Answers
Answer:
As406(s) + 4MnO_4^- (aq) → 4AsO_4^3- (aq) + 4Mn^2+ (aq)
Explanation:
Related Questions
The diagram below shows the different phase transitions that occur in matter.
Which arrow would most likely represent the phase change that involves the same amount of energy as arrow 1?

Answers
A material changes physically when its state changes. They don't involve any alterations to the matter's chemical composition and are reversible. State changes that occur frequently include melting, freezing, sublimation, deposition, condensation, and vaporization.
Which arrow would most likely represent the phase change that involves the same amount of energy as arrow 1?
"Arrow 4."
The energy required to go from a liquid to a gas is equivalent to that required to go from a gas to a liquid.
The process by which a substance transforms from the liquid phase to the gaseous state is known as boiling.
Diagram of water in phase:
Water changes from a liquid to a gas through the phase-shifting process of evaporation (water vapor).
Water changes its state from a gas to a liquid during the phase shift process known as condensation, and the resulting vapor turns into a cloud.
To know more about Matter, click on the link below:
https://brainly.com/question/17011982
#SPJ9
Answer: arrow 4
Explanation: just took the test ;-)
Virginia has 11 people in the House and 2 in the Senate. How many electoral votes does it have? ________________ Where is this addressed?
Answers
The total number of electoral votes does Virginia have is 13. This addressed in congressional apportionment.
When people cast their vote, means they actually voting for the group of people called electors. Senator and representatives are equal to the number of voters in each state . According to the question , Virginia has 11 people in the house and 2 senate means 11 + 2 = 13 . the total number of electoral votes are 13. These votes are assigned by united states electoral college. In the constitution the congressional apportionment is addressed.
Thus, Virginia has 11 people in the House and 2 in the Senate. The total number of electoral votes does Virginia have is 13. This addressed in congressional apportionment.
To learn more about Electoral votes here
https://brainly.com/question/28648659
#SPJ1
According to the Foliated Metamorphic Rock Chart slate, phyllite, schist, and gneiss can all have the same parent rock (shale). If this is true, what determines the difference between a slate and a gneiss rock that both are formed from shale? What role does the parent rock play in determining the type of metamorphic rock that will be formed?
Answers
According to the Foliated Metamorphic Rock Chart slate, phyllite, schist, and gneiss can all have the same parent rock (shale) is a true statement.
The parent rock, in this case shale, plays a significant role in determining the type of metamorphic rock that will be formed. The minerals and structure of the parent rock provide the starting material for the metamorphic rock, and the specific conditions under which the rock undergoes metamorphism determine the final characteristics of the metamorphic rock.What determines the difference between a slate and a gneiss rock that both are formed from shale?Slate, phyllite, schist, and gneiss are all types of metamorphic rocks that can be formed from shale, which is a sedimentary rock composed of clay and other fine-grained minerals. The specific type of metamorphic rock that is formed from shale depends on the conditions under which the shale undergoes metamorphism, including the temperature, pressure, and presence of fluids.
Slate is a fine-grained metamorphic rock with a uniform, flat surface and a layered structure. It is formed when shale undergoes low-grade metamorphism, which occurs at relatively low temperatures and pressures.
Therefore, Gneiss, on the other hand, is a medium- to coarse-grained metamorphic rock with a banded or wavy texture. It is formed when shale undergoes high-grade metamorphism, which occurs at higher temperatures and pressures.
Learn more about Metamorphic Rock from
https://brainly.com/question/1176274
#SPJ1
What is the molar mass of phosphoric acid, H3PO4?
Answers
Answer:
The molar mass is 97.99 g
True/False: The definition of an ion is when the number of neutrons of an atom changes from the original neutral state.
Answers
Answer:
False. a ion is an atom or molecule with a net electric charge due to the loss or gain of one or more electrons.
Explanation:
outline the synthesis of c6h5ch2ch2cooh starting from benzaldehyde and bromoacetate
Answers
Answer:
i gotchu
Explanation:
its the 3rd raikages will eassyyy
Which of the following shows the abbreviation for an Sl unit of density?
A. L/kg
B. g/mL
C.
g/cm
D. kg/m2
Answers
Explanation:
ur answer okkkkkkkkkkkkkkkkll
HELPP PLZZZ
What is the thermal energy of an object?
the total kinetic and potential energies of all its particles
the strength of the strong nuclear force in all of its atoms
the total potential energy of its particles
its total temperature
Answers
Answer:
the total kinetic andd potential energies of all its particles
Explanation:
other brainly questions
Answer:
Hey mate....
Explanation:
This is ur answer....
The thermal energy of an object is the energy contained in the motion and vibration of its molecules. Thermal energy is measured through temperature. The energy contained in the small motions of the object's molecules can be broken up into a combination of microscopic kinetic energy and potential energy.
Hope it helps you,
Mark me as the brainliest....
Follow me!
inorganic compound created by the removal or replacement of one, two, or all three hydrogen atoms in phosphoric acid; used in fertilizers and detergents and is a major cause of water pollution
Answers
The inorganic compound that is created by the removal or replacement of one, two, or all three hydrogen atoms in phosphoric acid and is commonly used in fertilizers and detergents, as well as a major cause of water pollution, is called "phosphate."
What do you mean by inorganic compound?
An inorganic compound is a chemical compound that does not contain carbon-hydrogen (C-H) bonds, which are the defining characteristic of organic compounds. Inorganic compounds can be composed of a variety of elements, including metals, non-metals, and metalloids.
The inorganic compound that is created by the removal or replacement of one, two, or all three hydrogen atoms in phosphoric acid and is commonly used in fertilizers and detergents, as well as a major cause of water pollution, is called "phosphate."
Phosphate compounds are essential nutrients for plant growth and are often added to fertilizers to improve soil fertility. However, when excess phosphate is added to water bodies, it can lead to eutrophication, a process where an excessive growth of algae and other aquatic plants occur, ultimately leading to oxygen depletion and harm to aquatic life.
Phosphates are also commonly used in detergents to aid in cleaning, but they can have similar negative impacts on water quality if they are not properly treated before being discharged into waterways. Therefore, regulations are in place to limit the amount of phosphate that can be added to detergents and other products in many countries to mitigate water pollution.
Learn more about inorganic compound click here:
https://brainly.com/question/26221159
#SPJ1
A bone taken from a garbage pile buried under a hill-side had 14C/12C ratio 0.477 times the ratio in a living plant or animal. What was the date when the animal was buried?
Answers
Answer:
The date when the animal was buried can be estimated by using the decay rate of Carbon-14. The half-life of Carbon-14 is 5,730 years, so the date when the animal was buried can be estimated by taking the half-life and multiplying it by the logarithm of the ratio of the 14C/12C ratio of the bone and the 14C/12C ratio of a living plant or animal. In this case, 5,730 years x log(0.477) = 45,906 years ago.
Explanation:
uranium-235 can be induced to undergo nuclear fission by the absorption of a neutron. how many neutrons are produced if the other fission products are xenon-134 and strontium-88? enter the whole number answer in the box.
Answers
In this reaction, two neutrons are produced.
The nuclear fission reaction of uranium-235 with the absorption of a neutron is as follows:
235U + 1n → 134Xe + 88Sr + 2n + energy
In this reaction, two neutrons are produced, so the answer to the question is two.
Nuclear fission is a process in which an atom splits into two or smaller atoms, releasing a large amount of energy in the process. In the case of uranium-235, the absorption of a neutron causes the nucleus to become unstable, leading to its splitting into two smaller atoms, xenon-134 and strontium-88.
This process also releases two additional neutrons, which can then go on to cause further fission reactions. The energy released in this process is what makes nuclear power plants so efficient. The process can be controlled by the use of moderators, which slow down the neutrons and allow for more precise control of the reaction.
For more questions like Nuclear fission click the link below:
https://brainly.com/question/28683212
#SPJ4

Solid magnesium reacts with aqueous copper(I) chloride to form aqueous magnesium chloride and solid copper. Write a balanced chemical equation for each chemical reaction.
Answers
Answer:
Explanation:
Mg(s) +2CuCl (aq) -> MgCl2(aq) + 2Cu(s)
Mg(s) +2CuCl (aq) → MgCl\(_2\)(aq) + 2Cu(s) is the balanced chemical equation for each chemical reaction.
What is balanced chemical equation?An equation for just a chemical reaction is said to be balanced if both the reactants as well as the products have the same number of atoms and total charge for each component of the reaction. In other words, both sides of a reaction have an equal balance of mass and charge.
The ingredients and outcomes of a chemical reaction are listed in an imbalanced chemical equation, but the amounts necessary to meet the conservation of mass are not specified. Mg(s) +2CuCl (aq) → MgCl\(_2\)(aq) + 2Cu(s) is the balanced chemical equation for each chemical reaction.
Therefore, Mg(s) +2CuCl (aq) → MgCl\(_2\)(aq) + 2Cu(s) is the balanced chemical equation for each chemical reaction.
To know more about balanced chemical equation, here:
https://brainly.com/question/15052184
#SPJ2
81.5 g of metal was heated from 11 degrees Celsius to 69 degrees Celsius. If 6739 joules of heat energy were used, what is the specific heat capacity of the metal?
Answers
Answer:
the metal become red hot
CHAPTER 8 FORCE AND MOTION
Choose the S.I. unit for pressure.
1. Watt
2. Joule
3. Pascal
4.Newton
Answers
Imagine a world in which all the elements existed, but they couldn't form any chemical bonds with eachother. What kinds of substances could exist in such a world? what kinds of substances could not exist? what do you think this world would be like?
Answers
Answer:
In a world where elements exist but are unable to form chemical bonds with each other, the most basic and purest form of elements would exist as individual atoms or ions. There would not be any molecules or compounds since the atoms would not be able to bind with each other.
Substances that could not exist in this world would be those that are composed of chemical bonds, such as gases, liquids, or solids. This would include common substances such as water, air, and most organic materials.
In this world, there would not be any reactions or chemical processes as the atoms would not be able to interact with each other. This would result in a world that is still and unchanging, with no movement or life. It would be a world of individual atoms and ions, floating aimlessly in a void. This world would be inhospitable to life and would not support any kind of chemical or biological processes.
Answer: All elements would just coexist and nothing would be created. This means that substances such as water, salt, etc. would not form. This world would have no life since chemical bonds would not be able to form.
Hope this helps or at least gets you started! Have a great day:)
What is the periodic trend for atomic size from top to bottom in a group ? From left to right in a period ?
Answers
Answer: (i)from top to bottom atomic size increases
(II) from left to right the atomic size decreases
Explanation:
(i) more shells are being opened as we go down hence size will increase
(II) number of shells is the same , but as we move from left to right charge density is increasing hence the outer electrons feels greater attraction from nucleus hence size decreases
contact 0775776762 thankful for this site
Write the empirical formula of at least four binary ionic compounds that could be formed from the following ions: Mg²+, Ni+, Br¯`, s²-
Answers
Answer:
Explanation:
What is the temperature of 2.60 mol of gas at a pressure of 102.63 atm and a volume of 341 mL
Answers
Answer:
164.04 K
Explanation:
PV = n RT R = gas constant = .082057 L-atm/(mol-K)
T will be in K
102.63 (.341) = 2.60 * .082057 * K
K = 164.04 K
Is HCI an acid or a base?
Answers
Answer:
Strong Acid
Explanation:
Hope this helps! :)
After a morning of cross-country skiing, you return to the chalet and you prepare a good broth.
You pour 250 mL (1g/mL) of broth into a cup at a temperature of 70°C (c = 4.18 J/g•°C).
To avoid burning yourself, you add 50 mL of cold water at 5°C to the cup. What will be the
final broth temperature?
Answers
The final broth temperature will be approximately 38.4°C.
When mixing two substances with different temperatures, we can use the principle of conservation of energy. The energy lost by the hot substance (broth) is equal to the energy gained by the cold substance (water), assuming no energy is lost to the surroundings. This can be expressed using the equation:
Q_lost = Q_gained
The energy lost by the broth can be calculated using the formula:
Q_lost = m_broth * c_broth * (T_final - T_initial)
where m_broth is the mass of the broth, c_broth is its specific heat capacity, T_final is the final temperature, and T_initial is the initial temperature of the broth.
Similarly, the energy gained by the water can be calculated using:
Q_gained = m_water * c_water * (T_final - T_initial)
Since the two substances reach thermal equilibrium, we can set Q_lost equal to Q_gained:
m_broth * c_broth * (T_final - T_initial) = m_water * c_water * (T_final - T_initial)
Plugging in the given values and solving for T_final, we find that the final temperature of the broth is approximately 38.4°C.
for such more questions on temperature
https://brainly.com/question/4735135
#SPJ8
What is the molar concentration of Zn2+ ions in a solution, if the electrode potential value is 59mV less than the standard electrode potential value at 298 K?
Answers
Molar concentration of Zn2+ions in a solution is 3.481 mol/lit
The electrode potential value is 59mV
Temperature=298k
What is electrode potential?
It is a force of galvanic cell. basically it is the difference between an electrolyte and electrode.equation formed- Zn → Zn2+ + 2e
from Nernst equation-
E=E cell - 0.059 log [Zn2+]
[zn2+]=3.481 mol/lit
hence, Molar concentration of Zn2+ions in a solution is 3.481 mol/lit
Learn more about electrode potential here:
https://brainly.com/question/15417662
#SPJ10
When a cloud becomes too heavy with water droplets it can rain hail or snow this is known as what?
Answers
Answer:sunny days
Explanation:meow
Answer:
This is condensation.
Explanation:
The process of water vapour turning back into liquid water.
For the reaction below taking place at STP_S + _O2 --> _SO3 How many L of O2 would be needed to produce with 9.1 L of SO3?Give # and unit and remember sig figs.
Answers
1) First, let's balance the chemical equation:
_S + _O2 --> _SO3
To balance the equation, we need to equal the number of atoms of each element on the reactants and products side.
2 S + 3 O2 --> 2 SO3
Reactants side:
S - 2
O - 6
Products side:
S - 2
O - 6
2) Now to calculate, we need to know the molar volume value. This is the space occupied, in liters, by 1 mole of any matter in a gaseous state and under normal conditions of temperature and pressure (STP). The value is 22.4 liters/mol
So let's transform 9.1 L of SO3 into mole:
22.4 liters --- 1 mol
9.1 liters ---- x mol
22.4x = 9.1
x = 0.406 moles of SO3
3) Now we use the reaction proportion to know how many moles of O2 is needed:
3 moles of O2 ----- 2 moles of SO3
x moles of O2 ----- 0.40625 moles of SO3
2x = 1.21875
x = 0.609375 moles of O2
4) Now let's transform moles of O2 into liters using the molar volume value:
22.4 L ---- 1 mol
x L ----- 0.609375
x = 13.7 liters of O2
Answer: It is needed 13.7 liters of O2.
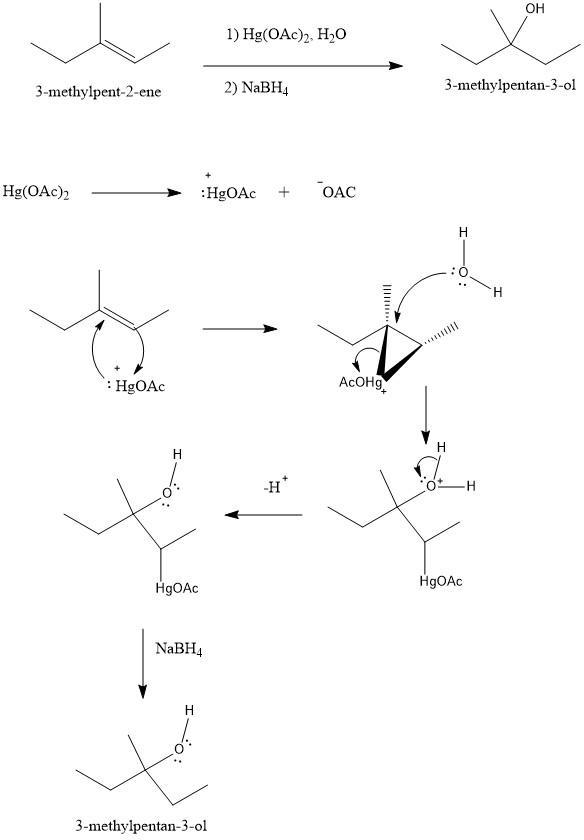
When 3-methylpent-2-ene is treated with mercury(II) acetate in methanol and the resulting product isreacted with NaBH4, what is the primary organic compound which results
Answers
Answer:
3-methylpentan-3-ol
Explanation:
In this case, we have an "Oxymercuration reaction". With this in mind, we will have to add an "OH" to the most substituted carbon of the double bond and we will obtain 3-methylpentan-3-ol. To understand how this molecule is produced we have to check the mechanism:
The mercury(II) acetate (\(Hg(OAC)_2\)) is an ionic substance. So, this substance can be ionized into his ions and we will have the cation \(HgOAc^+\) and the anion \(AcO^-\). The cation will attack the double bond and vice-versa to produce a "cyclic intermediate". Then a water molecule will attack the most substituted carbon and the cyclic compound would be broken producing a new bond C-O with a positive charge in the oxygen. Then a deprotonation step takes place and finally, the \(NaBH_4\) would reduce the compound to produce the final alcohol.
See figure 1
I hope it helps!
A gas has a volume of 50.0 mL at a temperature of 10.0 K and a pressure of 760. kPa. What will be the new volume when the temperature is changed to 20.0 K and the pressure is changed to 380. kPa?
Answers
To solve this problem using the gas laws, we need to use the Ideal Gas Law. This law states that the product of the pressure and the volume of a gas is proportional to the absolute temperature.
The equation of the Ideal Gas Law is the following:
\(\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{\dfrac{P_1V_1}{T_1}=\frac{P_2V_2}{T_2} } \end{gathered}$} }\)
Where:
P₁ = initial pressure = 760 kPaV₁ = initial volume = 50.0 mL = 0.050 LT₁ = initial temperature = 10.0 KP₂ = Final pressure = 380 kPaT₂ = final temperature = 20.0 KV₂ = Final volume = ?We clear for V₂:
\(\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{V_2=\frac{P_1V_1T_2}{P_2T_1 } } \end{gathered}$} }\)
Where:
P₁ = initial pressure V₁ = initial volumeT₁ = initial temperatureP₂ = Final pressureT₂ = final temperatureV₂ = Final volumeSubstituting the known values:
\(\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{V_2=\frac{760\not{kPa}\times0.050 \ L\times20.0\not{k} }{ 380\not{kPa}\times10.0\not{k} } } \end{gathered}$} }\)
\(\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{V_2=\frac{760 \ L}{3800 } } \end{gathered}$} }\)
\(\boxed{\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{V_2\approx0.2 \ Liters} \end{gathered}$} }}\)
When the temperature changes to 20.0 K and the pressure changes to 380 kPa, the new volume will be approximately 0.2 L (200.0 mL).What are the missing coefficients for C3H8 + o2 = Co2 +H2O
Answers
Answer: C3H8 + 5O2 = 3Co2 +4H2O
Explanation: Equations must be balanced
You must have the same amount of C
H and O on both sides of the equation
Please upload a worksheet related to Chemistry periodic table. Its also ok if its from your own textbook. I want to study and practice. HELP!!!!
Answers
Hi there, here is an unsolved worksheet for you to practice.
Study well.
Hope it helps you....
Answered by Benjemin ☺️
✅

what layers of protection can be used tot detect and respond to abnormal reactions or process conditions
Answers
In the body, the main protective layer is known as the epithelium, this can be found in the skin as well as other organs such as the lungs.
The body is often exposed to external factors that can damage it; due to this, throughout evolution, most animals and multicellular organisms have developed protective mechanisms.
In the case of humans and other animals, the protective layer is known as the epithelium, which is composed of epithelial cells. This protective layer can be found internally and externally.
External layer: This is the first protective layer, commonly known as skin, this layer protects the inner organs from extreme temperatures, changes in pressure, etc.
Internal layer: Organs such as the lungs or the ones in the gastrointestinal system are all covered by a thin epithelial layer that helps each organ to be protected.
Learn more about skin in: https://brainly.com/question/12057617
The photon used to measure the electron location and
velocity has a size and velocity similar to that of the
electron and will displace the electron.
TRUE
FALSE
Answers
An element has a mass number of 9 and 5 neutrons. What element is it?
Answers
Answer:
Fluorine
Fluorine is the element in question, as its atomic number is 9 . You would name this particular isotope using the mass number. It would be called fluorine-19.
Explanation:
Answer:
Fluorine
Fluorine is the element in question, as its atomic number is 9 . You would name this particular isotope using the mass number. It would be called fluorine-19.