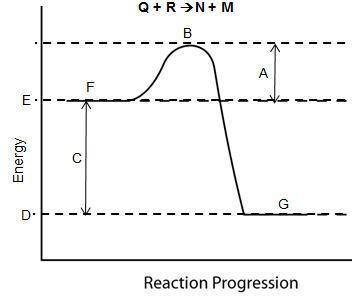
Answers
Answer:
A) enthalpy of reaction
Explanation:
The region C signifies the enthalpy of reaction.
This diagram is the energy profile of an endothermic reaction. In such reaction, heat is absorbed from the surrounding. At the end of the reaction, the heat of product is lesser than that of the reactants.
Enthalpy changes are heat changes accompanying a physical and chemical change. An enthalpy is the difference between the sum of the heat contents of products and sum of the heat contents of reactants.it is indeed A) enthalpy of reaction
Related Questions
If more energy is absorbed than what is released during bond breaking and forming,the reaction is blank
Answers
If more energy is absorbed than what is released during bond breaking and forming, the reaction is endothermic.
When bonds in the reactants are broken in endothermic reactions, greater energy is absorbed than emitted when new bonds are created in the products.
The energy required to break existing bonds in endothermic processes is more than the energy released when new bonds are generated. In an exothermic process, more energy is generated when new bonds are created than is consumed when old ones are broken.
If more energy is absorbed than what is released during bond breaking and forming, the reaction is endothermic.
Learn more about endothermic reactions, here:
https://brainly.com/question/28909381
#SPJ1
What is the most stable element in the universe (in terms of binding energy)?
A. uranium
B. gold
C. iron
D. hydrogen
E. helium
F. lead
G. water
Answers
Answer:
C).Iron
Explanation:
Iron has a binding energy of 8.8MeV
What is formed when a BASE dissociates in water, according to the Brønsted-Lowry definition?
a salt
a stronger acid
a conjugate base
a conjugate acid
Answers
A Conjugate acid is formed when a BASE dissociates in water, according to the Brønsted-Lowry definition.
hence, option (c) is correct
What is Bronsted-lowry definition ?
A Bronsted-Lowry acid is defined as a substance that gives up or donates hydrogen ions during a chemical reaction.
In contrast, a Bronsted-Lowry base accepts hydrogen ions.
Another way of looking at it is that a Bronsted-Lowry acid donates protons, while the base accepts protons
hence, option (c) is correct
Learn more about acid base concept here ;
https://brainly.com/question/14228337
#SPJ1
Answer:
D
Explanation:
what is the name given to the group in box? + explain please

Answers
The name given to the group delimited in the image is ethyl.
What is ethyl?It is a hydrocarbon from the alkyl functional group.
Ethyl is a substituent derived from ethane. The formula for ethane is \(C_2H_6\), whereas, ethyl has one H less than ethane.
Thus, the formula for ethyl is \(C_2H_5\).
Looking at the delimited portion of the image, 2 C atoms in C-C bond are bonded by 5 H atoms.
More on alky groups can be found here: https://brainly.com/question/15942698
#SPJ1
EASY CHEM, WILL GIVE BRAINLIEST!!

Answers
Hi there!
\(\large\boxed{4.04g}\)
To solve, we can use dimensional analysis to convert from molecules to grams.
We must use Avogadro's number (6.02 × 10²³) in converting from molecules to moles.
After converting, multiply by the atomic mass, or grams per mole.
\(1.204 * 10^{24} moles *\frac{1 mol}{6.02*10^{23}moles}* \frac{2.02g}{1mol }= 4.04g\)
If your equation includes 7(CrO4)2, how many Cr's are there?
If your equation includes 7(CrO4)2, how many O's are there?
Answers
If an equation includes 7(CrO₄)₂, the numbers of Cr's and O's atoms that are there are 14 and 56 respectively.
How to calculate number of atoms?The number of atoms present in a chemical compound can be calculated by multiplying the subscript of the particular element by any coefficient.
According to this question, 7 moles of chromate with the chemical formula; (CrO₄)₂ is given. The number of oxygen and chromium atoms in this compound can be calculated as follows:
Chromium = 7 (coefficient) × 2 = 14 atomsOxygen = 7 (coefficient) × 8 = 56 atomsLearn more about no of atoms at: https://brainly.com/question/14190064
#SPJ1
Chair and Boat Conformers of Cyclohexane (C6H12). Note it is impossible to place all the carbons in the same plane without straining the bonds. Take two opposite carbons and pull both of them up to make one conformation and then pull one of them down to make the other conformation.
a. Can you interconvert one conformer into the other without breaking any bonds?
b. Explain why these represent conformers and not isomers.
Answers
Answer:
See explanation
Explanation:
Conformation refers to the various spatial arrangements of atoms in a molecule that result from free rotation across the carbon-carbon single bond.
There are two possible conformations of cyclohexane. They are; the chair and boat conformations.
We can convert the molecule from one conformation to another by rotation of single bonds.
These conformations are not isomers. Isomers are different molecules while conformers are different spatial arrangements of the same molecule obtained by rotation across carbon-carbon single bonds. Isomers are not obtained by rotation across carbon-carbon single bonds.
Hence, the chair and boat conformers of cyclohexane are obtained by rotation across the carbon-carbon single bond hence they are conformers and not isomers.
The measure of the length of events and the duration of intervals between events
Answers
The measure of the length of events and the duration of intervals between events is time.
What is time?The duration of events or the gaps between them can be measured, compared, or even ordered using time. The lengthy period of time that the Earth's geologic history takes up is known as geologic time. Starting at the beginning of the Archean Eon formal geologic time runs until the present. Geology is defined as the "Science of the Earth."
Geology is the fundamental Earth science that examines how the earth created, its structure and composition, and the various forces acting on it. It is sometimes known as geoscience or earth science.
Learn more about time at;
https://brainly.com/question/479532
#SPJ1
How many moles of NaCl can be produced from 2.5 moles of BaCl_2.
NaOH+BaCl-> NaCl+BaOH2
Answers
Answer:
5 moles of NaCl
Explanation:
Here is the balanced reaction:
2NaOH + BaCl2 -> 2NaCl + Ba(OH)2
1 mole of BaCl2 will produce 2 moles of NaCl
so 2.5 moles of BaCl2 will produce 2.5 x 2 = 5 moles of NaCl
Draw out the skeletal structure of cis-2-methylcyclohexano
Answers
The chemical structure of the compound is shown in the image attached.
How do you draw a chemical structure?Ascertain the molecule's atomic composition and the types of bonds (covalent, ionic, etc.) that each atom forms.
The skeletal structure, which is a straightforward illustration of the molecule's framework, should be drawn first. To do this, a series of lines are drawn to symbolize the atoms' bonds.
By positioning the atoms at the ends of the bond lines, you may complete the skeleton framework.
Learn more about chemical structure:https://brainly.com/question/30261824
#SPJ1

cyanate ion waste solution from gold-mining operations can be destroyed by treatment with hypochlorite ion in basic solution. Write a balanced oxidation-reduction equation for this reaction. OCN^-(aq) +OCl^-(aq) --> CO2^-(aq)+N2(g)+Cl^-(aq)+H2O(l)
Answers
The balanced oxidation-reduction equation for the destruction of cyanate ion waste solution from gold-mining operations by treatment with hypochlorite ion in basic solution is:
OCN⁻(aq) + OCl⁻(aq) + 2OH⁻(aq) → CO₂⁻(aq) + N₂(g) + Cl⁻(aq) + H₂O(l)
In this reaction, the cyanate ion (OCN⁻) is oxidized to carbon dioxide (CO₂⁻) and nitrogen gas (N₂), while the hypochlorite ion (OCl⁻) is reduced to chloride ion (Cl⁻). The reaction takes place in basic solution, which provides the hydroxide ions (OH⁻) needed to neutralize the acidic H⁺ ions produced during the oxidation of the cyanate ion.
The reaction is exothermic, releasing heat energy as the products form. This reaction is an effective way to dispose of the cyanate ion waste generated by gold-mining operations, as it converts the hazardous waste into harmless gases and ions.
To learn more about oxidation-reduction equation, here
https://brainly.com/question/13699873
#SPJ1
If 22.00 mL of 2.00 M potassium iodide is needed to reach the equivalence point with 18.00 mL of lead (II) nitrate, determine the molarity of the lead (II) nitrate solution. Note: First write the balance equation between potassium iodide and lead (II) nitrate.
Answers
The molarity of the lead(II) nitrate solution if 22.00 mL of 2.00 M potassium iodide is needed to reach the equivalence point with 18.00 mL of lead (II) nitrate is 1.2M.
How to calculate molarity?The molarity of a solution can be calculated using the following formula:
CaVa/CbVb = na/nb
Where;
Ca = concentration of acidCb = concentration of baseVa = volume of acidVb = volume of basena = number of moles of acidnb = number of moles of baseThe balanced equation of the reaction is as follows:
Pb(NO3)2 (aq) + 2KI (aq) → 2KNO3 (aq) + PbI2 (s)
22 × 2/18 × Cb = 2/1
44/18Cb = 2
Cb = 44 ÷ 36
Cb = 1.2M
Therefore, the molarity of the lead(II) nitrate solution if 22.00 mL of 2.00 M potassium iodide is needed to reach the equivalence point with 18.00 mL of lead (II) nitrate is 1.2M.
Learn more about molarity at: https://brainly.com/question/356585
The substance krypton has the following properties normal melting point 115.9 K normal boiling point: 119.8 K triple point: 0.72 atm. 115.8 K critical point: 54.3 atm. 209.4 K A sample of krypton at a pressure of 1.00 atm and a temperature of 149.2 K is cooled at constant pressure to a temperature of 107.8 K Which of the following are true?
a) The final state of the substance is a solid
b) One or more phase changes will occur
c) The final state of the substance is a liquid
d) The sample is initially a gas
e) The solid initially present will vaporize.
Answers
Answer:
B. One or more phase change occur
C. the final state of substance is liquid
D. the sample initially gas
Explanation:
The pressure p = 1.00 atm
The temperature t = 149.2K
The temperature > 119.8K
119.8K being the normal boiling point.
This shows that the krypton is a gas
After it has cooled the pressure = 1.00 atm
The temperature T dropped to 107.8K
T < 115.9K
The melting point has been put as 115.9K
This is a liquid. The final state of the substance is a liquid. Since the temperature is less than the melting point and the pressure is 1.00 atm
Using Reaction A, how many grams of CO2 can be created from 5.67 moles of water?
127.6 g CO2
199.6 g CO2
81.65 g CO2
311.85 g CO2

Answers
Answer:
81.65 g CO2
Explanation:
1. 50.0 g of an unknown metal was heated to 95.0 °C and added to 45.0 g of water at 25.0 °C
in a calorimeter. Once the system reached equilibrium the final temperature was 28.5 °C.
Calculate the specific heat capacity of the metal with correct units.
2. 60.0 g of nickel heated to 100.0 °C was added to 55.0 g of water at 25.0 °C inside a
calorimeter and the system was allowed to reach equilibrium. What would be the final
temperature of the metal and water? (specific heat of Ni = 0.44 J/g °C)
.
3. One challenge during calorimetry is to minimize the heat lost to the environment during
the experiment. Suggest how you would minimize the heat loss during the experiment (read
the steps of the experiment before you answer the question)
Answers
Answer
Q = mcΔT
Qwater = -Qmetal
(100 g)(4.18 J/g°C)(25°C - 20°C) = -(50 g)c(25°C - 90.0°C)
2090 J = (3250 g °C)c
c = 0.643 J/g °C
The temperature of a sample of gas in a steel container at 25.0 kPa starts at -50 C and decreases by a factor of three. What is the final pressure inside the tank?
Answers
Answer: The final pressure inside the tank is 8.41 kPa.
Explanation: We can use the combined gas law to solve this problem, which relates the pressure, volume, and temperature of a gas:
(P1V1)/T1 = (P2V2)/T2
where P1, V1, and T1 are the initial pressure, volume, and temperature, respectively, and P2, V2, and T2 are the final pressure, volume, and temperature, respectively.
We are given P1 = 25.0 kPa, T1 = -50 C = 223 K, and V1 is unknown. We also know that the temperature decreases by a factor of three, so T2 = T1/3 = 223/3 K.
To find V2, we need to assume that the steel container is rigid and its volume remains constant. Therefore, V1 = V2, and we can cancel out the volume from the equation:
P1/T1 = P2/T2
Substituting the values, we get:
P2 = P1 * T2 / T1 = 25.0 * (223/3) / 223 = 8.41 kPa
Therefore, the final pressure inside the tank is 8.41 kPa.
Answer:
So if pressure of a gas is increased by 25%, the volume of a gas is decreased by 25%.
Explanation:
URGENT- please do by 14th July if possible!!!
1. How do metals react with acids?
2. What are the similarities and differences in the way different metals react with water and acids?
3. Why are some metal is more reactive than others
4. Why is the reactivity of metals so important to us?
5. What the displacement reactions?
6. Why do you displacement reactions happen?
7. Why are they important to us?
8. How are displacement reactions explained as redox reactions?
Thank you!
Answers
Answer:
Acids react with most metals to form hydrogen gas and salt. ... When an acid reacts with metal, salt and hydrogen gas are produced
If a pork roast must absorb 1500 kJkJ to fully cook, and if only 14% of the heat produced by the barbeque is actually absorbed by the roast, what mass of CO2CO2 is emitted into the atmosphere during the grilling of the pork roast?
Answers
You need to know the amount of heat generated by the combustion reaction.
Assuming propane as fuel, you can use thiis data:
C3H8(g)+5O2(g)---3CO2(g)+4H2O(g) ΔH= -2217 KJ
So when 3 moles of CO2 is emmitted 2217 kJ of heat is produced.
The molar wegiht of CO2 is 12 g/mol + 2 * 16 g/mol = 44 g/mol.
Then 3 mol * 44 g / mol = 132 g of CO2 are produced with 2217 kJ of heat.
Now you have to calculate how much energy you need to produce if only 12% is abosrbed by the pork
Energy absorbed by the pork = 12% * total energy =>
total energy = energy absorbed by the pork / 0.12 = 1700 kJ / 0.12 = 14,166.67 kJ.
Now, state the proportion:
132 g CO2 / 2217 kJ = x / 14,166.7 kJ =>
x = 14,166.67 * 132 / 2217 = 843.48 g CO2.
Answer: 843 g of CO2
If 2.47 g of CuNO3 is dissolved in water to make a 0.820 M solution, what is the volume of the solution in milliliters?
volume:
Answers
Answer:
23.4 milliliters
Explanation:
Note 1: This answer assumes that the volume of CuNO3 is negligible
Note 2: CuNO3 can't be produced in any meaningful quantities and can't be obtained by the average chemist, maybe you meant Cu(NO3)2 instead?
From the definition of molarity, molarity = moles / volume
the number of moles is the number of grams divided by the molar mass, or
2.47 divided by 125.55 which is 0.01967
The M and moles is known so volume can be found.
0.82 = 0.01967 / volume
0.82 * volume = 0.01967
volume = 0.01967 / 0.82 = 0.023988 liters = 23.4 milliliters
ties there khat is Chemical Compound?
Answers
Answer:
In chemistry, a compound is a substance made up of two or more different chemical elements combined in a fixed ratio. When the elements come together, they react with each other and form chemical bonds that are difficult to break.
Explanation:
brainlist
Drag the tiles to the correct boxes to complete the pairs. Not all tiles will be used.
Match each SI unit to the quantity it measures.
Answers
The SI unit to the quantity it measures are:
mass - kilogram, gramtemperature - kelvintime - second, nanosecondelectric current - ampereWhat is SI unit used for?Mass: The mass of an object is a measure of its amount of matter. The SI unit of mass is the kilogram (kg) or gram (g).
Temperature: Temperature is a measure of the average kinetic energy of the particles in a substance. The SI unit of temperature is the kelvin (K).
Time: Time is a measure of the interval between two events. The SI unit of time is the second (s).
Electric current: Electric current is a measure of the flow of electric charge. The SI unit of electric current is the ampere (A).
Find out more on SI unit here: https://brainly.com/question/16393390
#SPJ1
Complete question:
Drag the tiles to the correct boxes to complete the pairs. Not all tiles will be used.
Match each SI unit to the quantity it measures.

You weigh out a 0.470-sample of hydrated nickel (II) chloride, NiCl2·xH2O. Upon heating, the mass of the anhydrous salt that remains is 0.256 grams. What is the formula of the hydrate? What is the name of the hydrate?
Answers
The formula of the hydrate is NiCl2·4.27H2O. The name of the hydrate is nickel (II) chloride tetrahydrate.
To determine the formula and name of the hydrate, we need to first find the value of "x" in the formula NiCl2·xH2O using the given information.
Mass of water = Initial mass - Mass of anhydrous salt
Mass of water = 0.470 g - 0.256 g
Mass of water = 0.214 g
The molar mass of H2O is 18.015 g/mol, so the number of moles of water present in the hydrated salt can be calculated as:
Moles of water = Mass of water / Molar mass of water
Moles of water = 0.214 g / 18.015 g/mol
Moles of water = 0.0119 mol
The number of moles of anhydrous salt can be calculated by dividing its mass by its molar mass:
Moles of anhydrous salt = Mass of anhydrous salt / Molar mass of anhydrous salt
Moles of anhydrous salt = 0.256 g / (Ni: 58.69 g/mol + 2Cl: 2 x 35.45 g/mol)
Moles of anhydrous salt = 0.00279 mol
The ratio of moles of water to moles of anhydrous salt is equal to "x" in the formula NiCl2·xH2O:
Moles of water / Moles of anhydrous salt = x
0.0119 mol / 0.00279 mol = x
x ≈ 4.27
Therefore, the formula of the hydrate is NiCl2·4.27H2O. The name of the hydrate is nickel (II) chloride tetrahydrate.
learn more about hydrates here
https://brainly.com/question/16275027
#SPJ9
If He(g) has an average kinetic energy of 7450 J/mol
under certain conditions, what is the root mean square speed of F2(g) molecules under the same conditions?
Answers
The root mean square speed of F2(g) molecules under the same conditions is approximately 431.3 m/s.
How to solve for the rms speed of F2(g) molecules ?The root mean square (rms) speed of a gas molecule is related to its average kinetic energy (KE) by the following equation:
rms speed = √(3RT/M)
Where
R is the gas constantT is the temperature in KelvinM is the molar mass of the gasTo solve for the rms speed of F2(g) molecules, we need to know the temperature and molar mass of F2(g). Let's assume that the temperature is the same as the conditions in which He(g) has an average kinetic energy of 7450 J/mol. The molar mass of F2 is 2 x the molar mass of one fluorine atom, which is approximately 19 amu.
Substituting these values into the equation, we get:
rms speed = √(3RT/M)rms speed = √(3 x R x T / M)rms speed = √(3 x 8.314 J/mol·K x T / 38.00 g/mol)rms speed = √(24.942 J/K·mol x T / 38.00 g/mol)rms speed = √(0.6564 J/K x mol x T)Now we can solve for the rms speed by plugging in the given value of average kinetic energy for He(g) and solving for T:
7450 J/mol = (1/2) x (3/2) x R x T
T = 7450 J/mol / (1.5 x 8.314 J/mol·K)
T = 597 K
Substituting this value of T into the equation for rms speed, we get:
rms speed = √(0.6564 J/K x mol x 597 K / 1 mol)
rms speed = 431.3 m/s
Therefore, the root mean square speed of F2(g) molecules under the same conditions is approximately 431.3 m/s.
Learn more about average kinetic energy here : brainly.com/question/492249
#SPJ1
If you have a sample of 3.5 × 1022 atoms of tin (Sn), how many moles of tin do you have?
The correct answer according to the text is 0.058 mol Sn but no matter how I work it, I get 5.8 X 10^-2 mol Sn. I am using a scientific calculator. How did they get that answer?
Answers
The number of mole of tin (Sn) that you have is 0.058 mole
Avogadro's hypothesis6.02×10²³ atoms = 1 mole of tin (Sn)
With the above information, we can obtain the number of mole ot tin. Details below
How to determine the mole of tin that contains 3.5×10²² atomsWe can obtain the number of mole of tin as follow:
6.02×10²³ atoms = 1 mole of tin (Sn)
Therefore,
3.5×10²² atoms = (3.5×10²² atoms × 1 mole) / 6.02×10²³ atoms
3.5×10²² atoms = 0.058 mole of tin (Sn)
Thus, the number of mole of tin (Sn) you have is 0.058 mole
Learn more about mole and avogadro's number:
https://brainly.com/question/8512069
#SPJ1
You have a solid object of unknown composition and mass. You determined that when this object absorbed 1.000 X 10^2J, its temperature increased by 2.0K. Calculate the objects heat capacity
Answers
Answer:
100 rbed KJ |0| +2k
Explanation:
Venus's atmosphere, while primarily CO2, is also 3.5% nitrogen gas (i.e. mole fraction of 0.035). What is the partial pressure of nitrogen on Venus in kPa given that the total atmospheric pressure is 1334 psi?
Answers
The partial pressure of nitrogen on Venus is approximately 321.914 kPa.
To find the partial pressure of nitrogen on Venus, we need to calculate the partial pressure using the mole fraction of nitrogen and the total atmospheric pressure. First, we convert the total atmospheric pressure from psi to kilopascals (kPa) since the mole fraction is given in terms of kPa.
1 psi = 6.89476 kPa
Therefore, the total atmospheric pressure on Venus is:
1334 psi × 6.89476 kPa/psi = 9197.53 kPa
Next, we can calculate the partial pressure of nitrogen using the mole fraction. The mole fraction of nitrogen is given as 0.035, which means that nitrogen makes up 3.5% of the total moles of gas in the atmosphere.
The partial pressure of nitrogen is given by:
Partial pressure of nitrogen = Mole fraction of nitrogen × Total atmospheric pressure
Partial pressure of nitrogen = 0.035 × 9197.53 kPa
Partial pressure of nitrogen = 321.914 kPa
Therefore, the partial pressure of nitrogen on Venus is approximately 321.914 kPa.
It's important to note that the given atmospheric composition of Venus's atmosphere and the total atmospheric pressure are approximate values and can vary depending on specific conditions and measurements.
For more such question on partial pressure visit:
https://brainly.com/question/19813237
#SPJ8
During a volcanic eruption, lava flowed at a rate of 37 m/min. At this rate how far in kilometers
can lava travel in 45 minutes?
Answers
Explain the optical activity of malic acid?
Draw a pair of stereoisomers of malic acid.
Answers
Under standard temperature and pressure, the optical activity of malic acid changes in the following ways:
Wavelength of lightMagnitudeTemperatureConcentration of solvent.What is an optical activity?An optical activity is also referred to as optical rotation and it can be defined as the ability of a substance or chemical compound to rotate the plane of polarization of a beam of plane-polarized light that passes through it.
What is malic acid?Malic acid refers to an organic compound (dicarboxylic acid) that is typically made by all living organisms and its molecular formula is \(C_4H_6O_5\). Malic acid is considered to be one of the most satisfactory substances to be used when studying optical activity because of its chemical properties such as having single asymmetric carbon atoms.
Under standard temperature and pressure, the optical activity of malic acid changes in the following ways:
Wavelength of lightMagnitudeTemperatureConcentration of solvent.The stereoisomeric forms of malic acid.
In Science, there are two (2) stereoisomeric forms of malic acid and these include:
L-enantiomersD-enantiomersRead more on malic acid here: https://brainly.com/question/1538214

Which of the following amino acid functional groups will influence the pKa of an amino acid residue the least if it is on an adjacent residue?A. -OH.B. -NH3.C. -CH3.D. -COOH.
Answers
Answer:
C. -CH3
Explanation:
The pKa (acid dissociation constant) of an amino acid residue is primarily influenced by the functional groups attached to the adjacent end of the amino acid chain. The acidity of a functional group depends on how electronegative the functional group is. In the given functional groups' list, the functional group with the least influence on the pKa of the amino acid is the alkyl substituent (-CH3), because they have the least electron-withdrawing capacity.
PLEASE HELP QUICKK
Calculate the energy of combustion for one mole of butane if burning a 0.367 g sample of butane (C4H10) has increased the temperature of a bomb calorimeter by 7.73 °C. The heat capacity of the bomb calorimeter is 2.36 kJ/ °C.
Answers
The energy of combustion for one mole of butane to be approximately 2888.81 kJ/mol.
To calculate the energy of combustion for one mole of butane (C4H10), we need to use the information provided and apply the principle of calorimetry.
First, we need to convert the mass of the butane sample from grams to moles. The molar mass of butane (C4H10) can be calculated as follows:
C: 12.01 g/mol
H: 1.01 g/mol
Molar mass of C4H10 = (12.01 * 4) + (1.01 * 10) = 58.12 g/mol
Next, we calculate the moles of butane in the sample:
moles of butane = mass of butane sample / molar mass of butane
moles of butane = 0.367 g / 58.12 g/mol ≈ 0.00631 mol
Now, we can calculate the heat released by the combustion of the butane sample using the equation:
q = C * ΔT
where q is the heat released, C is the heat capacity of the calorimeter, and ΔT is the change in temperature.
Given that the heat capacity of the bomb calorimeter is 2.36 kJ/°C and the change in temperature is 7.73 °C, we can substitute these values into the equation:
q = (2.36 kJ/°C) * 7.73 °C = 18.2078 kJ
Since the heat released by the combustion of the butane sample is equal to the heat absorbed by the calorimeter, we can equate this value to the energy of combustion for one mole of butane.
Energy of combustion for one mole of butane = q / moles of butane
Energy of combustion for one mole of butane = 18.2078 kJ / 0.00631 mol ≈ 2888.81 kJ/mol
Therefore, the energy of combustion for one mole of butane is approximately 2888.81 kJ/mol.
In conclusion, by applying the principles of calorimetry and using the given data, we have calculated the energy of combustion for one mole of butane to be approximately 2888.81 kJ/mol.
For more questions on molar mass, click on:
https://brainly.com/question/837939
#SPJ8