Describe what happens on the molecular level when acetic acid dissolves in water.
Answers
Since most acetic acid molecules do not dissociate when a sample is dissolved in water, acetic acid and water molecule interactions are what determine how soluble a substance is. In its liquid state, water forms a network of hydrogen bonds among its molecules; when a material dissolves in water, this network of hydrogen bonds is broken.
What is acetic acid such a poor electrolyte?Since acetic acid is a weak acid, only a small portion of the acetic acid molecules react to produce ethanoate and hydronium ions, shifting the equilibrium position substantially to the left. Aqueous acetic acid is a weak electrolyte as a result of the existence of these few ions.
Why does acetic acid have a low conductivity?The majority ($> 99%) of the acetic acid molecules remain after dissolution, with only a very tiny portion deprotonating to form acetate anions. Only the latter are charged compounds, and as a result, only these increase the conductivity of the solution. They are few, and conductivity is low.
What makes acetic acid more potent than water?The equilibrium shifts to the left and the concentration of hydrogen ions decreases when a strong acid is added to the buffer solution. A strong base is also added, which causes the equilibrium to move to the left and results in a smaller pH increase. Because of this, acetic acid is a better buffer than water.
Learn more about Acetic acid
https://brainly.com/question/17081077
#SPJ4
Related Questions
Question 3 of 25
Which statement describes a question that can guide the design of a
scientific investigation?
OA. It asks about whether a controlled variable is necessary.
B. It asks about the preferred outcome of the investigation.
C. It asks about how the observations will be organized.
D. It asks about a cause-and-effect relationship between two
variables.
Answers
The statement that describes a question that can guide the design of a scientific investigation could be about the necessity of a controlled variable. The correct answer is A.
What is a controlled variable?It is a variable whose values are controlled by the researcher during the course of experimental investigations.
When designing an experiment, it is necessary to identify the different variables that would be involved for a successful investigation.
Variables can be independent, dependent, controlled, or constant. Identifying the necessary variables will help in designing the experiment.
More on variables can be found here: https://brainly.com/question/15740935
#SPJ1
Which statement below best describes a catalyst?
Question 3 options:
An item that can slow reactions rates
A molecule that is consumed in a chemical reaction
An item that can increase reaction rates
An item that increases the concentration of reactions
Answers
Canal rays was the first name given to what?
Answers
What always happens when an acid is neutralized by an alkali?
Acids and Alkalis:
An acid is a substance that liberates hydrogen ions (protons) when allowed to dissociate in aqueous media. Examples are hydrochloric acid, sulfuric acid, nitric acid, etc, all of which are very strong acids, and ethanoic acid, citric acid, etc, which are weak acids. An alkali generally gives off hydroxyl ions in aqueous media. Examples are sodium hydroxide, potassium hydroxide, ammonia, etc.
Answers
Answer:
An acid is a substance that liberates hydrogen ions (protons) when allowed to dissociate in aqueous media. Examples are hydrochloric acid, sulfuric acid, nitric acid, etc, all of which are very strong acids, and ethanoic acid, citric acid, etc, which are weak acids. An alkali generally gives off hydroxyl ions in aqueous media. Examples are sodium hydroxide, potassium hydroxide, ammonia, etc.An acid is a substance that liberates hydrogen ions (protons) when allowed to dissociate in aqueous media. Examples are hydrochloric acid, sulfuric acid, nitric acid, etc, all of which are very strong acids, and ethanoic acid, citric acid, etc, which are weak acids. An alkali generally gives off hydroxyl ions in aqueous media. Examples are sodium hydroxide, potassium hydroxide, ammonia, etc.
Explanation:
An acid is a substance that liberates hydrogen ions (protons) when allowed to dissociate in aqueous media. Examples are hydrochloric acid, sulfuric acid, nitric acid, etc, all of which are very strong acids, and ethanoic acid, citric acid, etc, which are weak acids. An alkali generally gives off hydroxyl ions in aqueous media. Examples are sodium hydroxide, potassium hydroxide, ammonia, etc.
a weather system moving through the american midwest produced rain with an average ph of 5.07. by the time the system reached new england, the rain it produced had an average ph of 4.66. 1st attempt see hint how much more acidic was the rain falling in new england? times more acidic
Answers
Rain in New England is 2.57 times more acidic than rain in the Midwestern United States.
The acidity of a solution depends on the concentration of H⁺ ions ([H¹⁺ ]). This concentration can be calculated from pH using the following formula:
pH = -log([H⁺])
american midwest
pH = -log([H⁺])
5.07 = -log([H⁺])
[H⁺] = antilogarithm (-5.07) = 8.51 × 10⁻⁶ M
new england
pH = -log([H⁺])
4.66 = -log([H⁺])
[H⁺] = antilog (-4.66) = 2.19 × 10⁻⁵ M
The concentration ratios are:
\(\frac{2.19 \times 10^{-5} M}{8.51 \times 10^{-6} M} =2.57\)
Rain in New England is 2.57 times more acidic than rain in the Midwestern United States.
learn more about acidity here https://brainly.com/question/11543614
#SPJ4
What happens when a piece of Mg ribbon is burnt? Name the
new substance formed. Write a balanced chemical equation.
Answers
Answer:
When a Magnesium Ribbon is burnt, a powdery substance called magnesium oxide is formed.
Explanation:
There has obviously been a chemical change because several chemical properties of the magnesium have been modified: the color, the texture and the mass.
The increase in mass is due to the fact that oxygen from the air has combined with the magnesium to make magnesium oxide, MgO.
The chemical equation, Mg + O2 MgO shows this reaction but it needs to be balanced to make 2Mg + O2 2MgO.
Using stoichiometry, we can convert this eqation into an equation with moles:
2 mol Mg + 1 mol O2 2 mol MgO.
Next, we convert to grams using atomic masses obtained from the periodic table:
48g Mg + 32g O2 80g MgO
Lastly, we determine the same thing in the proportions we used. In other words, we used only 0.15g of Mg (not 48g) so everything needs to be divided by 320. So 80 / 320 = 0.25 g. If we burn 0.15 g of Mg, we obtain 0.25 g of MgO.
Hope this helps!!!
This is my first answer.
ANSWERS PLEASE HELP
i need answers

Answers
Answer:
C. is the correct answer
Explanation:
what is solution, colloid, suspension?
Answers
Explanation:
SOLUTION:Solution is a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to what is called the limit of solubility.
COLLOID:A mixture in which one substance is divided into minute particles (called colloidal particles) and dispersed throughout a second substance
SUSPENSION:A suspension is a heterogeneous mixture of a finely distributed solid in a liquid. The solid is not dissolved in the liquid, as is the case with a mixture of salt and water.
A compound contains 29.27% carbon, 51.22% nitrogen, and 19.50% oxygen. What is the molecular formula if the molar mass is 570.5 grams?
Answers
Answer:
its either A or B most likley A if im wrong then im sorry.
Explanation:
have a wonderfull day
a closed piston-cylinder device contains 5l of water vapor are initially at 300 kpa and 200 deg c. the water undergoes some process taking it to a final state of 175 kpa and a quality of 50%. determine the change of enthalpy between the initial and final state
Answers
The change in enthalpy (Δh) between the initial and final state is \(-48270 kJ\)
The change in enthalpy (Δh) between the initial and final state can be calculated using the following equation:
Δ\(h = m (h2 - h1)\)
where m is the mass of water vapor, h1 is the enthalpy of the initial state and h2 is the enthalpy of the final state.
Since the initial state is at 300 kPa and 200°C, the enthalpy of the initial state (h1) can be calculated using the steam tables. From the steam tables, the enthalpy of the initial state (h1) is 2760 kJ/kg.
The final state is at 175 kPa and a quality of 50%. The enthalpy of the final state (h2) can also be calculated using the steam tables. From the steam tables, the enthalpy of the final state (h2) is 1703 kJ/kg.
Now, the mass of water vapor (m) in the closed piston-cylinder device can be calculated using the ideal gas law equation.The following equation is the ideal gas law:
\(PV = mRT\)
where R is the gas constant, T is the temperature, V is the volume, m is the mass, and P is the pressure.
Since the volume of the closed piston-cylinder device is 5 liters and the temperature is 200°C, the mass of the water vapor (m) can be calculated as follows:
\(m =\frac{ (300 kPa * 5 liters)}{ (0.287 *323.15 K)}\)
Therefore, the mass of the water vapor (m) is 41.6 kg.
Finally, the change in enthalpy (Δh) between the initial and final state can be calculated using the equation given above. Thus,
Δ\(h = 41.6 (1703 - 2760) = -48270 kJ\)
Therefore, the change in enthalpy (Δh) between the initial and final state
is \(-48270 kJ\).
learn more about enthalpy Refer:brainly.com/question/13996238
#SPJ4
What air mass is represented by the letters C and T?
Answers
Explanation:
Based on temperature: tropical (warm), polar (cold), arctic (extremely cold). Naming convention for air masses: A small letter (c, m) indicates the moist content followed by a capital letter (T, P, A) to represent temperature.
The Continental tropical air masses are represented by the letters C and T.
Air Mass:
It is defined as the volume of air that is distributed in thousands of square miles and adapts to the characteristics of the surface.
Air mass is based on the temperature and humidity in the air. There are 4 types of air masses arctic, tropical, polar, and equatorial.
Continental tropical (cT), is a type of air mass. They are very hot and dry. They formed during the summer over the Desert Southwest and northern Mexico.
Therefore, the Continental tropical air masses are represented by the letters c and T.
To know more about Continental tropical (cT):
https://brainly.com/question/18257713
Please balance the equation, putting the correct coefficient in each box. Do not leave any boxes blank! Enter "1" for formulas having a presumed coefficient of 1. F2 + P2 --> PF3
Answers
Answer:
3F2+P2_>2PF3now f is 6 on both sides and your p is also 2 on both sides
consider the following balanced equation for the complete neutralization of nitric acid of unknown concentration with aqueous 0.100 m potassium hydroxide in a titration reaction. hno3(aq) koh(aq) -----> kno3(aq) h2o(l) if 59.7 ml of the base are required to neutralize 21.4 ml of the acid, what is the molarity of the acid? enter only the numeric value for your answer (no units).
Answers
The molarity of the unknown nitric acid solution is 0.279 M.
Balanced chemical equation for the reaction will be;
HNO₃(aq) + KOH(aq) → KNO₃(aq) + H₂O(l)
According to the equation, 1 mole of KOH reacts with 1 mole of HNO₃ to produce 1 mole of KNO₃ and 1 mole of H₂O. Therefore, the number of moles of KOH used in the titration is equal to the number of moles of HNO₃ in the original solution.
Volume of HNO₃ solution used = 21.4 mL = 0.0214 L
Volume of KOH solution used = 59.7 mL = 0.0597 L
Molarity of KOH solution = 0.100 M
Number of moles of KOH used = molarity x volume = 0.100 M x 0.0597 L = 0.00597 moles
Since 1 mole of KOH reacts with 1 mole of HNO₃, the number of moles of HNO₃ in the solution is also 0.00597 moles.
Molarity of HNO₃ solution = number of moles / volume = 0.00597 moles / 0.0214 L
= 0.279 M
To know more about molarity here
https://brainly.com/question/8732513
#SPJ4
what is a metal that forms two types of oxides and rusts in moisture
Answers
Answer:
An example of a metal that rusts is Iron
Answer:
Iron
Explanation:
The metal that forms 2 types in moisture is iron.
Devin had a bucket of shredded metals the metals consist of aluminum steel and copper he tried sorting the mixture by running it through a filter but this did not sort the mixture into separate components what can devin conclude
Answers
Answer:
Devin's problem can be solved by exploiting the activity series of metals.
Explanation:
This determines what metals are more reactive and would be readily displaced in a reaction.
Seeing as they are both metals scraps of similar sizes, filtration isn't a solution. But since Copper is lower on the activity series than Aluminium, adding an acidic solution (say tetraoxosulphate VI solution) to the mixture, would have aluminium in solution and Cu as residue which can then be filtered off.
I hope this was helpful.
Why is it safe to use salt in your food if salt is made of two dangerous elements?
Answers
Because sodium and chlorine on their own have really poisonous/combustive characteristics. However, when they have been chemically bonded together, their characteristics change. They'll be completely harmless. =]
Do NOT ever try a mixture of the two (one that hasn't being chemically combined) it can have fatal results.
What is true about proportions? Select all that apply.
(Just keep in mind that this is for chemistry class, when trying to find the mass or volume of a substance in an equation.)
A- Proportions are the amount of a substance that contains 6.02 x 1023 particles.
B- Proportions have cross products that are equal.
C- Proportions can be written as equal fractions.
D- Proportions are sums that compare compounds.
Answers
The true statement about proportions in this context is that; "Proportions have cross products that are equal."
Proportions in stoichiometryThe term stoichiometry is umbrella term that encompasses all the calculatrions that are aimed at calculating the amount of substance using mass - volume relationships. Usually, stoichiometry makes use of proportions.
The true statement about proportions in this context is that; "Proportions have cross products that are equal."
Learn more about proportions: https://brainly.com/question/9743981
An atmospheric pressure, air-water mixture is at 375 °C and the
vapor pressure is 8.02 kPa. What is the relative humidity?
Answers
The relative humidity of the air-water mixture at 375 °C and with a vapor pressure of 8.02 kPa is 50.7%
To determine the relative humidity, we need to compare the actual vapor pressure (e) of water in the air-water mixture to the saturation vapor pressure (es) at the same temperature. The relative humidity (RH) is then given by the ratio of e to es, expressed as a percentage.
Given:
Temperature (T) = 375 °C
Vapor pressure (e) = 8.02 kPa
First, we need to find the saturation vapor pressure at 375 °C. The saturation vapor pressure can be calculated using the Antoine equation:
log10(es) = A - (B / (T + C))
For water, the Antoine equation constants are:
A = 8.07131
B = 1730.63
C = 233.426
Plugging in the values, we can calculate the saturation vapor pressure (es) at 375 °C.
log10(es) = 8.07131 - (1730.63 / (375 + 233.426))
log10(es) = 8.07131 - (1730.63 / 608.426)
log10(es) = 8.07131 - 2.84481
log10(es) = 5.2265
Taking the antilog of both sides:
es = 10^5.2265
es ≈ 15826.98 Pa (or 15.827 kPa)
Now we can calculate the relative humidity:
RH = (e / es) * 100
RH = (8.02 kPa / 15.827 kPa) * 100
RH ≈ 50.7%
Therefore, the relative humidity of the air-water mixture at 375 °C and with a vapor pressure of 8.02 kPa is approximately 50.7%.
Learn more about humidity from the given link
https://brainly.com/question/30765788
#SPJ11
which electron in sulfur is most shielded from nuclear charge?
Answers
Answer: 3p Orbitals
Explanation:
Electrons present in the 3p orbitals are farthest from the nucleus. Therefore, the electrons present in the 3p orbital will be shielded by the electrons present in the inner orbitals. Hence, 3p orbital in sulfur is most shielded from the nuclear charge".
An electron in 3p orbital of sulfur is most shielded from nuclear charge. It is true that the core electrons effectively protect the surrounding electrons from nuclear charge.
What is electron ?The elementary electric charge of the electron is a negative one, making it a subatomic particle. Due to their lack of known components or substructure, electrons, which are members of the first generation of the lepton particle family, are typically regarded to be elementary particles.
The atom is made up of the subatomic particles electron, proton, and neutron. The atom is made up of a core nucleus that has neutrons and protons in it. The nucleus is surrounded by electrons. Protons are positively charged, neutrons are neutral, and electrons are negatively charged.
The shielding ability of an electron in a s orbital is higher than that of an electron in a p or d orbital of the same shell. Additionally, electrons in s orbitals are less well protected by electrons in other orbitals due to their high penetrating nature.
Thus, An electron in 3p orbital of sulfur is most shielded from nuclear charge.
To learn more about electron follow the link below;
https://brainly.com/question/1255220
#SPJ2
Which statement best describes a solution containing a strong base?
A. the solution is dilute
B. The solution is concentrated
C. a small amount of the base dissociates in solution
D nearly all of the base dissociates in solution
Answers
of the following, which is true of primary batteries? select the correct answer below: primary batteries can always be recharged. an alkaline battery can deliver about thirty to fifty times the energy of a zinc-carbon dry cell of similar size. alkaline batteries are a type of primary battery prone to leaking potassium hydroxide. zinc-carbon dry cell batteries were designed as direct replacements for alkaline batteries.
Answers
Answer:
Alkaline batteries are a type of primary battery prone to leaking potassium hydroxide.
Explanation:
A primary battery is a single-use battery; with the exception of some alkaline batteries, they cannot be recharged. Alkaline batteries are a type of primary battery designed as a direct replacement for dry cell batteries. They can deliver about three to five times the energy of a zinc-carbon dry cell battery of similar size. However, alkaline batteries are prone to leaking potassium hydroxide, so they should be removed from devices for long-term storage.
The correct answer is that an alkaline battery can deliver about thirty to fifty times the energy of a zinc-carbon dry cell of similar size. This is because alkaline batteries use a more efficient chemical reaction to produce electricity than zinc-carbon dry cell batteries.
It is important to note that primary batteries, including alkaline and zinc-carbon dry cell batteries, cannot be recharged and must be replaced when their energy is depleted. Additionally, while alkaline batteries are prone to leaking potassium hydroxide if left unused for a long time or if damaged, proper handling and storage can prevent this issue. Zinc-carbon dry cell batteries were not designed as direct replacements for alkaline batteries, but rather as a lower-cost alternative.
The correct statement among the given options is: alkaline batteries are a type of primary battery prone to leaking potassium hydroxide. Primary batteries, such as alkaline and zinc-carbon dry cell batteries, are designed for single-use and cannot be recharged. Alkaline batteries do have a higher energy capacity compared to zinc-carbon dry cells, but not 30-50 times more. Lastly, zinc-carbon dry cell batteries were not designed as direct replacements for alkaline batteries, as they have different chemistries and performance characteristics.
To know more about batteries visit:
https://brainly.com/question/11670669
#SPJ11
1) Explain the change in conductivity that occurred when you diluted denatured ethanol to 20% by volume using deionized water. What does your data suggest about the deionized water that you are using in this experiment
Answers
When diluting denatured ethanol to 20% by volume using deionized water, the conductivity of the solution is expected to decrease. This is because deionized water has a lower concentration of ions compared to the denatured ethanol.
The lower ion concentration in deionized water leads to a decrease in conductivity. Therefore, the data suggests that deionized water is a good choice for dilution in this experiment as it minimizes the presence of ions in the solution.
Denatured ethanol is also known as denatured alcohol. It is ethanol (ethyl alcohol) that has been intentionally rendered unfit for human consumption by adding substances that are called denaturants and these denaturants are toxic or unpleasant-tasting compounds.
To know more about denatured ethanol, refer
https://brainly.com/question/31786352
#SPJ11
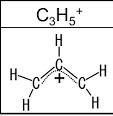
What can you conclude about the symmetry of the C3H5 ion as it actually exists, as regards the bond lengths and the distribution of the positive charge
Answers
In the allyl cation, all the bond lengths are equal and the positive charge is delocalized over all the three carbon atoms.
The allyl cation has the formula C2H5+. In the allyl cation, the positive charge is delocalized over the three carbon atoms as shown in the image attached. Hence, relevant resonance structures can be proposed for the allyl cation.
The bond lengths in the allyl cation are intermediate between the bond lengths of double bonds and single bonds. All the bond lengths are equal.
Learn more: https://brainly.com/question/12108425
The unbalanced basic equation for the reaction in most airbags is NaN3 --> Na + N2.
A normal airbag needs about 67 liters of Nitrogen gas to fully inflate. (At STP: 1 mole of gas = 22.4 liters of that gas.) How many grams of reactant is needed to produce that amount of gas?
Answers
Answer:52!
Explanation:
How are energy and work related? A. Energy is the force needed to do work B. Work times energy is force C. Energy is the capacity to do work D. Work and energy are the same
Answers
Answer:
I believe the answer is A
Explanation:
Work and energy are related because when you work, you cause displacement in the object you are exerting upon. While this happens, you transfer energy between the systems. Both work and energy share the same SI unit, called the joule.
PLEASE HELP ME!
Aspartame is an artificial sweetener that is 160 times sweeter than table sugar. It is marketed as Pal-Sweet. The molecular formula of aspartame is C¹⁴H18N²O5.
i) Name the elements that made up aspartame. [2 marks]
(ii) Calculate the relative molecular mass of aspartame. [2 marks]
(iii) Calculate the number of moles in 7.35g aspartame. [3 marks]
(iv) Calculate the number of molecules in 5.88 g aspartame. [2 marks]
(v) Calculate the mass in grams of one molecule of aspartame. (H=1, C=12, N=14, 0=16, N = 6 x 10²³mole) [3 marks]
Answers
Answer:
A. Carbon, Hydrogen, Nitrogen, and Oxygen
B. 12(14) + 1(18) +12(2)+16(5)= 294.20g
C. 7.35/294.3=0.0250mol
D. 5.88g/294.3=0.01997961264 × 6.02 × 10^2= 12.028
Explanation:
The answer is suppose to be B but I don’t understand why not D

Answers
How many protons are in neon
Answers
Answer:
10
Explanation:
Neons atomic # is 10 and the atomic number is the same as the # of electrons and protons
10 protons ........................Explanation:
How many atoms of Na are in the following formula: 3Na(SO4)2
1) 3
2) 6
3) 2
4) 4
Answers
Answer:
Only 3 atoms
Explanation:
The 3 came from the coefficient.
What volume will 50. 0 g of nitrogen gas occupy at STP?
Answers
Answer:
39.98 liters
Explanation:
hope this helps