Answers
a. Balancing the redox reaction in both acidic and basic mediums:
Fe²+ + \(MnO_4\)- --> Fe³+ + Mn²+.
b. Balancing the redox reaction in both acidic and basic mediums:
Cu + \(NO_3\)- --> Cu+2 +\(N_2O_4.\)
a. Fe²+ + \(MnO_4\)- --> Fe³+ + Mn²+
Balanced equation in acidic medium:
Fe²+ + \(MnO_4\)- --> Fe³+ + Mn²+
To balance the equation, we can follow these steps:
1)Assign oxidation numbers to each element:
Fe²+ (Fe has a +2 oxidation state)
\(MnO_4\)- (Mn has a +7 oxidation state)
2)Identify the element being reduced and the element being oxidized:
Fe²+ is being oxidized (from +2 to +3)
\(MnO_4\)- is being reduced (from +7 to +2)
3)Balance the atoms and charges for each half-reaction:
Oxidation half-reaction: Fe²+ --> Fe³+ (requires one Fe²+ and one electron)
Reduction half-reaction:\(MnO_4\)- --> Mn²+ (requires five electrons and eight H+ ions to balance charges)
4)Balance the number of electrons in both half-reactions:
Multiply the oxidation half-reaction by 5 and the reduction half-reaction by 1 to equalize the number of electrons in both half-reactions.
The balanced equation in acidic medium is:
5Fe²+ + \(MnO_4\)- + 8H+ --> 5Fe³+ + Mn²+ + 4H2O
Balanced equation in basic medium:
To balance the equation in a basic medium, we need to add OH- ions to both sides to neutralize the H+ ions.
The balanced equation in basic medium is:
5Fe²+ + \(MnO_4\)- + 8OH- --> 5Fe³+ + Mn²+ + 4\(H_2O\)
Overall charge balancing:
In both acidic and basic media, the overall charges are balanced, with an equal number of positive and negative charges on both sides of the equations.
b. Cu + \(NO_3\)- --> Cu+2 + N₂O4
Balanced equation in acidic medium:
Cu + \(NO_3\)- --> Cu+2 + N₂O4
To balance the equation, we can follow these steps:
1)Assign oxidation numbers to each element:
Cu (Cu has a 0 oxidation state)
\(NO_3\)- (N has a +5 oxidation state)
2)Identify the element being reduced and the element being oxidized:
Cu is being oxidized (from 0 to +2)
\(NO_3\)- is being reduced (from +5 to +4)
3)Balance the atoms and charges for each half-reaction:
Oxidation half-reaction: Cu --> Cu+2 (requires two electrons)
Reduction half-reaction: \(NO_3\)- --> N₂O4 (requires three electrons)
4)Balance the number of electrons in both half-reactions:
Multiply the oxidation half-reaction by 3 and the reduction half-reaction by 2 to equalize the number of electrons in both half-reactions.
The balanced equation in acidic medium is:
3Cu + 2\(NO_3\)- --> 3Cu+2 + N₂O4
Balanced equation in basic medium:
To balance the equation in a basic medium, we need to add OH- ions to both sides to neutralize the H+ ions.
The balanced equation in basic medium is:
3Cu + 2\(NO_3\)- + 6OH- --> 3Cu+2 + N₂O4+ 3\(H_2O\)
Overall charge balancing:
In both acidic and basic media, the overall charges are balanced, with an equal number of positive and negative charges on both sides of the equations
The complete question is :
Balance the following redox reactions in both acidic and basic medium using the ion-electron method.
Rubrics:
1pt balanced equation acidic medium.
1pt balanced equation basic medium.
1pt balance overall charges of acid and basic medium.
a. Fe²+ + \(MnO_4\)- --> Fe³+ + Mn²+
b. Cu + \(NO_3\) --> Cu +2 + N₂O4
Know more about acidic medium here:
https://brainly.com/question/24255408
#SPJ8
Related Questions
The reaction of C2H4 +Cl2 is what type of reaction.
Answers
Answer:
C2H4(g) + Cl2(g)→C2H4Cl2(g)
This is an example of synthesis reaction, when compounds are joined together.
Consider the following equilibrium system: H2(g) + 12(g) + 2H2(g) AG°=-16.77kJ At 298 K, HI is injected into an evacuated container. At equilibrium, the partial pressure of H2 is 0.103 atm. What is the partial pressure of HI? A) 9.24 atm B) 3.04 atm C) 870 atm 10) D) 0.870 atm E) 33.5 atm
Answers
The given equilibrium system is: H2(g) + 1/2I2(g) ↔ 2HI(g) ΔG° = -16.77 kJ
At equilibrium, the partial pressure of H2 is given as 0.103 atm.
According to the stoichiometry of the balanced equation, the mole ratio between H2 and HI is 1:2. This means that for every 1 mole of H2 that reacts, 2 moles of HI are formed.
Since the partial pressure of H2 is 0.103 atm, the partial pressure of HI will be twice that value, as determined by the mole ratio.
Partial pressure of HI = 2 × 0.103 atm = 0.206 atm
Therefore, the correct answer is B) 0.206 atm, which is the partial pressure of HI at equilibrium in the given system.
To know more about equilibrium click here:
brainly.com/question/30807709
#SPJ11
TOICHIOMETRY Using molarity to find solute moles and solution volume Calculate the volume in liters of a 2.58 x 10^* mm magnesium fluoride solution that contains 125 mmol of magnesium fluoride (MgF2). Be sure your answer has the correct number of significant digits. x I ?
Answers
The volume of the 2.58 x 10² mmol/L magnesium fluoride (MgF₂) solution that contains 125 mmol of MgF₂ is approximately 484.5 L.
To calculate the volume of the magnesium fluoride solution, we can use the relationship between moles, molarity, and volume. The equation for this relationship is:
moles = molarity * volume
Given that the solution has a molarity of 2.58 x 10² mmol/L and contains 125 mmol of MgF₂, we can rearrange the equation to solve for the volume:
volume = moles / molarity
Substituting the given values:
volume = 125 mmol / (2.58 x 10² mmol/L)
To ensure proper units, we need to convert the molarity from mmol/L to mol/L:
molarity = 2.58 x 10² mmol/L * (1 mol/1000 mmol) = 2.58 x 10⁻¹ mol/L
Now we can substitute the values into the equation:
volume = 125 mmol / (2.58 x 10⁻¹ mol/L)
Simplifying the expression:
volume = 125 mmol * (1 L / (2.58 x 10⁻¹ mol))
volume = 125 mmol * (1 L / 0.258 mol) = 484.5 L
Rounding to the correct number of significant digits, the volume of the solution is approximately 484.5 L.
To know more about molarity refer here:
https://brainly.com/question/31545539#
#SPJ11
7) Explain how the atomic number of an element identifies the element.
Answers
Answer:
The atomic number or proton number (symbol Z) of a chemical element is the number of protons found in the nucleus of every atom of that element. The atomic number uniquely identifies a chemical element. ... The sum of the atomic number Z and the number of neutrons N gives the mass number A of an atom.
Answer:
Atoms of each element contain a characteristic number of protons. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); the number of protons in an atom is called the atomic number.
Explanation:
Throughout history, scientists have been known to copy ideas from their peers. Dmitry Mendeleev was no exception—he arranged all of the elements known at the time according to their atomic masses, just as Johann Döbereiner and John Newlands did before him. However, Mendeleev intentionally left blank spaces in his working version of the periodic table of elements.
Which statement best explains the importance of the blank spaces in Mendeleev’s version of the periodic table of elements?
Answers
The statement which best explains the importance of the blank spaces in Mendeleev’s version of the periodic table of elements is that they predicted the existence of elements that had not yet been discovered.
What is Periodic table?This is referred to as the tabular arrangement of elements into groups and periods based on their common properties and features. There are 18 groups and seven periods in which elements are grouped under.
Mendeleev intentionally left blank spaces in his working version of the periodic table of elements because he predicted the existence of elements that had not yet been discovered.
Read more about Periodic table here https://brainly.com/question/1173237
#SPJ1
What would cause entropy to increase in a reaction?
A. The products becoming more spread out
B. The products forming an ordered pattern
C. The products forming fewer molecules
D. The products forming a more rigid structure
Answers
i think A but if I'm wrong sorry
then if increasing then becoming spread out so letter A
What would be the MAJOR organic product of the following reaction?
Answers
A compound or product that follows Markovnikov's criteria is referred to as a major organic product.
As a result, the Markovnikov Rule states that when more hydrogen is added to carbon, the main product will result. This suggests that the main result will be 2-bromopropane when hydrogen is added to carbon-1, which has a higher concentration of hydrogen, and bromine is added to carbon-2. This process is known as an addition of HBr to the alkene or a hydrogenation reaction. Bromine was added to the alkene according to Markownikoff's rule. The main organic product that results from the following reaction is 3-dimethylbutan-2-amine, option (B). It should be noted that an acidic buffer is used to conduct the reaction between the ketone and the main amine.
To learn more about Markovnikov click here https://brainly.com/question/29668964
#SPJ4
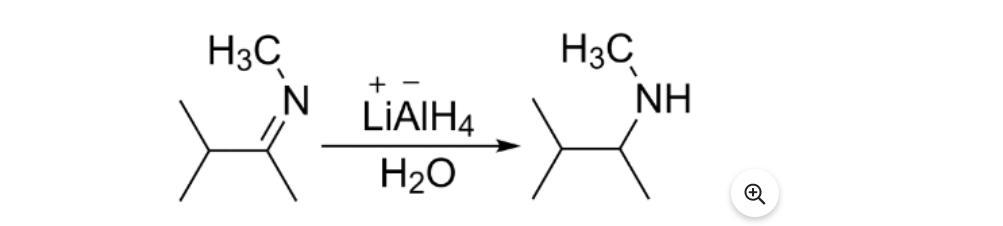
Help a person out please
Answers
Explanation:
here I haven't drawn the diagram but I have marked them where they will be .
So if it helps don't forget to like and Mark me

Some items, such as rubber, float on the water, while some items, such as gold, sink in
the water. If an item floats is it more or less dense than the water?
Answers
Answer:
If an item is less dense, it floats on the water.
Explanation:
We know this since the more dense something it usually is heavier than if it was less dense. Which ways it down resulting to it sinking.
Question 1 (1 point)
A party balloon is filled up under the following conditions: 1.1 atm 65°C, 1,55 liters. Then, the balloon is taken to an outdoor wedding with
sightly different conditions: 1.2 atm and 92°C. What is the new size of the balloon?
Do not enter a unit, just the number
Round your answer to 2 sig figs.
Answers
Answer:
.5
Explanation:
Answer:
1.5
Explanation:
1.5
how many molecules are there in 4dm³ of nitrogen gas
Answers
There are 1.078 x 10²³ molecules
Further explanationGiven
4 dm³ = 4 L Nitrogen gas
Required
Number of molecules
Solution
Assumptions on STP (1 atm, 273 K), 1 mol gas = 22.4 L, so for 4 L :
mol = 4 : 22.4
mol = 0.179
1 mol = 6.02 x 10²³ particles(molecules, atoms)
For 0.179 :
= 0.179 x 6.02 x 10²³
= 1.078 x 10²³
Aqueous solutions of compounds containing element X are blue. Element X could be (1) carbon (2) copper (3) sodium (4) potassium
Answers
The element X that could be responsible for the blue color in aqueous solutions of compounds is (2) copper.
Copper compounds are known to exhibit various shades of blue in aqueous solutions. This is due to the presence of copper ions (Cu2+) which absorb certain wavelengths of light, particularly in the blue region of the electromagnetic spectrum. The absorption of light by copper ions results in the reflection of blue light, giving the solution its characteristic blue color.
Copper is a transition metal that can form different oxidation states, including Cu2+. When copper ions are present in solution, they can interact with water molecules or other ligands to form complex ions, which contribute to the blue color. Copper compounds such as copper sulfate (CuSO4) and copper nitrate (Cu(NO3)2) are examples of substances that produce blue solutions when dissolved in water.
In contrast, carbon, sodium, and potassium compounds generally do not exhibit a blue color in aqueous solutions. Carbon compounds are typically colorless or exhibit other colors depending on their chemical structure. Sodium and potassium compounds are often colorless or may produce solutions with a slight yellow tint, but they do not typically produce a strong blue color.
To learn more about aqueous solutions click here: brainly.com/question/1326368
#SPJ11
O--H-H is a base, neither an acid nor a base, an acid

Answers
O--H-H represents a molecule of water (H2O) and it is neither an acid nor a base. Water is a neutral compound and does not have any excess H+ or OH- ions to classify it as an acid or a base.
Acids and Bases.A base is a substance that can accept protons (H+ ions) or donate electrons. Bases are characterized by a pH value greater than 7 and can react with acids to form salts and water. Some common examples of bases include hydroxide ions (OH-), ammonia (NH3), and sodium hydroxide (NaOH).
An acid is a substance that can donate protons (H+ ions) or accept electrons. Acids are characterized by a pH value less than 7 and can react with bases to form salts and water. Some common examples of acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and acetic acid (CH3COOH).
Learn more on Acids and Bases here https://brainly.com/question/9836972
#SPJ1
however, when the temperature increases, the solubility of gaseous solutes will _______?
Answers
Answer:
Decrease
Explanation:
Since the speed in which the gas molecules are faster as they are heated, they fly around in the container and logically, it is harder to insert a moving object into water than something more stationary or slower.
an active chemical in certain mushrooms that causes hallucinogenic effects is
Answers
Answer: Psilocybin (4-phosphoryloxy-N, N-dimethyltryptamine) is the active chemical in certain mushrooms that causes hallucinogenic effects.
Explanation:
An active chemical in certain mushrooms that causes hallucinogenic effects is psilocybin.
Some types of mushrooms, referred to as "magic mushrooms," contain psilocybin.
This chemical molecule, when consumed, is changed into psilocin, which causes the hallucinogenic experiences seen by users.
Mushrooms provide protein, vitamins, minerals, and antioxidants. These might offer several health benefits.
For instance, antioxidants are chemicals that help the body eliminate free radicals.
Free radicals are unfavourable byproducts of metabolism and other biological processes. If they accumulate, oxidative stress could start to appear in the body. This can harm the body's cells and result in a variety of diseases.
Some of the antioxidants found in mushrooms include the following:
Choline, selenium, and vitamin C.
To learn more about mushrooms, visit:
https://brainly.com/question/12430186
#SPJ11
Which of the following elements are found in the same period?
a) Cu and Fe
Ob) Cl and Kr
Oc) C and AI
d) B and Ga
e) Mg and Rb
Answers
Answer:
d
Explanation:
b and ga are same period
Use the Potential Energy Diagram to answer the questions.
Potential energy
1.What is the change in enthalpy (AH) for this reaction?
2. What is the activation energy?
3.Is this reaction endothermic or exothermic? Explain
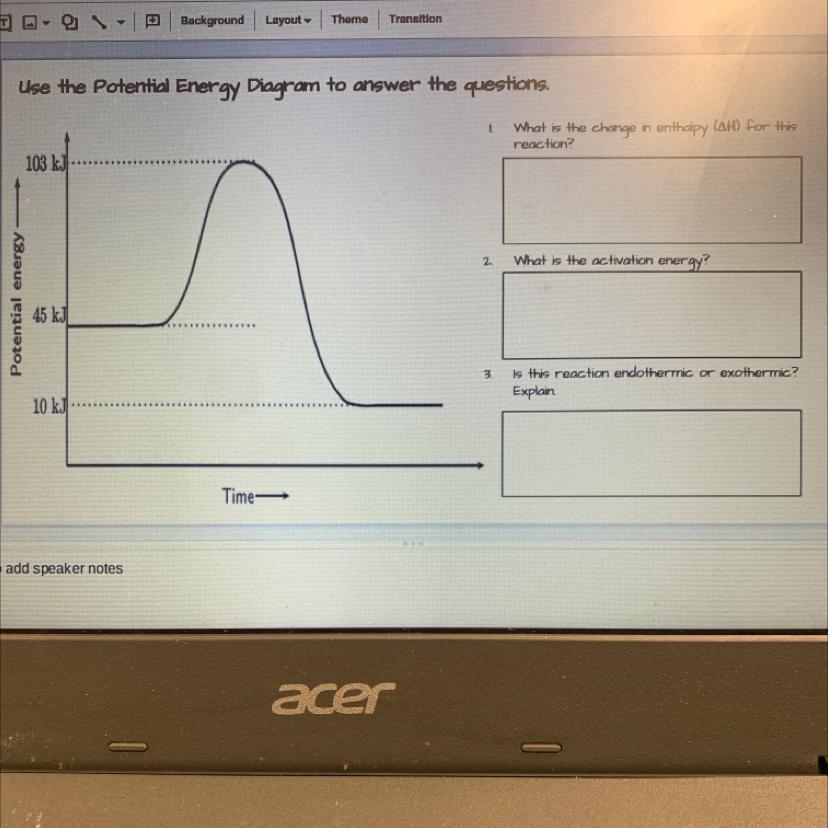
Answers
The enthalpy change for the reaction is -35kJ. The activation energy is 103 kJ. The reaction is exothermic
What is the potential energy diagram of a reaction?A potential energy diagram is a graphical representation of the potential energy changes that occur during a chemical reaction. It shows the energy of the reactants and products, as well as the activation energy, which is the minimum energy required for the reaction to occur.
The diagram typically has a horizontal axis that represents the progress of the reaction from the reactants to the products, and a vertical axis that represents the potential energy. The reactants are usually shown on the left-hand side, and the products on the right-hand side.
Learn more about exothermic reaction:https://brainly.com/question/10373907
#SPJ1
what species are produced at the positive and negative electrodes during the electrolysis of molten sodium chloride?
Answers
The species are produced at the positive and negative electrodes during the electrolysis of molten sodium chloride is at the positive and the species is Cl₂(g) and at the end of negative is Na(l).
The net effect of the passing the electric current to the molten salt in this cell that is to decompose the sodium chloride into the its elements, the sodium metal and the chlorine gas. The anode is called as the positive end and the cathode is called as the negative end.
During the process of the electrolysis the chlorine gas are produce at the positive end that is anode. The sodium metal is produce at the negative end that is the catode.
To learn more about electrolysis here
https://brainly.com/question/9977624
#SPJ4
How many different consitutional isomers are there for a compound having the molecular formula C3H6O
Answers
Answer:
that make 11 isomers....
0.92 lbm of water fills a container whose volume is 1.92 ft3. the pressure in the container is 100 psia. calculate the total internal energy and enthalpy in the container. use data from the steam tables. the total internal energy in the container is btu. the enthalpy in the container is btu.
Answers
The total internal energy in the container is 329.77 Btu and the enthalpy in the container is 385.14 Btu.
Using the steam tables, we can determine the specific volume of water at the given pressure and temperature. The specific volume of water is 0.01658 \(ft^3/lbm\).
The mass of water in the container is 0.92 lbm, so the total volume of the water is:
V = m/v = 0.92 lbm / 0.01658 \(ft^3/lbm\) = \(55.539 ft^3\)
Assuming the water is at saturation, we can find the total internal energy and enthalpy by using the values in the steam tables for saturated water at 100 psia.
From the steam tables, the total internal energy of saturated water at 100 psia is 358.05 Btu/lbm, so the total internal energy in the container is:
U = m * u = 0.92 lbm * 358.05 Btu/lbm = 329.77 Btu
From the steam tables, the enthalpy of saturated water at 100 psia is 419.02 Btu/lbm, so the enthalpy in the container is:
H = m * h = 0.92 lbm * 419.02 Btu/lbm = 385.14 Btu
Therefore, the total internal energy in the container is 329.77 Btu and the enthalpy in the container is 385.14 Btu.
Learn more about enthalpy :
https://brainly.com/question/13996238
#SPJ4
URGENT CAN SOMEONE ANSWER THIS QUESTION AND SHOW THEIR WORK PLEASE! How many moles of ammonia (NH) can be produced from the reaction of 4.0 liters of hydrogen at 50.0°C and 1.2atm of pressure with excess nitrogen?
Answers
Answer:
0.116 moles of ammonia can be produced from the reaction of 4.0 liters of hydrogen at 50.0°C and 1.2atm of pressure with excess nitrogen.
Explanation:
The balanced chemical equation for the reaction of hydrogen and nitrogen to form ammonia is:
N2(g) + 3H2(g) → 2NH3(g)
To determine how many moles of ammonia can be produced from the reaction of 4.0 liters of hydrogen at 50.0°C and 1.2atm of pressure with excess nitrogen, we need to use the ideal gas law equation:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature.
First, we need to convert the volume of hydrogen gas to moles using the ideal gas law equation:
n = PV/RT
where P is the pressure in atm, V is the volume in liters, R is the gas constant (0.0821 L·atm/mol·K), and T is the temperature in Kelvin.
n = (1.2 atm)(4.0 L)/(0.0821 L·atm/mol·K)(50.0°C + 273) = 0.174 mol H2
Since there is excess nitrogen, all of the hydrogen will react to form ammonia. Using the mole ratio between NH3 and H2 from the balanced chemical equation:
2 mol NH3 / 3 mol H2
we can calculate how many moles of NH3 will be produced:
n(NH3) = (0.174 mol H2) × (2 mol NH3 / 3 mol H2) = 0.116 mol NH3
Therefore, 0.116 moles of ammonia can be produced from the reaction of 4.0 liters of hydrogen at 50.0°C and 1.2atm of pressure with excess nitrogen.
B-
Why many nuclei like U234, U236 and
U238 undergo fission only by fast neutrons?
Answers
The nucleus more easily, increasing the probability of causing fission many nuclei like U234, U236, and U238 undergo fission only by fast neutrons.
Fission can be caused by slow as well as fast neutrons. It is the energy of the neutron which determines its effectiveness in causing fission. Fast neutrons are more effective in causing fission. Hence, many nuclei like U234, U236, and U238 undergo fission only by fast neutrons.
Fission is a nuclear reaction process in which the nucleus of an atom is split into two smaller nuclei with the release of a large amount of energy and two or three neutrons. Uranium-235 (U-235) and Plutonium-239 (Pu-239) are the most commonly used fissile materials, but other materials like U-234, U-236, and U-238 can also undergo fission. When a neutron is absorbed by the nucleus of a fissile material like U-235 or Pu-239, it becomes unstable and splits into two smaller nuclei with the release of a large amount of energy.
The fission process also releases two or three neutrons, which can cause further fission of other nuclei, leading to a chain reaction. The chain reaction can be controlled by using a neutron moderator, which slows down the fast neutrons, making them more effective in causing fission. The efficiency of the fission reaction depends on the energy of the neutron.
Fast neutrons are more effective in causing fission than slow neutrons, which have less energy. This is because fast neutrons can penetrate the nucleus more easily, increasing the probability of causing fission. Hence, many nuclei like U234, U236, and U238 undergo fission only by fast neutrons.
Fast neutrons are more effective in causing fission than slow neutrons, which have less energy.
This is because fast neutrons can penetrate the nucleus more easily, increasing the probability of causing fission. Hence, many nuclei like U234, U236, and U238 undergo fission only by fast neutrons.
Learn more about nucleus with the given link,
https://brainly.com/question/13061744
#SPJ11
The movement of the tectonic plates on the earth is caused by
Answers
Answer:
The movement of tectonic plates is caused by convection currents in the molten rock in Earth's mantle below the crust layer. Earthquakes and volcanoes are great examples of the tectonic movement's results.
Explanation:
20 Points!!
If I ran through the rain, what would my body use for energy?
•The steak I ate because it's a protein.
•RNA
•The pasta I ate because it's a •carbohydrate.
•DNA
Answers
Answer:
the steak I ate because its protein
CO₂ + H₂O → H₂CO3 → H* + HCO3 Review this formula and discuss the mechanisms involved in the forward and reverse components of the reaction by answering the following: 1. When CO₂ + H₂O
Answers
Forward component of the reaction When CO₂ is added to water, it dissolves and reacts to form carbonic acid (H₂CO3) in the forward reaction.
The formula CO₂ + H₂O → H₂CO3 → H* + HCO3 represents the carbon dioxide equilibrium. The forward and reverse components of the reaction can be explained as follows: H₂CO3 has two possible reactions: It either releases a hydrogen ion (H+) and forms bicarbonate (HCO3-) or it releases two hydrogen ions (2H+) to form carbonate (CO32-) and water (H₂O).
CO₂ + H₂O → H₂CO3 → H+ + HCO3Reverse component of the reactionWhen hydrogen ions (H+) are added to bicarbonate ions (HCO3-) or carbonate ions (CO32-), the reverse reaction takes place and carbonic acid (H₂CO3) is formed. Carbonic acid (H₂CO3) can also be decomposed into carbon dioxide (CO₂) and water (H₂O).
To know more about component visit:
https://brainly.com/question/30324922
#SPJ11
Which of the following is not an example of a fossil fuel being used? a. Coal being burned at a power plant. c. Driving a car with a full tank of gasoline. b. Using wood to build a fire. d. Using a natural gas furnace to heat your home.
Answers
Answer:
c, driving a car
Explanation:
got it right :)
have a nice day!~
HELP!! b. At the equivalence point, all of the acid has been neutralized by the base. Why does the pH change so
sharply around the equivalence point? (HINT: the pH is neutral, just like the water example in the
previous question.)
Answers
At equivalence there is no more HA and no more NaOH, for this particular reaction. So that means we have a beaker of NaA and H2O. The H2O contributes 1 x 10-7 M hydrogen ion and hydroxide ion. But NaA is completely soluble because group 1 ion compounds are always soluble. So NaA breaks apart in water and it just so happens to be in water. So now NaA is broken up. The Na+ doesn't change the pH but the A- does change the pH. Remember that the A anion is from a weak acid. That means it will easily attract a hydrogen ion if one is available. What do you know? The A anion is in a beaker of H+ ions! So the A- will attract H+ and become HA. When this happens, it leaves OH-, creating a basic solution, as shown below.
how should you write the volume dispensed by a 5 ml volumetric pipet?
Answers
When writing the volume dispensed by a 5 ml volumetric pipet, it should be written as 5.00 mL.
A volumetric pipet is a laboratory instrument utilized to dispense very accurate and precise volumes of liquid. It is commonly used in analytical chemistry to make up solutions or to dilute stock solutions. Volumetric pipettes, also known as transfer pipettes or bulb pipettes, are single-volume liquid measuring instruments. They are meant to deliver a precise volume of liquid at a fixed temperature when the tip is slightly below the liquid surface.
It is important to write the volume with two decimal places to indicate the precision of the pipette.
Volumetric pipettes are utilized to prepare and dilute solutions. They are made of glass, with a round or conical end. They are intended to provide a precise volume of liquid, such as a certain number of milliliters or milligrams of a substance. Because of its accuracy, a volumetric pipet is used to create a standard solution.
For more such questions on volumetric pipet, click on:
https://brainly.com/question/28945018
#SPJ11
Calculate the maximum mass of aluminium which can be extracted from 10 kg of
aluminium oxide
2AlO3+ 3C ----->4Al + 3CO2
Answers
Answer:
Hrishikesh. bshjsjbd. jwjjja
silver (I) nitrate reacts with nickel (II) chloride to produce silver (I) chloride and nickel (II) nitrate wright the balanced chemical equation for this
Answers
Answer: 2 AgNO3(aq) + NiCl2(aq) ⇒ 2 AgCl(s) + Ni(NO3)2 (aq)
Explanation:
