calculate the volume in milliliters of a zinc nitrate solution that contains of zinc nitrate . round your answer to significant digits.
Answers
The volume in milliliters of a 1.30 M zinc nitrate solution that contains 100.g of zinc nitrate. The volume of solution is 406. mL
To calculate the volume of solution, we use the equation used to calculate the molarity of solution:
Molarity of solution = \(\frac{Mass of Solute * 100}{Molar Mass of solute * Volume of solution(in Ml) }\)
We are given:
Molarity of solution = 1.30 M
Given mass of zinc nitrate = 100. g
Molar mass of zinc nitrate = 189.4 g/ml
Putting values in above equation, we get:
1.3M = \(\frac{100 * 1000}{189.4 * Volume of solution}\)
⇒ Volume of solution = \(\frac{100 * 1000}{189.4* 1.3}\)
= 406 mL
Hence, the volume of solution is 406. mL
Learn more about Zinc Nitrate:
https://brainly.com/question/29355805
#SPJ4
Related Questions
someone help me please

Answers
Ionization of beryllium (Be) produces a +2 charge. Be now has a net positive charge of +2 after losing two electrons and gaining two fewer negative charges in its ionized state.
BerylliumThe chemical element beryllium (Be) has an atomic number of four. In its innermost and outermost shells, it has two electrons each. Beryllium needs to shed two electrons from its outermost shell in order to reach a stable electron configuration.Beryllium becomes a positively charged ion known as a beryllium ion (Be2+) when it loses two electrons. The ion has a net positive charge of +2 due to the loss of two electrons, leaving only two positively charged protons in the nucleus.Due to its smaller electron cloud and lower number of electrons, the beryllium ion is smaller than the neutral beryllium atom.learn more about beryllium here
https://brainly.com/question/1820862
#SPJ1
What is a good example of ACCURACY and PRECISION? *
Answers
Answer:
Bow and arrow
Explanation:
A bow and arrow need accuracy to hit a target. It needs precision to hit the small target
How many grams of NaCI must be added to 3 liters of H2O to create a solution with a 1.8 molarity?
Answers
Explanation:
Refer to pic.............

Which unit is used for measuring atomic mass? O A. atomic mole O B. grams/mole Ос. grams O D. atomic mass unit ОЕ. atomic mass weight
Answers
What is the difference between heterochromatin and euchromatin.
Answers
Answer:
The main difference between the two is euchromatin is genetically active while heterochromatin is genetically inactive
Answer:
Heterochromatin is the part of the chromosome without DNA coding genes and Euchromatin is the part of the chromosome with coding genes.
Explanation:
Euchormatin is the part of the chromosome which is rich with gene concentration and participates in the transition process. Heterochromatin is th area with a darkly stained DNA specific strand and is a condensed state.
Using the following equation, determine the males of sucrose (CisH55011 ) produced given 100 g of 02.
12 CO, + 11 H,0 - C2H2,0, + 12 0

Answers
Answer:
A --0.26 mol
Explanation:
using the stoichiometry ratio
1mol of sucrose -----> 12 moles of O2
no of mole of O2 = mass in g / mm
mm of O2 =2(16)
=32
no of mole of O2= 100/32
= 3.125 mol
recall
1 mol of sucrose ==> 12 mol of O2
1 mol of sucrose = 3.125/12
=== 0.2604 mol ✅✅
you can support by rating brainly it's very much appreciated ✅✅
how many microliters of 1.000 mnaoh solution must be added to 25.00 ml of a 0.1000 m solution of lactic acid ( ch3ch(oh)cooh or hc3h5o3 ) to produce a buffer with ph = 3.75?
Answers
First, we need to calculate equation the concentration of CH3CH(OH)COO- and HCH3CH(OH)COOH needed to produce a buffer solution at a given pH.
We will use the Henderson-Hasselbalch equation for this purpose. Henderson -Hasselbalch are equatio pH = pKa + log [CH3CH(OH)COO-] / [HCH3CH(OH)COOH]pH = 3.75 (given)pKa for lactic acid (HC3H5O3) =
We can assume that the volume of the resulting buffer solution is 25.00 ml (the same as the original volume of lactic acid), so we will add only a tiny amount of NaOH to it. The concentration of NaOH is given as 1.000 M.
To know more about equation Visit;
https://brainly.com/question/27984374
#SPJ11
How many carbon atoms are in the following compound:
10Fe2(CO3)3
Answers
which molecule or ion in the following list has the greatest number of unshared electron pairs around the central atom
Answers
The molecule or ion with the greatest number of unshared electron pairs around the central atom is XeF₄ (xenon tetrafluoride).
To determine the number of unshared electron pairs around the central atom in a molecule or ion, we need to consider its Lewis structure. In the Lewis structure, we represent the valence electrons as dots or lines around the atoms.
XeF₄ has a central xenon atom (Xe) surrounded by four fluorine atoms (F). Xenon has 8 valence electrons, and each fluorine atom has 7 valence electrons.
When we draw the Lewis structure for XeF₄, we place one fluorine atom on each side of the xenon atom, and we connect them with single bonds (represented by lines).
The remaining 4 valence electrons of xenon are placed as unshared electron pairs (represented by dots) around the xenon atom.
The Lewis structure of XeF₄ is as follows:
F F
\ /
Xe
/ \
F F
In this structure, xenon has 4 unshared electron pairs (dots) around it. Therefore, XeF₄ has the greatest number of unshared electron pairs around the central atom compared to the other molecules or ions in the list.
Conclusion: XeF₄ (xenon tetrafluoride) has the greatest number of unshared electron pairs (4) around the central atom.
To know more about electron pairs, visit,
brainly.com/question/13899233
#SPJ11
What is the meaning of the word neutral?
Answers
Explanation:
having no strongly marked or positive characteristics or features.
Write a net ionic equation to show that ethylamine, C2H5NH2 behaves as a Bronsted-Lowry base in water. (For organic molecules enter elements in order they are given in the question.) Write a net ionic equation to show that benzoic acid, C6H5COOH, behaves as a Bronsted-Lowry acid in water.
Answers
The net ionic equation for the behavior of ethylamine (C₂H₅NH₂) as a Bronsted-Lowry base in water is:
C₂H₅NH₂ + H₂O → C₂H₅NH₃⁺ + OH⁻
The net ionic equation for the behavior of benzoic acid (C₆H₅COOH) as a Bronsted-Lowry acid in water is:
C₆H₅COOH + H₂O → C₆H₅COO⁻ + H₃O⁺
In water, ethylamine (C₂H₅NH₂) can act as a Bronsted-Lowry base by accepting a proton (H⁺) from water. The reaction can be represented by the net ionic equation: C₂H₅NH₂ + H₂O → C₂H₅NH₃⁺ + OH⁻. In this equation, ethylamine (C₂H₅NH₂) accepts a proton from water (H₂O) to form the ethylammonium ion (C₂H₅NH₃⁺) and hydroxide ion (OH⁻). This shows the base behavior of ethylamine as it accepts a proton.
On the other hand, benzoic acid (C₆H₅COOH) can act as a Bronsted-Lowry acid in water by donating a proton (H⁺) to water. The reaction can be represented by the net ionic equation: C₆H₅COOH + H₂O → C₆H₅COO⁻ + H₃O⁺.
In this equation, benzoic acid (C₆H₅COOH) donates a proton to water (H₂O) to form the benzoate ion (C₆H₅COO⁻) and hydronium ion (H₃O⁺). This demonstrates the acid behavior of benzoic acid as it donates a proton.
To know more about Bronsted-Lowry acid refer here:
https://brainly.com/question/29317749#
#SPJ11
A student places a sample of a pure metal in a crucible and heats it strongly in air. Data from the experiment are given in the table above. The final mass was determined after the sample was cooled to room temperature. Which of the following statements related to the experiment is correct? (See attached table)
a.) The mass of the sample decreased, so physical changes occurred as the metal first melted and then boiled out of the crucible.
b.) The mass of the sample increased, so a chemical change occurred when bonds formed between the metal and another substance.
c.) There was nothing for the metal to react with, so only a physical change could have occurred.
d.) The sample was only heated, so neither a physical nor a chemical change occurred.
Answers
The correct answer is option A. The mass of the sample decreased, so physical changes occurred as the metal first melted and then boiled out of the crucible. The decrease in mass is a result of the metal melting and then vaporizing as it is heated.
What is physical changes?Physical changes refer to changes in the physical properties of a substance without altering its chemical composition. Examples of physical changes include melting, freezing, condensation, vaporization, sublimation, and physical state changes. Physical changes occur when a substance changes from one state of matter to another without a chemical reaction taking place.
This is an example of a physical change, as the material is changed from a solid to a liquid and then to a gas, but its chemical composition remains the same.
To learn more about physical changes
https://brainly.com/question/11370755
#SPJ4
What is the frequency of a wave with a wavelength of 5.4 x 10^-5 cm?
Answers
Velocity = Frequency x Lambda
Assuming that this is referring to an electromagnetic wave. The velocity of light is 2.998 x 10^8 m/s.
Convert the wavelength from cm to m. So divide it by 100 and you get 5.4x10^-7 meters.
2.998 x 10^8 = 5.4 x 10^-7 (λ)
Solve for λ and you get 5.55 x 10^14 Hz
The frequency of the wave is equal to 5.55 ×10¹⁴ s⁻¹.
What are frequency and wavelength?Frequency can be defined as the number of oscillations of a wave in one second. The frequency of the wave has S.I. units which are per second or hertz.
Wavelength can be described as the distance between the two most adjacent points in phase with each other. Two adjacent crests or troughs on a wave are separated by a distance of a single wavelength.
The relationship between frequency, speed of light, and wavelength is:
c = νλ
Given, the wavelength of the wave, λ = 5.4 ×10⁻⁵ cm
Then the speed of the wave is speed of light, c = 3 ×10¹⁰ m/sc
The frequency of the waves can be calculated from the above mentioned relationship:
The frequency of the given wave will be equal to:
ν = c/λ = 3× 10¹⁰/5.4 ×10⁻⁵ = 5.55 ×10¹⁴ s⁻¹
Therefore, the frequency of the wave is equal to 5.55 ×10¹⁴ s⁻¹.
Learn more about wavelength and frequency, here:
brainly.com/question/18651058
#SPJ2
A student has a piece of aluminum metal what is the most reasonable assumption a student can make about the metal
Answers
Answer:
- It could be stretched into a thin wire.Explanation:
As per the question, the most rational claim that the student can make about the aluminum metal is that 'it could be stretched into a thin wire' without breaking which shows its ductility. It is one of the most significant characteristics of a metal. Metals can conduct electricity in any state and not only when melted. Thus, option A is wrong. Options C and D are incorrect as metals neither have the same shape always nor do they break on hitting with a hammer. Therefore, option E is the correct answer.
Answer:
Yes, the other person is correct; the answer is E. It could be stretched into a thin wire.
Explanation:
Here is why it is NOT B:
"A briskly burning wood fire is plenty hot enough to melt aluminum. If it's just smoldering (like a lot of campfires) it won't create enough heat to melt aluminum."
Therefore, CAMPFIRES are not likely to melt aluminum if it is thrown in. Aluminum's melting point is 1,221 ° F. Typical campfires are about 900 ° F.
You're Welcome
~Kicho [nm68]
The amount of mass within the system remained constant
during a process for____
Answers
The amount of mass within the system remained constant during the process for a closed system. A closed system refers to a system that does not exchange matter with its surroundings but allows energy transfer across its boundaries. It undergoes internal energy changes but maintains a constant mass.
A closed system, in thermodynamics, is a physical system that doesn't interact with anything outside the system's boundaries. It can only exchange energy with its environment. In a closed system, there is no exchange of matter across the system's boundaries. Because there is no external exchange, the system's mass remains constant, making it a constant mass system.
When there is no exchange of mass with the environment, the amount of mass within the system remains constant throughout the process. The mass of a closed system remains constant because, in a closed system, the total quantity of mass and energy remains constant. In conclusion, the amount of mass within the system remained constant during the process for a closed system.
To know more about closed system please refer:
https://brainly.com/question/13453484
#SPJ11
The percentage of salicylic acid is 60.78% carbon 4.38% hydrogen and the rest is oxygen calculate the empress formula of salicylic acid
Answers
Answer: c2h2o
Explanation:
help i could use all the help and will be posting more questions later

Answers
you need to add 9L of water to 10mL of the solution of HCl with a pH of 3 to change the pH of 6.
First, calculate the amount of HCl present in 10mL of the solution with a pH of 3:
10mL x 0.1M = 1 mmol HCl
Then, calculate the amount of HCl required to raise the pH to 6:
1 mmol HCl x (10^3 - 10^6) = 9 mmol HCl
Finally, calculate the amount of water required to add 9 m mol of HCl to the solution:
9 mmol HCl x (1L/1000 mmol HCl) = 9L water
Hence, you need to add 9L of water to 10mL of the solution of HCl with a pH of 3 to change the pH of 6.
What is ph?
pH is a measure of acidity and alkalinity. It is measured on a scale of 0 to 14, with 0 being the most acidic and 14 being the most basic (alkaline). A pH of 7 is neutral.
Therefore, you need to add 9L of water to 10mL of the solution of HCl with a pH of 3 to change the pH of 6.
To learn more about ph
Here: https://brainly.com/question/12609985
#SPJ1
Pls help I will give u points pls

Answers
Answer:
i think D
Explanation:
Which has the greater mass, 1 liter of water or I liter of gasoline? The density of water is 1.00 g/mL and that of gasoline is approximately 0.68 g/mL.
Answers
Answer:
Water
Explanation:
Gas= 0.689/ml x 1000 ml = 680 g
Water= 1/ml x 1000 ml = 1000 g (grater)
1 liter of water would have greater mass than the I liter of gasoline if the density of water is 1.00 grams/milliliters and that of gasoline is approximately 0.68 grams/milliliters.
What is density?It can be defined as the mass of any object or body per unit volume of the particular object or body. Generally, it is expressed as in gram per cm³ or kilogram per meter³.
By using the above formula for density
ρ = mass / volume
As given in the problem we have to find which has the greater mass, 1 liter of water or I liter of gasoline if The density of water is 1.00 g/mL and that of gasoline is approximately 0.68 g/mL
1 Litre = 1000 milliliter
1 liter of water = 1000 milliliter
mass of 1 liter of water = density of water ×volume of water
= 1.00 grams/milliliters×1000 milliliter
=1000 grams
Similarly;y for the gasoline ,
1 liter of gasoline = 1000 milliliter
mass of 1 liter of gasoline = density of gasoline ×volume of gasoline
= 0.68 grams/milliliters×1000 milliliter
=680 grams
Thus we calculated that 1 liter of water would have a mass of 1000 grams while the I liter of gasoline has mass of 680 grams.
Learn more about density from here
brainly.com/question/15164682
#SPJ6
C7H6O2 + 02 →
CO₂ +
H₂O
how to balance this equation
Answers
Answer:
7 C7H6O2 + 12 O2 → 14 CO2 + 6 H2O
Explanation:
To balance this equation, you need to make sure that the number of atoms of each element on both sides of the equation are equal. In this equation, the number of carbon atoms (7) and hydrogen atoms (12) on the left side must be equal to the number of carbon atoms (1) and hydrogen atoms (2) on the right side. To balance this equation, you need to add coefficients of 7, 12, 2, and 14, respectively, in front of the compounds C7H6O2, O2, CO2, and H2O. This results in the equation: 7 C7H6O2 + 12 O2 → 14 CO2 + 6 H2O
Which of the following people believed the atom to be indivisible?
Democritus
Dalton
Bohr
Rutherford
Answers
Dalton believed that atom in not visible it is an invisible particle and whole universe is made up of atom only.
What is an atom?Atom is a visible particle consist of proton neutron and electrons and can be seen under the electron microscope only and it is the smallest unit of matter from which everything of the universe made up of.
Dalton says that atom is the particle cannot be seen but all matter is made up of that only which is not true and not even possible because matter is visible to us.
After some time with the help of experiments Rutherford performed bombarding experiment on the gold foil with the help of that he concluded that atom is visible.
Therefore, Dalton believed that atom in not visible it is an invisible particle and whole universe is made up of atom only.
Learn more about Atom, here:
https://brainly.com/question/14214017
#SPJ5
You may assume the following combustion event locations when analyzing the figures: \begin{tabular}{|l|c|c|} \hline & Design 1 & Design 2 \\ \hline Spurk Crask Angle & −10 deg & −20 deg \\ \hline 10\% MFB Crank Angle & 0 deg & −5 deg \\ \hline 500 MFB Crank Angle & 10 deg & 10 deg \\ \hline 904 MFB Crank Angle & 25 deg & 30 deg \\ \hline \end{tabular} Note that the data series plotted in c) through f) begin at the spark timing. Please compare the two engines at part load unless told otherwise. For (b) through ( g), a complete discussion will include competing factors that affect the primary and secondary parameters of each process, including those that make an event more or less likely, or make a characteristic increase or decrease for each engine design. (a) Construct a table comparing the important parameters of the two designs. At a minimum, the table should highlight the differences in the compression ratio, Φ,EGR level, intake
Answers
A table comparing the important parameters of the two engine designs is shown below:ParametersDesign 1Design 2Compression Ratio 18:118:1Φ (Equivalence Ratio)0.75 (Richer)0.65 (Leaner)EGR Level 10%15%Intake Temperature 80°C100°C Intake Pressure 1 bar0.5 bar Intake Valve Closing −15 deg BTDC−25 deg BTDC.
The compression ratio is the ratio of the volume of the combustion chamber from its largest capacity to its smallest capacity. The compression ratio in Design 1 is 18:1, while in Design 2, it is 18:1. The higher compression ratio is seen in Design 1, which will lead to a higher engine efficiency since more fuel energy is converted into useful work.
The Φ (equivalence ratio) is the ratio of the actual air/fuel ratio to the stoichiometric air/fuel ratio. A Φ value of 1.0 indicates a stoichiometric mixture, whereas values less than 1.0 indicate a lean mixture and values greater than 1.0 indicate a rich mixture. The Equivalence ratio in Design 1 is 0.75, which means it is running rich, while in Design 2, it is 0.65, which means it is running lean.
The design 2 engine has a higher Φ (equivalence ratio) level, which may lead to lower NOx emissions due to lean-burn combustion.The EGR (Exhaust Gas Recirculation) level in Design 1 is 10%, whereas in Design 2, it is 15%. The EGR level in Design 2 is higher than that in Design 1, which can help to reduce NOx emissions by lowering the temperature of combustion.
Intake temperature is 80°C in Design 1, whereas in Design 2, it is 100°C. The higher intake temperature in Design 2 means that the engine will have a higher thermal efficiency due to the increased Carnot efficiency.Intake pressure is 1 bar in Design 1, while in Design 2, it is 0.5 bar. The higher intake pressure in Design 1 results in more air being compressed into the combustion chamber, allowing for more fuel to be burned and greater efficiency.
Intake Valve Closing (IVC) is −15 deg BTDC in Design 1 and −25 deg BTDC in Design 2. The IVC timing is responsible for trapping the charge in the combustion chamber and influencing the engine's breathing. The lower IVC timing in Design 2 provides less resistance to flow, resulting in greater volumetric efficiency.
Learn more about equivalence ratio here,
https://brainly.com/question/1706633
#SPJ11
HELP!! ILL MARK BRAINLIEST!! Why do shows about ghosts often feature mostly nighttime scenes? This is the only time of day ghosts are present. This time of day produces more dramatic footage. This is the time of day when it is easiest to see ghosts. This is the only time spiritual energy can be measured.
Answers
We sense "ghosts" that aren't there because of our heightened state of anxiety, our incapacity to discern nighttime stimuli effectively, our belief that bad entities should favor the dark, and our relative lack of nighttime experience.
The correct option is (B) This time of day produces more dramatic footage.
In ghost stories, ghosts frequently manifest at night for a variety of reasons:
Because our inclination to fear the dark is inherent. In the daytime, we can see nearby predators; at night, we cannot. We are more exposed.Because it's difficult for humans to see in the dark, it's simpler to imagine things that aren't actually there. Our brain is capable of putting together several pieces of data to construct an experience that didn't actually take place. For instance, at darkness, we can hear things but not see them.Because nightfall is nasty and dangerous, it makes sense that wicked things would emerge at this time of day. It seems sense that spirits would prefer the night over the day, at least on the surface.We have a lot of experience not seeing ghosts during the day and far less experience seeing ghosts at night. That instance, if someone were to say, "X tends to happen around 4pm," you would respond, "no, it doesn't," but if they said, "X tends to happen at 4am," you may be a little unsure. There are moments of the night when you aren't awake all that often.Learn more about the Anxiety with the help of the given link:
https://brainly.com/question/3253078
#SPJ1
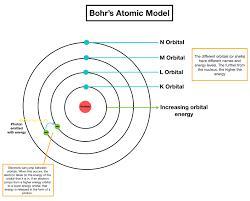
Why is electron filling similar to going up the stairs?.
Answers
If we look into the Bohr's atomic model, it is like a staircase.
Where every next orbit is like a stair.
As we can not rest in air, we either have to stay on level 1 or can go up with enough energy to climb next level.
Same way, electron can either stay on one orbit or can go to higher orbits with enough energy to jump from lower energy level to the higher energy level, can say orbit.
According to Bohr, atom is a system consisting of a dense nucleus surrounded by orbiting electrons.
He compared his atomic structure with the structure of the Solar System.
To know more about Bohr's atomic model:
brainly.com/question/3964366
#SPJ4
Can u please answer if you know.

Answers
Answer:
plants
Explanation:
they are plant cells, happy Thanksgiving :)
Answer: photosynthetic tissues
Explanation: Chloroplasts are present in the cells of all green tissues of plants and algae. Chloroplasts are also found in photosynthetic tissues that do not appear green, such as the brown blades of giant kelp or the red leaves of certain plants.
Amino acids can be classified as A. Hydrophobic amino acids with nonpolar R groups B. Polar amino acids with neutral R groups but the charge is not evenly distributed C. Positively charged amino acids with R groups that have positive charge at physiological pH. D. Negatively charged amino acids with R groups that have a negative charge at physiological pH.
Answers
The correct answer is A. Based on the given options, amino acids can be classified into different categories based on the properties of their R groups.
it are organic compounds serve as the building blocks of proteins. They consist of a central carbon atom (alpha carbon) bonded to an amino group (-NH2), a carboxyl group (-COOH), a hydrogen atom, and an R group (side chain) that varies among different amino acids.
classified R groups amino acids. The given options represent different classifications:
A. Hydrophobic amino acids with nonpolar R groups: These amino acids have R groups that are nonpolar and do not readily interact with water molecules. They tend to be hydrophobic (water-fearing) and are often found in the interior of proteins away from water.
B. Polar amino acids with neutral R groups but the charge is not evenly distributed: These amino acids have polar R groups that contain functional groups such as hydroxyl (-OH) or amine (-NH2). Although they are polar, the charge is not evenly distributed within the R group.
C. Positively charged amino acids with R groups that have positive charge at physiological pH: These amino acids have R groups that contain positively charged functional groups, such as amino groups (-NH3+), at physiological pH (around 7.4).
D. Negatively charged amino acids with R groups that have a negative charge at physiological pH: These amino acids have R groups that contain negatively charged functional groups, such as carboxylate groups (-COO-), at physiological pH.
Based on the given options, amino acids can be classified into different categories based on the properties of their R groups. Amino acids with hydrophobic nonpolar R groups are classified as hydrophobic amino acids.
To know more about amino acids, visit:
https://brainly.com/question/14351754
#SPJ11
Which statement below is true?
The mass of each planet is different, so they each orbit their own moons.
The mass all the planets together is greater than the mass of the sun.
The mass of all planets is the same, so they all orbit the Sun.
The mass of each planet is less than that of the Sun, so they all orbit the Sun.
Answers
Answer:The mass of each planet is less than that of the Sun, so they all orbit the Sun.
Explanation:
An airplane travels 200 km/h in 4 hours going to Zambales. What will be the distance and displacement?
Answers
Answer:
See explanation
Explanation:
Recall that;
Speed = Distance/time
Distance = Speed * time
Speed = 200 km/h
Time = 4 hours
Distance = 200 km/h * 4 hours = 800 kilometres
Displacement has to do with distance covered in a specified direction, in this case, the direction is towards Zambales.
Hence, the displacement is 800 kilometres towards Zambales.
For the chemical reaction
Na2CO3+Ca(NO3)2⟶CaCO3+2NaNO3
how many moles of calcium carbonate (CaCO3) are produced from 6.0 mol of sodium carbonate (Na2CO3)?
number of moles:
mol
Answers
6.0 mol calcium carbonate
In order to solve questions of stoichiometry, such as this, it is important to look at the mole-to-mole ratio of the equation. This ratio can be found by the coefficient placed in front of the molecule. Calcium carbonate has a coefficient of 1, as does sodium carbonate. In that case, the conversion ratio is 1:1, creating the same amount of moles for calcium carbonate as for sodium carbonate.
The number of moles of Calcium Carbonate produced from 6.0 mol of Sodium Carbonate (Na2CO3) is also 6.0 moles.
The balanced chemical reaction between Sodium carbonate and Calcium Nitrate is given as
Na2CO3+Ca(NO3)2 ⟶ CaCO3+2NaNO3
The mole ratio between sodium carbonate and calcium carbonate is 1:1. Hence, the number of moles of calcium carbonate (CaCO3) produced from 6.0 mol of sodium carbonate (Na2CO3) will also be 6.0 mol.
The balanced chemical reaction is as follows:
Na2CO3+Ca(NO3)2 ⟶ CaCO3+2NaNO3
From the above-balanced chemical reaction, we can say that one mole of Sodium Carbonate (Na2CO3) reacts with one mole of Calcium Carbonate (CaCO3). This means, if we have 6.0 moles of Sodium Carbonate, then 6.0 moles of Calcium Carbonate will be produced. Hence, the number of moles of Calcium Carbonate produced from 6.0 mol of Sodium Carbonate (Na2CO3) is also 6.0 moles.
To know more about moles refer to:
https://brainly.com/question/29367909
#SPJ11
what is conditional reflexes??
Answers
Answer:
Actions that you have trained yourself to perform.
they are obtained by experience. they are not passed from generation. they can be learnt or abolished. Higher center's of brain is responsible for conditional reflexes
Explanation:
example riding a bike, swimming, typing