers
Answer:
Compaction.
Explanation:
All the others are before this.
Related Questions
PLZ HELP ASAP! I need this done tonight please
The importance of wearing a seatbelt while riding in the car can best be explained using:
A) Newton's First Law (Law of Inertia)
B) Newton's Second Law (F=ma)
C) Newton's Third Law (Action-Reaction)
D none of the above
Answers
Explanation:
this is a chemistry question ???
anyway, it is clearly A, as seatbelts try to counteract the inertia a moving body (our bodies riding in a car) has, when the environment (the car) of this body is suddenly coming to a stop.
this inertia would otherwise try to move the body still forward resulting in a headfirst crash into and often through the windshield. which causes much more damage to the body than the bruises caused by the pressure of the seatbelts against the body counteracting the body's inertia.
but also clearly, all 3 laws play a role.
the force with which the moving body has to deal when smashing into the windshield and potentially then other objects outside the car is described by the second law.
and for any action (crash) there are corresponding reactions (like the conversion of the crash energy into some absorbing reaction : damaged soft and hard tissue in the human body, crumbled car bodies,...).
Which of the following bulk properties of substances are affected by intermolecular forces? Select all that apply.
A ductility
B. boiling point
C volatility
D. malleability
Emelting point
Answers
We have that the properties of substances are affected by inter molecular forces is
boiling pointOption BFrom the question we are told
Which of the following bulk properties of substances are affected by intermolecular forces
Molecules
Generally inter molecular forces increase as the boiling point of a substance increase
Therefore
it is only right to say
boiling point
Option B
For more information on Molecules visit
https://brainly.com/question/19922822
what is the full meaning of UPAC
Answers
International Union of Pure and Applied Chemistry is known as IUPAC.
The International Union of Pure and Applied Chemistry is a global federation of National Adhering Organizations that aims to advance the chemical sciences, particularly through developing nomenclature and terminology. The International Science Council includes it along with its members.Describe the IUPAC formula.The nomenclature (naming scheme) of the IUPAC (International Union of Pure and Applied Chemistry) is applied for naming organic compounds. This will ensure that the names are consistent. Additionally, it allows for the naming of each compound, which is not possible with the common names now in use.How are IUPAC names written?In conclusion, the compound's name is written out with the substituents listed alphabetically before the base name (derived from the number of carbons in the parent chain). Between numbers and letters are separated by dashes and commas, respectively. The name has no spaces.
To learn more about IUPAC visit:
https://brainly.com/question/16631447
#SPJ9
Vertical columns are know as
Answers
Answer:
The vertical columns on the periodic table are called groups or families because of their similar chemical behavior. All the members of a family of elements have the same number of valence electrons and similar chemical properties. The horizontal rows on the periodic table are called periods
Explanation:
glucose 6‑phosphate+H2O⟶glucose+Pi glucose 6‑phosphate+H2O⟶glucose+Pi K′eq1=270 K′eq1=270 ATP+glucose⟶ADP+glucose 6‑phosphate ATP+glucose⟶ADP+glucose 6‑phosphate K′eq2=890 K′eq2=890 Using this information for equilibrium constants determined at 25∘C,25∘C, calculate the standard free energy of hydrolysis of ATP. standard free energy:
Answers
Answer:
-30.7 kj/mol
Explanation:
The standard free energy for the given reaction that is the hydrolysis of ATP is calculated using the formula: ∆Go ’= -RTln K’eq
where,
R = -8.315 J / mo
T = 298 K
For reaction,
1. K′eq1=270,
∆Go ’= -RTln K’eq
= - 8.315 x 298 x ln 270
= - 8.315 x 298 x 5.59
= - 13,851.293 J / mo
= - 13.85 kj/mol
2. K′eq2=890
∆Go ’= -RTln K’eq
= - 8.315 x 298 x ln 890
= - 8.315 x 298 x 6.79
= - 16.82 kj/mol
therefore, total standard free energy
= - 13.85 + (-16.82)
= -30.7 kj/mol
Thus, -30.7 kj/mol is the correct answer.
When carbon is burned in air, it reacts with oxygen to form carbon dioxide. When 20.4 g g of carbon were burned in the presence of 70.4 g
of oxygen, 16.0 g of oxygen remained unreacted. What mass of carbon dioxide was produced?
Answers
Answer:
C + O2 -> CO2
C 20.4/12 = 1.7
O 70.4-16.0/16=3.4
CO2 1.7 mol * 44 = 74.8
At point a the object has 30 joules of energy. how much energy does the object have at point b?
Answers
Answer:
It’s 10
Explanation:
I looked it up
To solve this we must be knowing each and every concept related to energy and its calculations. Therefore, at point b, 10joule of energy is present.
What is energy?Energy is defined in physics as the ability to accomplish work or heat items. It's a scalar measurement with magnitude but no direction. Energy is maintained, which implies it can change forms but is not generated or destroyed. potential energy transforms into kinetic energy.
One type of energy can be transferred into another without breaking a thermodynamic rule. Not all of these energy sources are equally beneficial in practical applications. At point a the object has 30 joules of energy. At point b, 10joule of energy is present.
Therefore, at point b, 10joule of energy is present.
To learn more about energy, here:
https://brainly.com/question/29763772
#SPJ5
How many atoms are there in 90.2 g of selenium?
Answers
Answer:First calculate the moles of selenium by multiplying the mass x molar mass 90.2 × 78.96 = 7122.2 moles. Number of moles multiplied by ...
Explanation:
At 25∘C, the Henry's law constant for CO2 is 0.034Matm. What pressure of carbon dioxide is needed to maintain a CO2 concentration of 0.80 M?
Answers
Answer:
P = (Henry's law constant) * (CO2 concentration)
P = (0.034 M/atm) * (0.80 M) = 0.027 atm.
In order to maintain a CO_2 concentration of 0.80 M at 25 degrees Celsius, a pressure of about 23.53 atm would be needed according to Henry's Law.
In the field of Chemistry, Henry's law is used to describe the solubility of gases in liquids. This law states that at a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with the liquid.
Using the given values from the problem, where the Henry's Law constant (KH) is 0.034 M⋅atm and the desired CO_2 concentration (C) is 0.80 M, we can insert these into the formula for Henry's law: P = C/KH.
By substituting the relevant values, you get P = 0.80 M / 0.034 M⋅atm which equates to approximately 23.53 atm. Therefore, a pressure of about 23.53 atm of carbon dioxide would be needed to maintain a CO_2 concentration of 0.80 M at 25 degrees Celsius.
Learn more about Henry's Law here:
https://brainly.com/question/35540348
#SPJ2
A sample is found to contain 57.2 % N a H C O 3 NaHCOX3 by mass. What is the mass of NaHCO 3 in 4.25 g of the sample
Answers
Answer:
The mass of N a H C O 3 present is 2.431 g
Explanation:
The sample contains 57.2 % N a H C O 3 by mass.
To find the mass of N a H C O 3 in the sample, we need to find what the equivalent of 57.2 %.
Mass of N a H C O 3 = Percentage Composition * Mass of sample
Mass of N a H C O 3 = 57.2 / 100 * 4.25
Mass of N a H C O 3 = 2.431 g
The mass of N a H C O 3 present is 2.431 g
The mass of the compound (NaHCO₃) contained in 4.25 g is 2.431 g.
The given parameters;
percentage composition of the compound, = 57.2%mass of the compound (NaHCO₃) = 4.25The mass of the compound (NaHCO₃) contained in 4.25 g is calculated by the finding its equivalent in the given percent composition as follows;
\(mass \ = \frac{57.2}{100} \times 4.25\\\\mass = 2.431 \ g\)
Thus, the mass of the compound (NaHCO₃) contained in 4.25 g is 2.431 g.
Learn more about percentage composition here: https://brainly.com/question/20065048
What is the mass in grams of 33.3 moles of NaCl?
Answers
Answer:
33.3 moles of NaCI = 1946.144241 grams
Explanation:
SORRY THIS IS SO LATE YOU PROBABLY GOT IT ALREADY BUT ANYWAY I HOPE YOU HAVE AN AMAZING DAY AND GOOD BYE
Answer:
1946.144241
Explanation: I searched it up it is correct
electrons are transferred from atoms of phosphorus to atoms of sodium
Answers
Electrons are transferred from atoms of sodium to atoms of phosphorous The sodium atom looses electron and the phosphorus atoms gains electrons.
What is an electron ?The electron is a subatomic particle with an elementary electric charge of -1. Electrons are the first generation of the lepton particle family and are widely regarded as elementary particles due to the lack of known components or substructure.
This transfer causes the sodium atoms to acquire positive charge and phosphorous to acquire negative charge .
Thus, the sodium and phosphorus atoms strongly attract with each other.
To learn more about an electron, follow the link;
https://brainly.com/question/1255220
#SPJ1
Your question is incomplete, most probably, your question was
Complete the paragraph about the formation of sodium phosphide.
Electrons are transferred from atoms of
and the phosphorus atoms
to atoms of
C. This transfer makes the sodium atoms
As a result, the sodium and phosphorus atoms strongly
each other.
Two observations that indicate an organic liquid is dry when using the drying agent:
Answers
Two observations that can indicate that an organic liquid is dry when using the drying agent are:
When it's observed that the drying agent no longer clumps.When the organic layer isn't cloudy anymore (has become clear).Organic liquid is liquid that contains volatile organic compounds (often shorten to VOCs), such as crude oils and petroleum distillates. It is used in many stuff, ranging from paints, lacquers, glues, and adhesives; even in the production of dyes, textiles, and pharmaceuticals.
Drying agents are things that absorb moisture from their vicinity. They can be used to remove trace amounts of water from an organic solution. Some examples of drying agents are sodium metal or calcium hydride.
Learn more about drying agents at https://brainly.com/question/24174657
#SPJ4
_Pb(OH)2+_HCl -> _H2O+_PbCl2
Answers
Answer:
20 + CIPb
Explanation:
Use the commutateive proptery to reoder the terms
Answer:
PB(OH)2+ 2HCl --> 2H2O + PbCl2
PLEASE HELP QUICKLY!!!
HI gas is removed from the system
at equilibrium below. How does the
system adjust to reestablish
equilibrium?
51.8 kJ + H₂(g) + 1₂(g) = 2HI(g)
A. The reaction shifts to the right (products) and the concentrations
of I, and H₂ decrease.
B. The reaction shifts to the left (reactants) and the concentrations
of H₂ and I increase.
C. The reaction shifts to the right (products) and the concentrations
of I, and H₂ increase.
D. The reaction shifts to the left (reactants) and the concentration of
HI increases.

Answers
Answer:
A. The reaction shifts to the right (products) and the concentrations of I and H₂ decrease.
Explanation:
If gas is removed from the system at equilibrium, the system will try to compensate for the loss by shifting the reaction in a direction that produces more gas molecules. This is known as Le Chatelier's principle, which states that a system at equilibrium will respond to a disturbance by shifting in a way that minimizes the effect of the disturbance.
In this case, since gas is being removed from the system, the reaction will shift to the side that produces more gas molecules. Looking at the balanced equation, we can see that 2HI(g) has a greater number of gas molecules compared to H₂(g) and I₂(g). Therefore, the system will shift to the right (products) to produce more HI(g) and reestablish equilibrium.
13.41g of Fe(NO3)2 is dissolved in 83.0mL of water. Calculate the molarity of the solution.
Answers
Answer:
whats the question in this
Explanation:
Look at sample problem 19.10 in the 8th ed Silberberg book. Write the Ksp expression. Find the concentrations of the ions you need (in this case Ca2 and F-). Put those concentrations into the Ksp expression to calculate Q. Compare Q to K A common laboratory method for preparing a precipitate is to mix solutions containing the component ions. Does a precipitate form when 10. ml of 0.0010 M Ca(NO3)2 is mixed with 10. ml of 0.00010 M NaF
Answers
Answer:
Explanation:
From the information given:
\(CaF_2 \to Ca^{2+} + 2F^-\)
\(Ksp = 3.2 \times 10^{-11}\)
no of moles of \(Ca^{2+}\) = 0.01 L × 0.0010 mol/L
no of moles of \(Ca^{2+}\) = \(1 \times 10^{-5} \ mol\)
no of moles of \(F^-\) = 0.01 L × 0.00010 mol/L
no of moles of \(F^-\) = \(1 \times 10^{-6}\ mol\)
Total volume = 0.02 L
\([Ca^{2+}}] = \dfrac{1\times10^{-5} \ mol}{0.02 \ L} \\ \\ \\ \[[Ca^{2+}}] = 0.0005 \ mol/L\)
\([F^{-}] = \dfrac{(1\times 10^{-6} \ mol)}{0.02 \ L}\)
\([F^{-}] = 5 \times 10^{-5} \ mol/L\)
\(Q = [Ca^{2+}][F^-]^2 \\ \\ Q = 0.0005 \times (5\times 10^{-5})^2 \\ \\ Q = 1.25 \times 10^{-12}\)
Since Q<ksp, then there will no be any precipitation of CaF2
Metric system is also known as the (use the amount of letters in 16 across) WILL MARK BRAINLIEST:))

Answers
Answer:
Si System
hope this helped!
What does the Coriolis effect impact?
O amount of radiation from the sun
O wind direction
O precipitation levels
o wind speed
Answers
It should be wind direction
Explanation:
I hope this helps. Let me know if I am wrong
Based on the "Reactivity in Substitution Reactions" experiment, which molecule would be expected to react the fastest using AgNO3 in water-ethanol ?
Answers
Answer:
C) EtOH 1% AgNO3
An endergonic reaction with a Δh and Δs can be changed into an exergonic reaction.
a. True
b. False
Answers
Full question:
This question is incomplete, here it is completed:
An endergonic reaction with a ______ ∆H and a ______ ∆S can be changed into an exergonic reaction by decreasing the temperature.
Option A: negative, positive
Option B: negative, negative
Option C: positive, positive
Option D: positive, negative
Answer:
Option B: negative, negative
Explanation:
The change in free energy (ΔG) of a system for a constant-temperature process is
ΔG = ΔH - TΔS
free energy is the energy available to do work. Thus, if a particular reaction is accompanied by a release of usable energy (that is, ΔG is negative), it is said to be exergonic. On the other hand, if a reaction consumes energy (that is, ΔG is positive), it is said to be endergonic.
Looking at the equation, we can see that if ΔH is negative and ΔS is negative, then ΔG will be negative only when TΔS is smaller in magnitude than ΔH. This condition is met when T is small.
ΔG = ΔH - TΔS
- -
This means that the reaction proceeds spontaneously at low temperatures. At high temperatures, the reverse reaction becomes spontaneous. An example of that would be the following reaction:
NH₃(g) + HCl(g) → NH₄Cl(s)
Does sound travel faster in a warm room or a cold room? Explain your answer.
plssss help me I need the answer

Answers
Answer:
Sound travels faster in a warm room than a cold room because particles bump into each other more often, increasing the chances of energy transfer, and the waves travel faster.
Consider an element Z that has two naturally occurring isotopes with the following percent abundances: the isotope with a mass number of 19.0 is 55.0% abundant; the isotope with a mass number of 21.0 is 45.0% abundant. What is the average atomic mass for element Z?
Average atomic mass of Z = [mass]
Answers
Answer:
Average atomic mass = 19.9 amu
Explanation:
Isotopes can be defined as two or more forms of a chemical element that are made up of equal numbers of protons and electrons but different numbers of neutrons.
Generally, the isotopes of a chemical element have the same chemical properties because of their atomic number but different physical properties due to their atomic weight (mass number).
Given the following data;
Relative abundance of Z-19 = 55%
Relative abundance of Z-21 = 45%
Atomic mass of Z-19 = 19 amu
Atomic mass of Z-21 = 21 amu
To find the average atomic mass;
Average atomic mass = 19 * (55/100) + 21 * (45/100)
Average atomic mass = 19*0.55 + 21*0.45
Average atomic mass = 10.45 + 9.45
Average atomic mass = 19.9 amu
Therefore, the average atomic mass for element Z is 19.9 amu.
7 grams of oxygen gas is reacted with excess C4H8. How many grams of CO2 gas at STP are produced?
Answers
Why are some chemical substances, like oil and coal, considered nonrenewable?
the process that forms them stops working after a decade
the process that forms them has not worked for millions of years
the process that forms them is very fast
the process that forms them is very slow
Answers
Answer:
The process of the formation of coal and oil is a very gradual and slow process.
Explanation:
The process of the formation of coal and oil is a very gradual process that takes up to millions of years.
Inter conversion of glucose and fructose occurs with an eqilibrium constant of 1.0. glicose isomerase catalyzes this reaction. The final concentration of fructose at equilibrim from 40 mM glucose is .
Answers
Inter-conversion of glucose and fructose occurs with an equilibrium constant of 1.0. Glucose isomerase catalyzes this reaction. The final concentration of fructose at equilibrium from 40mM glucose is a. 40 mM.
How to find the final concentration of fructose?Using this formula to find the final concentration of fructose
Final concentration of fructose =Equilibrium from glucose/ Equilibrium constant
Where:
Equilibrium constant = 1.0
Equilibrium from glucose = 40 mM
Let plug in the formula
Final concentration of fructose = 40mM / 1.0
Final concentration of fructose = 40mM
Therefore we can conclude that the correct option is A.
Learn more about Final concentration of fructose here:https://brainly.com/question/14041283
#SPJ1
The complete question is:
Inter-conversion of glucose and fructose occurs with an equilibrium constant of 1.0. Glucose isomerase catalyzes this reaction. The final concentration of fructose at equilibrium from 40mM glucose is
a. 40 mM
b. 20 mM
c. 10 mM
d. 0 mM
To measure the amount of nickel in some industrial waste fluid, an analytical chemist adds sodium hydroxide solution to a sample of the fluid and collects the solid nickel(II) hydroxide product. When no more is produced, he filters, washes and weighs it, and finds that has been produced. The balanced chemical equation for the reaction is:
Answers
Answer:
The final balanced equation is
Ni2+ + 2NaOH --> Ni (OH)2 + 2Na+
Explanation:
It is given that sodium hydroxide is added to collect the solid nickel(II) hydroxide product
The empirical equation for this statement is
Ni2+ + NaOH --> Ni (OH)2 + Na+
We will first balance the hydroxide molecule. On the right side there are two OH molecules.
Thus, on the left side we will take 2 sodium hydroxide
Ni2+ + 2NaOH --> Ni (OH)2 + Na+
Now we will balance the sodium ion which are 2 in numbers on the left side and 1 on the right side
Ni2+ + 2NaOH --> Ni (OH)2 + 2Na+
So, the final balanced equation is
Ni2+ + 2NaOH --> Ni (OH)2 + 2Na+
Identify the activated complex in the following reaction.
a. CuFeSO
b. FeFe
c. FeCuSO4
d. FeSO4
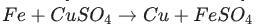
Answers
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. Option C)
An activated complex is a structure that exists temporarily during a chemical reaction and corresponds to the top of the energy barrier that must be overcome for the reaction to proceed to completion.
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. It is the structure with the greatest energy within the reaction process and is used to determine the rate at which the reaction occurs. An activated complex exists when the energy required to break the old bonds and form new ones has been absorbed. It has a specific configuration and energy content that is precisely defined.
A chemical reaction is the process by which atoms or groups of atoms in molecules interact to form new molecules. A chemical reaction is caused by the motion of electrons, which are negatively charged particles that surround atomic nuclei. The reaction proceeds through the formation of an intermediate species known as the transition state or activated complex. Reaction mechanisms are the sequence of steps involved in a chemical reaction. These steps describe the intermediate species formed as the reactants are converted to products. Hence option C) is correct.
for more questions on reaction
https://brainly.com/question/25769000
#SPJ8
A sample of a concentrated hydrochloric acid is 11.8Molarity and has a density 1.19 gram per mili liter calculate the mass percentage of HCl?
Answers
The mass percentage of HCl 11,900x/118 g and molality of solution is 9.915 mol L^-1.
Molarity = 11.8 M
Molarity = (moles of HCl) / (litres of solution)
Molality = (moles of HCl) / (kilograms of solvent)
=> Let number of moles of HCl present be x moles.
So, 11.8 = x/litres of solution
litres of solution = x/11.8 litres.
So, volume in mL = x/11.8 * 1000 mL =>10,000x/118 mL
Density of solution = 1.19 g/ml
So, D = m/v
Mass of solution = D * v => 1.19 * 10,000x/118 => 11,900x/118 g
=> (11,900x/118)/1000 L => 11.9x/118 litres
Moles of HCl = x moles.
Molality = x/( 11.9x/118)
=> 118x/11.9x => 118/11.9 => 9.9156 mol L-1
=> Molality of solution = 9.915 mol L-1 (approx.)
To learn more about molality visit:https://brainly.com/question/26921570
#SPJ9
Using the reading "Fossil Fuels" from lesson 12 describe the environmental and economic benefits and drawbacks of fossil fuels. Second, looking over the benefits and drawbacks, in your opinion, what do you think will happen to mining of fossil fuels in the next 50 years?
Answers
Fossil fuels are essential part of the power generation in the world. They are easily combustible and more reliable and cheaper. However, the burning of fossil fuels releases toxic gases to the environment.
What are fossil fuels ?Fossil fuels are fuel generated from the decomposition materials. Petroleum, coal, natural gas etc. are fossil fuels which are excavating from the earth.
Fossil fuels are non-renewable sources of energy. Hence, as the existing fossil sources are exhausted no more fossil fuel can be made. It is cheaper, reliable and easy to use.
However, the toxic hydrocarbon gases released from the burning of fossil fuels make the environment polluted. Therefore, overuse of fossil fuel definitely rise the atmospheric pollution.
Its use over the next 50 years, will increase the global warming and more of it will be exhausted.
Find more on fossil fuels :
https://brainly.com/question/3371055
#SPJ1