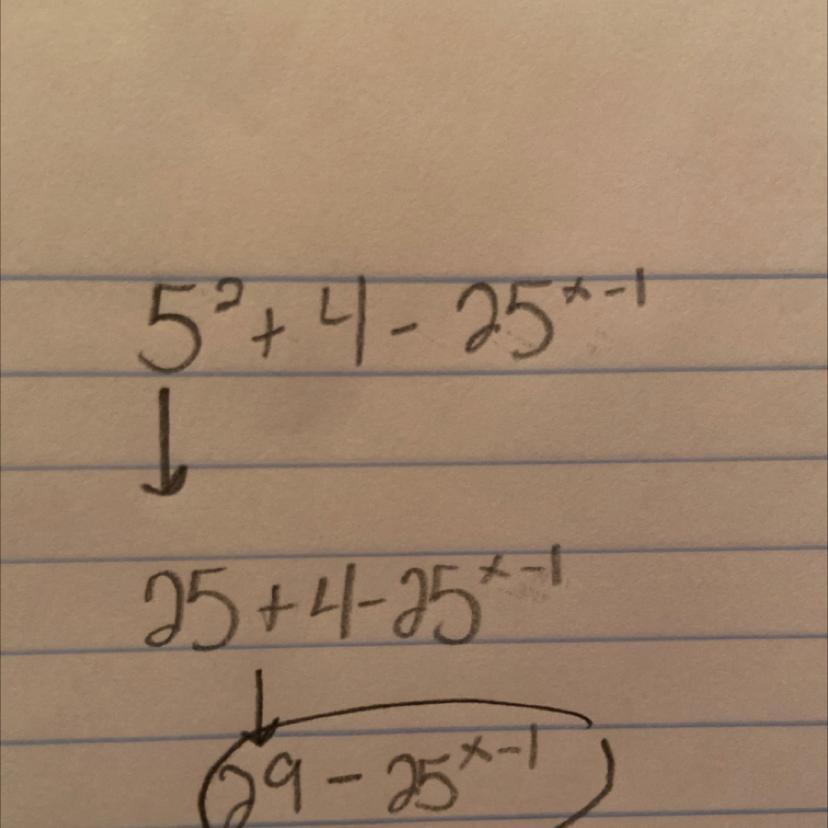distinguish among a mixture, a solution, and a compound.
Answers
Solution is two or more su substances that stay evenly mixed.
Compound is when two or more elements are chemically bonded together.
Related Questions
what's the difference between ionic and covalent bonding
Answers
Answer:
There are primarily two forms of bonding that an atom can participate in: Covalent and Ionic. Covalent bonding involves the sharing of electrons between two or more atoms. Ionic bonds form when two or more ions come together and are held together by charge differences.
Explanation:
how does kinetic energy at the bottom of a hill compared to its potential energy at the top
Answers
Answer:
Kinetic energy would have to work ten times harder than potential energy.
Explanation:
Let’s say you have a boulder and your asked to push it up the hill it would be difficult right? But lets reverse the situation what if you are asked to roll the boulder down the hill it wouldn’t be much work because the hill is basically doing all the work For you. Hope this Helps:)
1. A train travels North at 103 km/h. Is this an example of speed, velocity, or acceleration. speed velocity accelleration
Answers
Answer:
velocity
Explanation:
velocity has both speed AND direction.
Answer:
speed
Explanation:
Speed is the rate at which something covers distance. Velocity is the rate of change of its position, ex: 70km/h to north. Acceleration is the rate at which an object changes its velocity.
Perceived control over a stressful event results in Group of answer choices a. less reported stress b. more frustration regarding the stressful event c. less motivation to solve the problem causing the stress d. increased arousal
Answers
Perceived control over a stressful event typically results in a. less reported stress.
When an individual believes they have control over a situation, they tend to feel more empowered and confident in their ability to manage or resolve the stressor. This perception of control allows them to develop effective coping strategies and focus on problem-solving rather than being overwhelmed by the stress. Consequently, this sense of control often leads to a reduction in overall stress levels.
Contrary to options b, c, and d, perceived control does not typically lead to more frustration, less motivation to solve the problem, or increased arousal. Instead, having a sense of control often allows individuals to approach the stressful event in a more positive and proactive manner, ultimately reducing the negative impact of the stressor on their mental and emotional well-being. Thus, fostering a sense of control over stressful events can be a crucial factor in managing stress effectively. So therefore the correct answer is a. less reported stress.
Learn more about stress at
https://brainly.com/question/31366817
#SPJ11
_____ are considered dietary proteins.
A. apples
B. oils
C. eggs
D. potatoes
Answers
Answer:
Eggs
Explanation:
Apple = fruit
Oils = fat
Potatoes = Carbs
And eggs are the only proteins
During a process called photoact, ________ give up an electron as a part of the light-dependent reactions.
Answers
Answer:
Chloroplasts?
Explanation:
14. How many liters would 5 moles of oxygen gas take up?
a.0.22L
b.112
c.27.4L
d.55L
Answers
Answer:
a 0.22L
Explanation:
Answer:
A
Explanation:
1 moles of oxygen is 0.044L
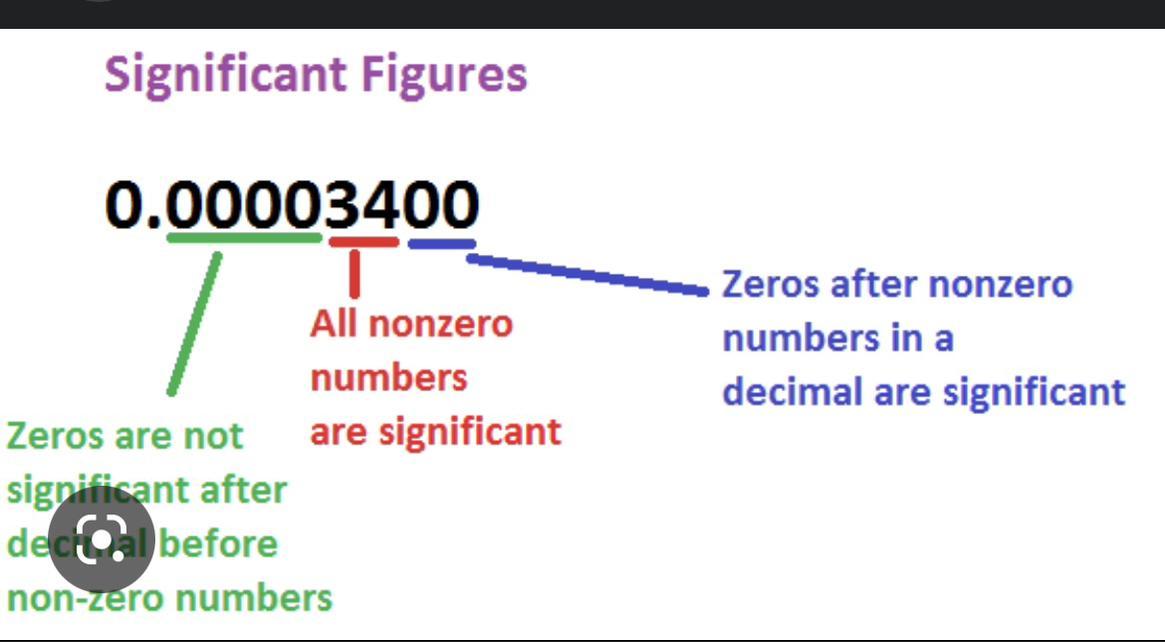
how would you round 34.9279 if there was 3 sig figs and 4 sig figs
Answers
which unit is closest in size to the radius of an atom
Answers
An atom's radius is well under 1 nanometer, or one billionth of a meter.
What is an atom?An atom is a matter particle that defines a chemical element uniquely. An atom is made up of a central nucleus and one or more negatively charged electrons. The nucleus is positively charged and contains one or more protons and neutrons, which are relatively heavy particles.An element is made up of only one type of atom. Atoms are further subdivided into subatomic particles known as electrons, protons, and neutrons. Chemical reactions allow elements to combine to form molecules.The distance between the nuclei of two identical atoms bonded together is used to calculate atomic radius. Atoms' atomic radius decreases from left to right across a period. Atoms' atomic radius generally increases from top to bottom within the atom.To learn more about atom refer to :
https://brainly.com/question/17545314
#SPJ4
Calculate the oxidation number of Cao
Answers
Answer:+2 -2
Explanation:
Which types of posted signs convey information about chemical storage? (Select all that apply)
Hazard signs such as "Flammable," "Oxidizer," and "Corrosive"
Exit sign
Gas Cylinder sign
National Fire Protection Association (NFPA) diamond
Safety equipment signs such as "Safety Shower" and "Eyewash Station"
Answers
Hazard signs such as "Flammable," "Oxidizer," and "Corrosive" National Fire Protection Association (NFPA) diamond Gas Cylinder sign posted signs convey information about chemical storage.
Pictograms are pictorial symbols that are used to express particular information about a chemical's risks. OSHA mandates pictograms on principal labels to indicate chemical dangers. The exact OSHA danger categorization determines each pictogram(s). There are several forms of risks in the workplace, including chemical, ergonomic, physical, and psychological hazards, to mention a few. Dangerous situations. Employees who operate with machinery or on construction sites are more likely to be exposed to safety concerns. Biological dangers. Biological dangers are exceedingly hazardous Physical dangers Ergonomic risks Chemical risks Workload risks.
learn more about Pictograms here:
brainly.com/question/29898314
#SPJ4
Metals in general have:
a. High electrical conductivity
b. High thermal conductivity
c. High density
d. All of the above
Answers
Answer:
d. All of the above
Explanation:
Sulfur Dioxide (SO^2) emissions from smokestacks are reduced by a scrubbing mechanism in which SO^2 gas reacts with crushed limestone (CaCO) to produce removable solid waste. The quation for the balanced reaction can be found below. How many grams of CaCO, are needed to completely react with 1250 g of SO^2
Answers
3906.114 grams of CaCO₃ is required to completely react with 1250 grams of SO₂.
What is Scrubbing mechanism?Scrubbing mechanism is a method of removing pollutants, such as sulfur dioxide (SO₂), from industrial exhaust gases. In this mechanism, a substance, such as limestone or lime, is added to the exhaust gases, which react with the pollutants to form solid waste products that can be easily removed.
Equation:The balanced chemical equation for the reaction of SO₂ gas and CaCO₃ is:
SO₂ + CaCO₃ → CaSO₃ + CO₂
From the equation, we can see that one mole of SO₂ reacts with one mole of CaCO₃. Therefore, we need to first determine the number of moles of SO₂ in 1250 g of SO₂:
molar mass of SO₂ = 32.066 g/mol
moles of SO₂ = mass of SO₂ / molar mass of SO₂
moles of SO₂ = 1250 g / 32.066 g/mol
moles of SO₂ = 39.012 mol
Since one mole of SO₂ reacts with one mole of CaCO₃, we need 39.012 moles of CaCO₃ to react with the 39.012 moles of SO₂. The molar mass of CaCO₃ is 100.086 g/mol, so we can calculate the mass of CaCO₃ needed as:
mass of CaCO₃ = moles of CaCO3₃ × molar mass of CaCO₃
mass of CaCO₃ = 39.012 mol × 100.086 g/mol
mass of CaCO₃ = 3906.114 g
Therefore, we need 3906.114 grams of CaCO₃ to completely react with 1250 grams of SO₂.
To know more about scrubbing mechanism, click here
https://brainly.com/question/30207790
#SPJ1
A LOT OF POINTS!!
30.0g sample of calcium sulfide contains 16.4g of calcium. What is the % of sulfur by mass in the compound
Answers
Answer:
there you go man
Explanation:


Rainwater is acidic because CO 2
( g) dissolves in the water, creating carbonic acid, H 2
CO 3
(K a1
=4.3×10 −7
,K a2
=5.6×10 −11
). If the rainwater is too acidic, it will react with limestone and seashells (which are principally made of calcium carbonate, CaCO 3
). - Part A Review I Constants I Periodic Table Calculate the concentrations of carbonic acid, bicarbonate ion (HCO 3
−
)and carbonate ion (CO 3
2−
) that are in a raindrop that has a pH of 5.10, assuming that the sum of all three species in the raindrop is 1.0×10 −5
M. Express your answers using two significant figures separated by commas. Submit Request Answer
Answers
The pH value of rainwater is approximately 17.62.
Rainwater is acidic because of the presence of carbon dioxide gas in it. The carbon dioxide gas is present in the atmosphere, and it dissolves in rainwater to form carbonic acid. The carbonic acid, in turn, lowers the pH of the rainwater, making it acidic. The concentration of carbon dioxide gas in the atmosphere is responsible for the acidity of rainwater.
The concentration of carbon dioxide gas in the atmosphere is expressed in terms of its molarity. The molarity of carbon dioxide gas in the atmosphere is given as 5.6 x 10^-11 M. The molarity of carbon dioxide gas in rainwater is the same as that in the atmosphere since it dissolves directly into the water.
The acidity of rainwater can be determined by calculating its pH value. The pH value is a measure of the acidity or basicity of a solution. A pH value of 7 indicates that the solution is neutral, while values below 7 indicate that the solution is acidic, and values above 7 indicate that the solution is basic.
The pH value of rainwater can be calculated using the formula pH = -log[H+], where [H+] is the concentration of hydrogen ions in the solution. Since rainwater is acidic, the concentration of hydrogen ions is higher than that of hydroxide ions. Thus, the pH value of rainwater is less than 7.
Using the given molarity of carbon dioxide gas in rainwater, the concentration of hydrogen ions can be calculated using the equation [H+] = Ka * [CO2]. Here, Ka is the acid dissociation constant of carbonic acid and is equal to 4.3 x 10^-7. Substituting these values into the equation gives [H+] = 2.408 x 10^-18 M.
Finally, the pH value of rainwater can be calculated using the formula pH = -log[H+]. Substituting the value of [H+] obtained above into this equation gives pH = 17.62.
for more questions onrainwater
https://brainly.com/question/28770892
#SPJ8
why does helium fusion require higher temperatures than hydrogen fusion
Answers
Helium fusion requires higher temperatures than hydrogen fusion because of the increased electrostatic repulsion between helium nuclei.
Helium has two protons, while hydrogen only has one, the strong nuclear force, which binds the atomic nuclei together, is powerful but short-ranged. To overcome the electrostatic repulsion and allow the strong nuclear force to act, helium nuclei must come very close to each other. At higher temperatures, the particles have greater kinetic energy, which increases the chances of helium nuclei colliding with enough force to overcome the repulsion.
The temperature required for helium fusion, known as the triple-alpha process, is around 100 million Kelvin, significantly higher than the 15 million Kelvin needed for hydrogen fusion through the proton-proton chain reaction. In summary, the increased electrostatic repulsion between helium nuclei and the need for a closer approach for the strong nuclear force to take effect result in helium fusion requiring higher temperatures than hydrogen fusion.
Learn more about hydrogen fusion at
https://brainly.com/question/3501435
#SPJ11
Calculate the density of an object, if the object has a mass of 30 grams and the volume is 10.0 ml.
Answers
Answer:
3.0grams/ml
density is a ratio of mass to volume
that implies 30grams/10ml
you can get a different answer depending on the unit
Running an HPLC assay using a column heated to approximately 60 °C can have what benefits over running the assay room temperature? Select all that are true.
increased back pressure
increased column life
decreased run time
more precise retention times
flucuating retention times
more precise quantitation
greatly enhanced peak shape
Answers
The following benefits can be achieved by running an HPLC assay using a column heated to approximately 60 °C:
Decreased run time: Increasing the temperature of the column can enhance the efficiency of the separation process by promoting faster analyte diffusion and interaction with the stationary phase. This can result in shorter run times for the assay.
More precise retention times: Heating the column can improve the reproducibility and precision of retention times, making it easier to identify and analyze specific peaks in the chromatogram accurately.
More precise quantitation: With improved retention time precision, the quantification of analytes becomes more accurate and reliable, leading to precise quantitation of the target compounds in the sample.
Greatly enhanced peak shape: Heating the column can help to eliminate or minimize peak tailing, which is often caused by interactions between analytes and the stationary phase. Improved peak shape enhances the accuracy and resolution of the assay.
On the other hand, the following options are not true:
Increased back pressure: Heating the column does not necessarily result in increased back pressure. Back pressure is primarily influenced by factors such as particle size, flow rate, and solvent viscosity, rather than column temperature.
Increased column life: While temperature can affect the column performance, it does not necessarily lead to increased column life. Factors such as sample composition, pH, and flow rate have more significant impacts on the longevity of the column.
Fluctuating retention times: By heating the column, the goal is to achieve more consistent and reproducible retention times. Fluctuating retention times are more likely to occur when temperature control is poor or when other experimental variables are not adequately controlled.
Learn more about temperature here:
https://brainly.com/question/15520591
#SPJ11
you need to prepare 100.0 ml of a diluted solution from your concentrated stock solution. assuming that the accuracy of the concentration is important, which type of glassware would be best for making the solution? How Should the correct amount of stock solution be obtained?
a. Measure out x mL using a graduated cylinder
b. Measure out x mL using a volumetric pipet
c. Measure out x g on a balance
Answers
The accuracy of the concentration is important, the type of glassware would be best for making the solution is to measure out x mL using a volumetric pipet. Option B is correct.
If accuracy of the concentration is important, the best type of glassware for making the solution is a volumetric flask. Volumetric flasks are designed to contain a precise volume of solution at a specific temperature, typically 20°C. They have a single graduation mark on the neck of the flask that indicates the calibrated volume, such as 100.0 ml.
To obtain the correct amount of stock solution, a volumetric pipette should be used. Volumetric pipettes are designed to dispense a precise volume of liquid, and are calibrated to deliver a specific volume at a specific temperature.
To use a volumetric pipette, it should be rinsed with the stock solution and allowed to drain, and then the stock solution should be drawn up to the calibrated mark using a pipette bulb or other aspiration device. The solution should then be transferred to the volumetric flask and diluted to the mark with solvent, typically distilled water.
Hence, B. Measure out x mL using a volumetric pipet is the correct option.
To know more about stock solution here
https://brainly.com/question/17018950
#SPJ4
experiment 1: what would happen to the measured cell potentials if 30 ml of solution was used in each half-cell instead of 25 ml?
Answers
The electrochemical reaction's cell potential remained constant when the volume in each half cell was decreased from 30 mL to 25 mL.
The difference between the potentials of the two half cells in the electrochemical reaction has been given as the cell potential.
The concentration of solutions in the oxidation and reduction halves of the two cells has been set.
Changes in cell potentialWhen the potential of the half cells changes, the cell potential has also altered.
The solution was added at a volume of 30 mL, which caused a cell potential difference of x.
There has been a difference in the potential being similar to the 30 mL solution with the volume of 25 mL, i.e. x.
As a result, the electrochemical reaction's cell potential remained constant when the volume in each half cell was decreased from 30 mL to 25 mL.
Learn more about 'cell potential' refer to
brainly.com/question/1313684
#SPJ4
Identify the most and the least acidic compound in each of the following sets.
Leave the remaining answer in each set blank.
a) difluoroacetic acid: _______ fluoroacetic acid: _______ trifluoroacetic acid: _______
b) cyclohexanol: _______ phenol: _______ benzoic acid: _______
c) oxalic acid: _______ acetic acid: _______formic acid: _______
Answers
a) difluoroacetic acid: most acidic fluoroacetic acid: least acidic trifluoroacetic acid : middle acidity. b) cyclohexanol: least acidic phenol: middle acidity benzoic acid: most acidic. c) oxalic acid: most acidic acetic acid: middle acidity formic acid: least acidic. Thus, the most acidic and least acidic compound in each set is identified as given above.
In the given question, we are given sets of compounds and we have to identify the most and the least acidic compound in each set. The acidic character of the compound depends upon its tendency to donate hydrogen ion. The compound that easily donates hydrogen ion is acidic in nature, while the compound that does not donate hydrogen ion easily is basic in nature.
The compound that donates hydrogen ion in a moderate way is neutral in nature.a) difluoroacetic acid: most acidic fluoroacetic acid: least acidic trifluoroacetic acid: middle acidity b) cyclohexanol: least acidic phenol: middle aciditybenzoic acid: most acidic.
Know more about cyclohexanol here:
https://brainly.com/question/7141113
#SPJ11
Molarity of Kool Aid solutions can be calculated by comparing the concentrations of Kool Aid powder and sugar added to a given volume of water. The molar mass of Kool Aid will be the same as that of sugar for our purpose. The molecular formula for sugar is C12H22O11- Your objective for this lab will be to calculate the molarity of Kool Aid desired based on package directions. You will then be provided two concentrated Kool Aid solutions. You will use dilution calculations to determine the amount of water and concentrated solution you will need in order to prepare 65 mL of the desired molarity.
Calculate the molarity of Kool Aid desired based on the following information from the package directions.
1 package Kool Aid powder = 4. 25 grams 1 cup sugar = 192. 00 grams
2. 00 quarts of water (1. 06 quarts = 1 liter)
Answers
The amount of concentrated solution needed is (0.286 M)(65 mL) / C M, and the amount of water needed is 65 mL minus the volume of the concentrated solution.
To calculate the molarity of Kool Aid desired, we need to determine the number of moles of Kool Aid powder and sugar in the package. Since the molecular formula for sugar is C12H22O11, we can calculate its molar mass as follows:
Molar mass of C12H22O11 = (12 * 12.01) + (22 * 1.01) + (11 * 16.00)
= 144.12 + 22.22 + 176.00
= 342.34 g/mol
Given that the package contains 4.25 grams of Kool Aid powder, we can calculate the number of moles of Kool Aid powder using its molar mass:
Number of moles of Kool Aid powder = Mass / Molar mass
= 4.25 g / 342.34 g/mol
≈ 0.0124 mol
Similarly, for the sugar, which has a molar mass of 342.34 g/mol, we can calculate the number of moles of sugar using its mass:
Number of moles of sugar = Mass / Molar mass
= 192.00 g / 342.34 g/mol
≈ 0.5612 mol
Now, to calculate the molarity of the desired Kool Aid solution, we need to determine the volume of water. Given that 1.06 quarts is equal to 1 liter, and we have 2.00 quarts of water, we can convert it to liters as follows:
Volume of water = 2.00 quarts * (1.06 liters / 1 quart)
= 2.12 liters
To find the molarity, we use the formula:
Molarity (M) = Number of moles / Volume (in liters)
Molarity of Kool Aid desired = (0.0124 mol + 0.5612 mol) / 2.12 L
≈ 0.286 M
To prepare 65 mL of the desired molarity, we can use dilution calculations. We need to determine the volume of concentrated solution and the volume of water needed.
Let's assume the concentration of the concentrated Kool Aid solution is C M. Using the dilution formula:
(C1)(V1) = (C2)(V2)where C1 is the initial concentration, V1 is the initial volume, C2 is the final concentration, and V2 is the final volume.
Given that C1 = C M and V1 = V mL, and we want to prepare a final volume of 65 mL (V2 = 65 mL) with a final concentration of 0.286 M (C2 = 0.286 M), we can rearrange the formula to solve for the volume of the concentrated solution:
(C M)(V mL) = (0.286 M)(65 mL)
V mL = (0.286 M)(65 mL) / C M
So, the amount of concentrated solution needed is (0.286 M)(65 mL) / C M, and the amount of water needed is 65 mL minus the volume of the concentrated solution.
For more such questions on concentrated visit:
https://brainly.com/question/28564792
#SPJ8
Which form of emission is commonly not written in nuclear equations because they do not affect charges, atomic numbers, or mass numbers?
alpha particle
beta particle
gamma ray
Answers
Answer: It is Gamma ray
Answer:
C. gamma ray
(Photo for proof at the bottom.)
Explanation:
A gamma ray is a type of radiation that occurs when an unstable nucleus emits a photon, the smallest unit of light. A photon has no mass or charge. Atomic and mass numbers are written based on the mass of the atom. But since no mass is lost when a photon is emitted, writing the emission of it isn't necessary.
Here's a photo of Edge, good luck.

What type of adaptations are these? Compare and contrast the adaptations of the Dart Frog and the
Hosta Plant. Your answer should be 3–4 sentences long.
Answers
The dart frog and hosta plant have different types of adaptations. The dart frog has physical adaptations such as bright colors, toxic skin, and webbed feet that help it survive in its environment.
These adaptations protect the frog from predators and enable it to move easily through its habitat. In contrast, the hosta plant has physiological adaptations such as the ability to tolerate shade and grow in moist soil. These adaptations help the plant to survive in its environment by conserving water and maximizing photosynthesis in low-light conditions. Overall, while both species have evolved adaptations to survive in their respective environments, the type of adaptations they have developed differ depending on their specific needs and challenges in their habitats.
To learn more about photosynthesis visit;
https://brainly.com/question/29764662
#SPL9
be sure to answer all parts. give a systematic name for the following formula: [pt(en)2br2](clo4)2
Answers
The systematic name for the given formula Pt(en)2Br22 is bis(ethylenediamine)dibromidoplatinum(IV) perchlorate.
In the systematic name, the prefix "bis" indicates that there are two ethylenediamine ligands, "dibromido" signifies the presence of two bromide ligands, "platinum(IV)" denotes the oxidation state of the platinum ion, and "perchlorate" represents the anion ClO4-. The overall coordination complex is enclosed in square brackets to indicate its unity.
The ethylenediamine ligand (en) is a bidentate ligand, meaning it coordinates to the metal ion through two nitrogen atoms. The presence of two bromide ligands and two perchlorate anions further completes the coordination sphere of the platinum ion.
To learn more about systematic
https://brainly.in/question/18770057
#SPJ11
an n-alkane of molecular formula c28h58 has been isolated from a certain fossil plant. write a condensed structural formula for this alkane.
Answers
The task is to write a condensed structural formula for an n-alkane with the given molecular formula.An n-alkane is an unbranched alkane.CH3(CH2)26CH3.
Which four forms of hydrocarbons are there?Alkanes, alkenes, alkynes, & aromatic hydrocarbons are the four subcategories that are typically used to classify hydrocarbons. These compounds might have relatively basic structures or relatively complicated ones.
The first four hydrocarbons are what?Typically, hydrogen atoms form a protective shell around carbon atoms to form molecules.Hydrocarbons are categorized into four primary groups: alkanes, alkenes, alkynes, and aromatic hydrocarbons.
To know more about molecular formula visit:
https://brainly.com/question/1247523
#SPJ4
rank the following in order to show the precipitation of halite from water. place the first event at the top and last event at the bottom.
Answers
The order to show the precipitation of halite from water are given below.
What is precipitation ?
Precipitation is the process of changing a dissolved substance from a super-saturated solution to an insoluble solid in an aqueous solution. Precipitate refers to the produced solid. The chemical agent that initiates the precipitation in an inorganic chemical process is referred to as the precipitant.
Step 1: Seawater starts to evaporate.
Step 2: Na cations and Cl anions concentrate as water molecules leave the liquid state and are left behind.
Step 3: Cl anions and Na cations move toward one another and start to bind.
Step 4: The resulting NaCl pairs join and start to form a crystal of halite, an ordered structure.
To learn more about Precipitation reaction click on the link below:
https://brainly.com/question/17074350
#SPJ4
Question:
Rank the following in order to show the precipitation of halite from water. Place the first event at the top and the last event at the bottom.
What is the formula for Water?
Name full name element's name
Answers
Answer:
2 Hydrogen atoms and 1 Oxygen atoms makes water
Explanation:
Hope this helps^^
Answer: Its chemical formula H2O, indicates that each of its molecules contains one oxygen and two hydrogen atoms,
Explanation: i hopes it helps :)
A bowling ball with a radius of 8.0 cm weighs 2722g. what is the density of the bowling ball?
Answers
The density of the bowling ball is 3.384 g/cm^3. The mass of a substance per unit of volume is its density. Density is most frequently represented by the symbol, however Latin letter D may also be used.
What is Volume?A closed surface or object's volume is measured in three dimensions and is expressed mathematically. Cubic measurements of volume include m3, cm3, in3, etc. The term "volume" is sometimes used to refer to "capacity." A three-dimensional space's occupied volume is measured. It is often numerically quantified using a variety of imperial units or SI-derived units, such as the cubic meter and liter (such as the gallon, quart, and cubic inch). The link between volume and length (cubed) is symbiotic. It is more common to think of a container's volume as its capacity rather than as the quantity of room it occupies. The amount of fluid (liquid or gas) that the container can hold is defined as its volume, in other words.Therefore,
The radius of the bowling ball = 8 cm
Weight of the ball = 2722 g
Volume of the bowling ball = 4 π r^2 = 4 x 3.142 x 8 x 8 = 804.352 cm^3
Density = Mass/Volume
⇒ Density of the ball = 2722/804.352
⇒ Density = 3.384 g/cm^3
Therefore, the density of the bowling ball is 3.384 g/cm^3.
To learn more about Volume, refer to:
https://brainly.com/question/1578538
#SPJ13
The density of the bowling ball is 3.384 g/cm^3. The mass of a substance per unit of volume is its density. Density is most frequently represented by the symbol, however Latin letter D may also be used.
What is Volume?A closed surface or object's volume is measured in three dimensions and is expressed mathematically. Cubic measurements of volume include m3, cm3, in3, etc. The term "volume" is sometimes used to refer to "capacity."
A three-dimensional space's occupied volume is measured. It is often numerically quantified using a variety of imperial units or SI-derived units, such as the cubic meter and liter (such as the gallon, quart, and cubic inch). The link between volume and length (cubed) is symbiotic.
It is more common to think of a container's volume as its capacity rather than as the quantity of room it occupies. The amount of fluid (liquid or gas) that the container can hold is defined as its volume, in other words.
Therefore,
The radius of the bowling ball = 8 cm
Weight of the ball = 2722 g
Volume of the bowling ball = 4 π r^2 = 4 x 3.142 x 8 x 8 = 804.352 cm^3
Density = Mass/Volume
⇒ Density of the ball = 2722/804.352
⇒ Density = 3.384 g/cm^3
Therefore, the density of the bowling ball is 3.384 g/cm^3.
To learn more about Volume, refer to:
brainly.com/question/1578538
#SPJ9
solve for x 5 to the power 2 x + 4 - 25 to the power x - 1 is equals to
Answers
Answer:
[][][][][][][][][][][][][][]