Arrange these elements according to electron affinity.
You are currently in a ranking module. Turn off browse mode or quick nav, Tab to move, Space or Enter to pick up, Tab to move items between bins, Arrow Keys to change the order of items, Space or Enter to drop.
Most energy released by gaining an electron Most energy absorbed by gaining an electron
Answer Bank
He, C F
Answers
The arrangement of the elements according to electron affinity is F > C > He.
Electron affinity is the energy released when one electron is added in the outer most shell of the gaseous element.
For moving left to right electron affinity increase because the force of attraction of nucleus on electron increase and size decrease so electron affinity increase.
So F has higher electron affinity because more energy is released after adding the electron.
In carbon electron negativity is less than F so electrons are less strongly attracted by nucleus than F so electron affinity of F > C
In case of He outermost shell is completely filled so electrons are not gained so has minimum electron affinity.
So electron affinity order is F > C > He
More energy released by gaining an electron is F > C > He
More energy absorbed by gaining an electron is He > C > F
So, F > C > He is the energy released by gaining an electron.
He > C > F absorbs more energy by gaining an electron.
To learn more about Electron affinity, Here :
https://brainly.com/question/24300752?referrer=searchResults
#SPJ4
Related Questions
Which statement about pure substances and molecules is correct?
O All pure substances are molecules.
O All molecules are pure substances.
o Molecules cannot be pure substances.
O Pure substances cannot be molecules.
Answers
A molecule must be a pure substance hence the correct answer is " All molecules are pure substances."
A pure substance is one which contains only one element or compound. A molecule is the smallest unit of a compound that can exist independently.
When we refer to a molecule of a compound, we are referring to that pure substance that contains only the element(s) that compose the compound.
Hence; All molecules are pure substances.
Learn more: https://brainly.com/question/19922822
This image shows a mixture of steam and carbon monoxide reacting to reversibly produce carbon dioxide and hydrogen gas. Write the balanced chemical equation which is taking place in the mixture.
Be sure to include the physical states of each species.

Answers
The reversible production of carbon dioxide and hydrogen gas as a result of a reaction between steam and carbon monoxide. The chemical formula for a balanced reaction is CO (g) + H2O (g) CO2 (g) + H2(g).
Does reversible carbon monoxide reaction result in the production of hydrogen and carbon dioxide gases?Carbon dioxide (CO2) and hydrogen gas are created when carbon monoxide (CO) and water mix (H2O). The process is reversible and every molecule in the balanced equation has a coefficient of 1.
What are the two processes that replenish the atmosphere with carbon in the form of CO2?Significant amounts of carbon are released into the atmosphere as a result of the burning of fossil fuels, changing land use, and the production of concrete with limestone.
To learn more about Carbon Monoxide visit:
brainly.com/question/22530423
#SPJ1
Calculate the work done when a force of 4 N pulls a box along the floor for a distance of 0.3 m.
Answers
Answer:
1.2 Joulesolution,
Force=4 N
Distance=0.3 m
Now,
\(work = f \times d \\ \: \: \: \: \: \: \: \: \: \: \: = 4 \times 0.3 \\ \: \: \: \: \: \: \: \: \: \: \: = 1.2 \: joule\)
hope this helps...
Good luck on your assignment....
Two asteroids are 75,000 m apart one has a mass of 8 x 10^7 N what is the mass of the other asteroid
Answers
The mass of the asteroid is C. 1.2 x \(10^{12}\) Kg
To find the mass of the other asteroid, we can rearrange the equation for the gravitational force between two objects:
F = (G * m1 * m2) / \(r^{2}\)
where F is the force of gravity, G is the gravitational constant, m1 and m2 are the masses of the two asteroids, and r is the distance between them.
Given that the distance between the asteroids is 75000 m, the force of gravity between them is 1.14 N, and one asteroid has a mass of 8 x \(10^{7}\) kg, we can substitute these values into the equation and solve for the mass of the other asteroid (m2):
1.14 N = (6.67430 × \(10^{-11}\) N \(m^{2}\)/\(Kg^{2}\) * 8 x \(10^{7}\) kg * \(m2\)) / \((75000 m)^{2}\)
Simplifying and solving the equation, we find that the mass of the other asteroid (m2) is approximately 1.2 x \(10^{12}\) kg. Therefore, Option C is correct.
The question was incomplete. find the full content below:
Two asteroids are 75000 m apart one has a mass of 8 x \(10^{7}\) kg if the force of gravity between them is 1.14 what is the mass of the asteroid
A. 3.4 x \(10^{11}\) kg
B. 8.3 x \(10^{12}\) kg
C. 1.2 x \(10^{12}\) kg
D. 1.2 x \(10^{10}\) kg
Know more about gravitational force here:
https://brainly.com/question/72250
#SPJ8
What is the total number of moles of reactants and products in the
chemical reaction listed below:
2 H₂S +30₂2 H₂O + 2 SO₂
Answers
The total number of moles of reactants and products in the chemical reaction given is 9 moles
How do i determine the total number of moles?The total number of mole of reactants and products in the chemical reaction can be obtained as follow:
2H₂S + 3O₂ -> 2H₂O + 2SO₂
The following were obtained from the above equation:
Mole of H₂S = 2 molesMole of O₂ = 3 molesMole of H₂O = 2 molesMole of SO₂ = 2 molesMole of reactants = Mole of (H₂S + O₂) = 2 + 3 = 5 molesMole of products = Mole of (H₂O + SO₂) = 2 + 2 = 4 molesTotal number of moles =?Total number of mole = Mole of reactants + mole of products
Total number of mole = 5 mole + 4 moles
Total number of mole = 9 moles
Thus, we can say that the total number of mole is 9 moles
Learn more about mole:
https://brainly.com/question/13314627
#SPJ1
What is the amino group in a protein?
Answers
An amino group is a functional group consisting of an amino (-NH2) and a carboxyl (-COOH) group, which is found in proteins.
It is essential for the formation of peptide bonds between amino acids, which are the building blocks of proteins. The amino group is partially positively charged and is therefore able to interact with other molecules through hydrogen bonding and electrostatic interactions.
The amino group is also able to donate a hydrogen atom, making it an important component in many biochemical reactions.
Furthermore, it plays an important role in the folding of proteins, as the electrostatic interactions between the amino and carboxyl groups help stabilize the protein's three-dimensional structure. In addition, the presence of the amino group can influence the solubility of proteins in different solutions.
To know more about proteins, click below:
https://brainly.com/question/10058019
#SPJ4
What are the causes and consequences of climate change
Answers
Answer: Humans are increasingly influencing the climate and the earth's temperature by burning fossil fuels, cutting down forests and farming livestock.
Explanation: This adds enormous amounts of greenhouse gases to those naturally occurring in the atmosphere, increasing the greenhouse effect and global warming.
Balance each of the following equations according to the half-reaction method: (a) Zn(s)+NO3−(aq)⟶Zn2+(aq)+N2(g)(in acid) (b) Zn(s)+NO3−(aq)⟶Zn2+(aq)+NH3(aq)(in base) (c) CuS(s)+NO3−(aq)⟶Cu2+(aq)+S(s)+NO(g)(in acid) (d) NH3(aq)+O2(g)⟶NO2(g)(gas phase) (e) H2O2(aq)+MnO4−(aq)⟶Mn2+(aq)+O2(g)(in acid) (f) NO2(g)⟶NO3−(aq)+NO2−(aq)(in base) (g) Fe3+(aq)+I−(aq)⟶Fe2+(aq)+I2(aq)
Answers
The balanced equation of the redox reactions by the half-reaction method is as follows:
(a) Zn(s) + 4 H+(aq) + NO₃⁻(aq) ⟶ Zn²⁺ (aq) + 2 H₂O(l) + N₂(g)
(b) Zn(s) + 2 OH⁻(aq) + NO₃⁻(aq) ⟶ Zn(OH)₂(aq) + NH₃(aq)
(c) CuS(s) + 6 H⁺(aq) + 2 NO₃⁻(aq) ⟶ Cu²⁺(aq) + S(s) + 2 NO(g) + 3 H₂O(l)
(d) 4 NH₃(aq) + 5 O₂(g) ⟶ 4 NO₂(g) + 6 H₂O(l)
(e) 2 H₂O₂(aq) + 2 MnO₄⁻(aq) ⟶ 2 Mn²⁺(aq) + 5 O₂(g) + 4 H₂O(l)
(f) 3 NO₂ (g) + 2 OH⁻ (aq) ⟶ 3 NO₃⁻ (aq) + NO₂⁻ (aq) + H₂O(l)
(g) 2 Fe³⁺ (aq) + 2 I⁻ (aq) ⟶ 2 Fe²⁺ (aq) + I₂ (aq)
What are half-reactions in redox reactions?Half reactions refer to the separate reactions that represent the oxidation and reduction processes occurring in the overall redox reaction.
Considering the given redox reactions:
(a) Zn(s) + 4 H+(aq) + NO₃⁻(aq) ⟶ Zn²⁺ (aq) + 2 H₂O(l) + N₂(g)
Half-reaction (oxidation): Zn(s) ⟶ Zn²⁺(aq) + 2 e⁻
Half-reaction (reduction): 4 H+(aq) + NO₃⁻(aq) + 3 e⁻ ⟶ 2 H₂O(l) + N₂(g)
(b) Zn(s) + 2 OH⁻(aq) + NO₃⁻(aq) ⟶ Zn(OH)₂(aq) + NH₃(aq)
Half-reaction (oxidation): Zn(s) + 4 OH⁻(aq) ⟶ Zn(OH)₂(aq) + 2 e⁻
Half-reaction (reduction): NO₃⁻ aq) + 8 H₂O(l) + 6 e⁻ ⟶ NH₃(aq) + 9 OH⁻ (aq)
(c) CuS(s) + 6 H⁺(aq) + 2 NO₃⁻(aq) ⟶ Cu²⁺(aq) + S(s) + 2 NO(g) + 3 H₂O(l)
Half-reaction (oxidation): CuS(s) ⟶ Cu²(aq) + S(s) + 2 e⁻
Half-reaction (reduction): 6 H⁺(aq) + 2 NO₃⁻(aq) + 6 e⁻ ⟶ 2 NO(g) + 3 H₂O(l)
(d) 4 NH₃(aq) + 5 O₂(g) ⟶ 4 NO₂(g) + 6 H₂O(l)
Half-reaction (oxidation): 4 NH₃(aq) ⟶ 4 NO₂(g) + 8 H⁺(aq) + 8 e⁻
Half-reaction (reduction): 5 O₂(g) + 10 H₂O(l) + 10 e⁻ ⟶ 20 OH⁻(aq)
(e) 2 H₂O₂(aq) + 2 MnO₄⁻(aq) ⟶ 2 Mn²⁺(aq) + 5 O₂(g) + 4 H₂O(l)
Half-reaction (oxidation): 2 H₂O₂(aq) ⟶ 4 H⁺(aq) + 4 e⁻ + O₂(g)
Half-reaction (reduction): 2 MnO₄⁻(aq) + 16 H⁺ (aq) + 10 e⁻ ⟶ 2 Mn²⁺ (aq) + 8 H₂O (l)
(f) 3 NO₂ (g) + 2 OH⁻ (aq) ⟶ 3 NO₃⁻ (aq) + NO₂⁻ (aq) + H₂O(l)
Half-reaction (oxidation): 3 NO₂(g) + 6 OH⁻(aq) ⟶ 3 NO₃⁻ (aq) + 3 e⁻ + 3 H₂O(l)
Half-reaction (reduction): 3 NO₂ (g) + 2 e⁻ ⟶ 3 NO₂⁻ (aq)
(g) 2 Fe³⁺ (aq) + 2 I⁻ (aq) ⟶ 2 Fe²⁺ (aq) + I₂ (aq)
Half-reaction (oxidation): 2 Fe³⁺ (aq) ⟶ 2 Fe²⁺ (aq) + 2 e⁻
Half-reaction (reduction): 2 I⁻ (aq) ⟶ I₂ (aq) + 2 e⁻
Learn more about half-reactions at: https://brainly.com/question/26411933
#SPJ1
What size volumetric flask would you use to create a 1.00M solution using 166.00 g of KI?
Answers
Answer:
A 1 liter volumetric flask should be used.
Explanation:
First we convert 166.00 g of KI into moles, using its molar mass:
Molar mass of KI = Molar mass of K + Molar mass of I = 166 g/mol
166.00 g ÷ 166 g/mol = 1 mol KIThen we calculate the required volume, using the definition of molarity:
Molarity = moles / litersLiters = moles / molarity
1 mol / 1.00 M = 1 LWhat state of matter is every compound in for the chemical reaction? CH4 (g)+2 0₂ (g) -> CO₂(g) + 2 H₂0 (g
Answers
Answer:
CH4 (g) and O2 (g) are both in the gaseous state, while CO2 (g) and H2O (g) are also in the gaseous state.
In the above reaction, the reactants are in the gaseous state, and the products formed are also in the gaseous state.
Every compound in a chemical reaction can be in any state of matter like solid, liquid, or gas. In the reaction of methane and oxygen, the initial state of the reactants is in the gaseous form. The chemical reaction of methane and oxygen is given by the equation CH4 (g) + 2 O2 (g) -> CO2 (g) + 2 H2O (g).Here, methane and oxygen are the reactants, and carbon dioxide and water are the products. Methane (CH4) and oxygen (O2) react together in the presence of a spark or heat to produce carbon dioxide (CO2) and water (H2O).In the reaction, the methane gas combines with oxygen gas, which causes the release of heat energy and forms carbon dioxide gas and water vapor. Methane gas is a colorless and odorless gas that burns cleanly and is one of the primary components of natural gas.
The oxygen gas required for the reaction is available in the atmosphere. Carbon dioxide is a colorless gas with a faint odor and taste and is a significant component of the Earth's atmosphere. Water is a colorless, odorless, and tasteless liquid that is essential to life.The state of matter of every compound in a chemical reaction can change depending on the conditions in which the reaction occurs. For instance, a substance that is in the solid state at a lower temperature may melt into a liquid or boil into a gas at a higher temperature. Similarly, a liquid may freeze into a solid or vaporize into a gas under different conditions.
for such more questions on reactants
https://brainly.com/question/26283409
#SPJ8
What causes blood cells to shrink?
Answers
Calculate ΔHrxn for the following reaction: C(s) + H2O(g) --> CO(g) + H2(g) Use the following reactions and given ΔH values: C (s) + O2 (g) → CO2 (g), ΔH = -393.5 kJ 2 CO (g) + O2 (g) → 2 CO2 (g), ΔH= -566.0 kJ 2 H2 (g) + O2 (g) → 2 H2O (g), ΔH= -483.6 kJ Express your answer numerically, to four significant figures and in terms of kJ.
Answers
Answer:
ΔH = 130.5 kJ
Explanation:
Hello,
In this case, by using the Hess law, we compute the enthalpy of the required reaction:
C(s) + H2O(g) --> CO(g) + H2(g)
Thus, the first step is to keep the following reaction unchanged:
C (s) + O2 (g) → CO2 (g), ΔH = -393.5 kJ
Next, we invert and halve this reaction:
2 CO (g) + O2 (g) → 2 CO2 (g), ΔH= -566.0 kJ
So the enthalpy of reaction is inverted and halved:
CO2 (g) → CO (g) + 1/2 O2 (g) ΔH= 283 kJ
Then, we also invert and halve this reaction:
2 H2 (g) + O2 (g) → 2 H2O ΔH= -483.6 kJ
So the enthalpy of reaction is inverted and halved as well:
H2O → H2 (g) + 1/2 O2 (g) ΔH= 241.8 kJ
Finally, we add the three reactions to obtain the required reaction:
= C (s) + O2 (g) + CO2 (g) + H2O → H2 (g) + 1/2 O2 (g) + CO (g) + 1/2 O2 (g) + CO2 (g)
= C (s) + O2 (g) + CO2 (g) + H2O → H2 (g) + O2 (g) + CO (g) + CO2 (g)
= C (s) + H2O → H2 (g) CO (g)
So enthalpy is computed by:
ΔH = -393.5 kJ + 283 kJ + 241.8 kJ
ΔH = 130.5 kJ
Best regards.
Considering the Hess's Law, the enthalpy change for the reaction is 131.3 kJ.
Hess's Law indicates that the enthalpy change in a chemical reaction will be the same whether it occurs in a single stage or in several stages. That is, the sum of the ∆H of each stage of the reaction will give us a value equal to the ∆H of the reaction when it occurs in a single stage.
In this case you want to calculate the enthalpy change of:
C(s) + H₂O(g) → CO(g) + H₂(g)
You know the following reactions, with their corresponding enthalpies:
Equation 1: C (s) + O₂(g) → CO₂ (g) ΔH = -393.5 kJ
Equation 2: 2 CO (g) + O₂ (g) → 2 CO₂ (g) ΔH= -566.0 kJ
Equation 3: 2 H₂ (g) + O₂ (g) → 2 H₂O (g) ΔH= -483.6 kJ
First stepFirst, to obtain the enthalpy of the desired chemical reaction you need one mole of C(s) on reactant side and it is present in first equation so let's write this as such.
Second stepNow, 1 mole of CO(g) must be a product and is present in the second equation. Since this equation has 2 moles of CO(g) on the reactant side, it is necessary to locate this component on the product side (invert it) and divide it by 2 to obtain 1 mole of CO(g).
When an equation is inverted, the sign of ΔH also changes.
And since enthalpy is an extensive property, that is, it depends on the amount of matter present, since the equation is divided by 2, the variation of enthalpy also is divided by 2.
Third step
Finally, 1 mole of H₂O(g) must be a reactant and is present in the third equation. Since this equation has 2 moles of CO(g) on the product side, it is necessary to locate this component on the product side (invert it) and divide it by 2 to obtain 1 mole of H₂O(g).
So, the sign of ΔH also changes and the variation of enthalpy is divided by 2.
In summary, you know that three equations with their corresponding enthalpies are:
Equation 1: C (s) + O₂(g) → CO₂ (g) ΔH = -393.5 kJ
Equation 2: CO₂ (g) → CO (g) + \(\frac{1}{2}\) O₂ (g) ΔH= 283 kJ
Equation 3: H₂O (g) → H₂ (g) + \(\frac{1}{2}\) O₂ (g) ΔH= 241.8 kJ
Adding or canceling the reactants and products as appropriate, and adding the enthalpies algebraically, you obtain:
C(s) + H₂O(g) → CO(g) + H₂(g) ΔH= 131.3 kJ
Finally, the enthalpy change for the reaction is 131.3 kJ.
Learn more:
brainly.com/question/5976752?referrer=searchResults brainly.com/question/13707449?referrer=searchResults brainly.com/question/13707449?referrer=searchResults brainly.com/question/6263007?referrer=searchResults brainly.com/question/14641878?referrer=searchResults brainly.com/question/2912965?referrer=searchResultsA balloon is filled with 266 L of He gas, measured at 38 °C and 0.995 atm. What will its volume be when the temperature is lowered to −76 ° C and the pressure is 0.561 atm?
Answers
When the temperature is lowered to -76 °C and the pressure is 0.561 atm, the volume of the balloon will be approximately 179 L.
To solve this problem, we can use the combined gas law equation, which relates the initial and final conditions of a gas sample:
(P1 * V1) / (T1) = (P2 * V2) / (T2)
where P1, V1, and T1 are the initial pressure, volume, and temperature, respectively, and P2, V2, and T2 are the final pressure, volume, and temperature, respectively.
Substituting the given values:
(P1 * 266 L) / (38 + 273.15 K) = (0.561 atm * V2) / (-76 + 273.15 K)
Simplifying the equation:
(0.995 atm * 266 L) / (311.15 K) = (0.561 atm * V2) / (197.15 K)
Solving for V2:
V2 = [(0.995 atm * 266 L) / (311.15 K)] * (197.15 K / 0.561 atm)
V2 ≈ 179 L
For more such questions on temperature
https://brainly.com/question/27988898
#SPJ8
Use electronegativity values to classify the bonds in each of the following compounds as ionic, polar covalent, or nonpolar covalent.
(a) MgCl2
(b) CO2
(c) H2S
(d) NO2
Answers
On basis of electronegativity the nature of bonds in compounds are
a) Ionic Bond
b) Polar Covalent Bond
c) Non-Polar Covalent Bond
d) Non-Polar Covalent Bond
Electronegativity is tendency of an atom in a molecule to attract the shared pair of electrons towards itself. If polarity of compound increases the effect of Hydrogenbonding become more stronger and high amount of heat required to break a bond and hence Melting point increases. Electronegativity chart is present below :
Element Symbol Electronegativity
Hydrogen H 2.2
Carbon C 2.55
NItrogen N 3.04
Oxygen O 3.44
Magnesium Mg 1.31
Sulphur S 2.58
Chlorine Cl 3.16
On the basis of electronegativity difference :
Ionic Compound = E.N difference > 1.7.
Polar- covalent Compound = EN difference > 0.4 but EN difference < 1.7.
Non-polar Covalent Compound = EN difference < 0.4. Now, check the difference in provide compounds
1 ) MgCl₂ = 3.16 - 1.31 = 1.85 --> Ionic Bond
2 ) CO₂ = 3.44 - 2.55 = 0.89 --> Polar Covalent Bond
3 ) H₂S = 2.58 - 2.2 = 0.38 --> Non polar Covalent Bond
4 ) NO₂ = 3.44 - 3.04 = 0.40 --> Non polar Covalent bond.
To learn more about electronegativity, refer:
https://brainly.com/question/18258838
#SPJ4
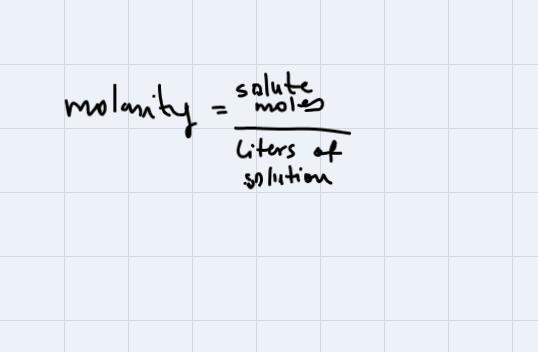
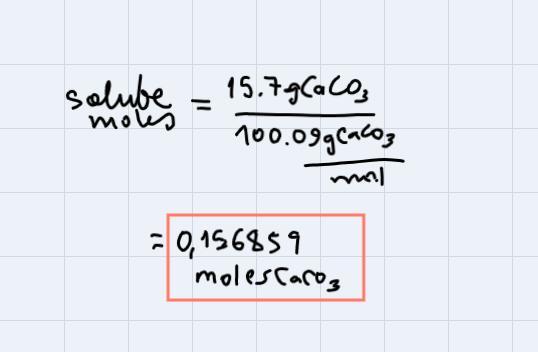
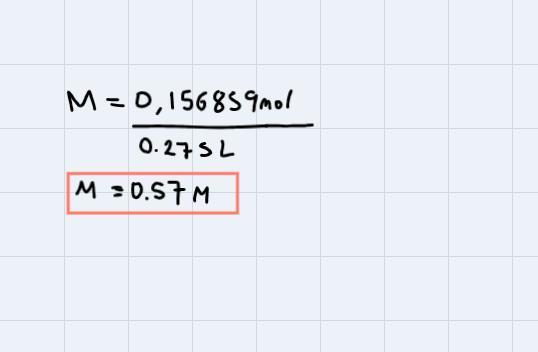
Calculate the molarity of a solution that contains 15.7 g of CaCO3 dissolved in enough water to make 275 mL of solution. (MW: CaCO3=100.09 g/mol
Answers
We can find molarity using the following equation:
In this problem, our solute is CaCO3, and we have 15.7 g of it. To express this amount in moles, what we do is to divide by the molar weight of CaCO3. (Always that you want to pass from grams to moles you do this, just to divide the amount that you have by the molar weight of the substance).
This is:
Now, we have 275mL of solution and this is 0.275L of solution. (To pass from mL to L we just divide by 1000).
Finally, replacing in the formula to find molarity, we have:
The answer is 0.57M.
\( \sf{\blue{«} \: \pink{ \large{ \underline{Q\orange{U} \red{E} \green{S} \purple{TI} \pink{{ON}}}}}}\)
What is the difference between an acid and a base? Provide examples of each.
Answers
Answer:
An acid is any hydrogen-containing substance that is capable of donating a proton (hydrogen ion) to another substance. A base is a molecule or ion able to accept a hydrogen ion from an acid.
Answer:
Acids::1.Sour in taste
2. Tum blue litmus into red
3. Acids change methyl orange to red
4.Phenolphthalein remains colourless
5. Acids do not give soapy touch
6. Give hydrogen ions in solution
Bases::Bitter in taste
Bitter in tasteTurn red litmus blue Bases change methyl orange to yellowPhenolphthalein gives pink colour Soapy to touchGive hydroxyl ions in solution if it helped uh please mark me a BRAINLIEST :-))Which statement about geologic times are true? ~Pick multiple answers~

Answers
Answer:
the correct answers are A,D,E, and G
Explanation:
HN5O3+Mg reaccion de sustitucion sencilla
Responder por favor lo necesito rapido
Answers
The balanced equation of the reaction could be written as; \(HN_{5} O_{3} + Mg ------ > MgO + HNO_{3}\)
What is a reaction?We know that in chemistry, the term reaction has to do with the combination of two or more species to give a product. Now the process of the reaction would alter the chemical composition of the reactants as we move on the way to get the ne substances called the products. There would be a breaking down of the bonds that holds the reactant molecules together and then there is a recombination of the atoms in a different way so as to obtain the products of the reaction. This is how a chemical reaction takes place in a given system.
In this case we have the oxidation of the compound called \(HN_{5} O_{3}\). In this case, the compound is actually being oxidized as we can see from the process that would be shown in the reaction equation accurately.
Learn more about reaction equation:https://brainly.com/question/3588292
#SPJ1
9. It says its wrong? someone help!

Answers
The balanced net ionic equation for the reaction between potassium sulfide and lead(II) nitrate is Pb²⁺(aq) + S²⁻(aq) → PbS(s)
Writing balanced net ionic equation for a reactionFrom the question, we are to write the balanced net ionic equation for the given chemical reaction.
The given chemical reaction is
K₂S(aq) + Pb(NO₃)₂(aq) →
The between potassium sulfide and lead(II) nitrate will produce potassium nitrate and lead sulfide.
That is,
K₂S(aq) + Pb(NO₃)₂(aq) → KNO₃(aq) + PbS(s)
Now, balance the equation
K₂S(aq) + Pb(NO₃)₂(aq) → 2KNO₃(aq) + PbS(s)
Write the complete ionic equation
2K⁺(aq) + S²⁻(aq) + Pb²⁺(aq) + 2NO₃⁻(aq) → 2K⁺(aq) + 2NO₃⁻(aq) + PbS(s)
Cancel out the spectator ions
S²⁻(aq) + Pb²⁺(aq) → + PbS(s)
Hence, the balanced net ionic equation is
Pb²⁺(aq) + S²⁻(aq) → PbS(s)
Learn more on Writing balanced net ionic equation here: https://brainly.com/question/28837770
#SPJ1
50 points plz simple answers just zinc-carbon battery or alkaline battery
What are the electrodes made of?
What is the electrolyte made of?
What is a common usage of this type of battery?
What are the advantages of this type of battery?
Options Zinc-carbon battery or alkaline battery
Answers
Explanation:
-The most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water or alcohol.
-battery electrolyte is a solution inside batteries. it transports positively charged ions between the cathode and anode terminals.
-The main advantage of solid electrolytes is that they eliminate the risk of leaking and eliminate flammability, which is a safety risk in batteries with liquid electrolytes.
-it is a alkaline battery
Infer Before 1937, scientists had not found element 43. Chemists predicted the properties of element 43. How was it possible for chemists to predict those propertis
Answers
Answer:
chemists has their way in doing alot of stuffs
if you have 4.0 moles of nitrogen and 5.0 moles of hydrogen, what is the maximum amount of ammonia that you can produce?

Answers
Answer:
Explanation:
Here, we want to get the maximum amount of ammonia that can be produced
From the question, we have it that:
1 mole nitrogen gave 2 moles ammonia
Thus:
4 mole nitrogen will give 8 moles ammonia
Furthermore:
3 moles of hydrogen gave 2 moles of ammonia
5 moles of hydrogen will give:
\(\frac{5\times2}{3}\text{ = }\frac{10}{3}\)The maximum amount of ammonia that can be produced is thus 8.0 moles
What is the symbol for the ion that contains 12 protons, 10 electrons, and 12 neutrons?
Answers
list 10 concepts/words associated with motion
Answers
A list of the ten (10) concepts or words that are associated with motion include the following:
VelocityTimeAccelerationGravityPositionEnergyKepler’s Law of Elliptic Motion.Newton's First Law of Motion.Newton's Second Law of Motion.Doppler effect.What is a motion?Motion can be defined as a change in the location (position) of a physical object or body with respect to a reference point and time, especially due to the action of an external force.
The types of motion.In Science, there are different types of motion and these include the following:
Translational motionPeriodic motionRotational motionCircular motion Oscillatory motionLinear motion Uniform motionNon-Uniform motionGenerally, there are different concepts, phenomena and words that are associated with motion. A list of this ten (10) concepts or words include the following:
VelocityTimeAccelerationGravityPositionEnergyKepler’s Law of Elliptic Motion.Newton's First Law of Motion.Newton's Second Law of Motion.Doppler effect.Read more on motion here: brainly.com/question/26048315
#SPJ1
Laser fusion Group of answer choices uses chemical reactions to produce energy uses nuclear reactions to produce energy implodes a fuel pellet. causes most of the fuel pellet to produce energy. rotates a fuel pellet.
Answers
Answer:
Uses nuclear reactions to produce energy
Implodes a fuel pellet
Explanation:
Laser fusion is a method of initiating nuclear fusion reactions through heating, and compressing fuel pellets containing deuterium and tritium using high energy density laser beams. Lase fusion is also known as inertial confinement fusion and the energy produced by the process is known as Laser Inertial Fusion Energy, LIFE.
During the process of laser fusion, small pellets of deuterium-tritium (DT) isotopes mixture are fed into a blast chamber where they are compressed to high densities using a number of amplified laser beams in the chamber.
The high energy density of the beams as well as the heat produced due to compression, induces the thermonuclear explosion ignition resulting in the production of high energetic products such as charged particles, x-rays and neutrons. The energy produced is absorbed and stored as heat in a blanket that is then used in a steam thermal cycle to generate electrical power.
There are two methods of compression of the DT pellet: direct and indirect-drive laser fusions.
However, there are a number of limitations to energy production by this process. One limitation is that the process is extremely inefficient in energy energy production. Also, the heat produced by the flashtubes results innthe deformation of the laser glass.
how does an objects mass affect its kinetic energy?
Answers
If 16.4 grams of copper (II) bromide react with 22.7 grams of sodium chloride, how many grams of sodium bromide are formed?

Answers
The amount of sodium bromide that would be formed from the reaction will be 7.5524 grams
Stoichiometric calculationLooking at the equation of the reaction:
\(CuBr_2 + 2NaCl --- > CuCl_2 + 2NaBr\)
The mole ratio of CuBr2 and NaCl is 1:2.
Mole of 16.4 grams of CuBr2 = 16.4/223.37
= 0.0734 moles
Mole of 22.7 grams of NaCl = 22.7/58.44
= 0.3884 moles
Equivalent mole of NaCl = 0.1468 moles
Thus, NaCl is in excess while CuBr2 is limiting.
Mole ratio of CuBr2 and NaBr = 1:1
Mass of 0.0734 mole NaBr = 0.0734 x 102.894
= 7.5524 grams
More on stoichiometric calculation can be found here: https://brainly.com/question/8062886
PLZ HELP ASAP
Which would increase the reaction rate?
Check all that apply.
A. Stirring the reaction
B. Raising the activation energy
O C. Adding a catalyst
D. Raising the temperature
Answers
Answer:
adding a catalyst will increase the reaction rate.
Explanation:
im like 95% sure thats right.
Answer:
A. C. D
Explanation:
ap ex
High-pressure liquid chromatography (HPLC) is a method used in chemistry and biochemistry to purify chemical substances. The pressures used in this procedure range from around 500 kilopascals (500,000 Pa) to about 60,000 kPa (60,000,000 Pa). It is often convenient to know the pressure in torr. If an HPLC procedure is running at a pressure of 5.05×10^8 Pa , what is its running pressure in torr?
Answers
Answer:
3787500 Torr
Explanation:
1 Pascal = 0.0075 Torr
So:
5.05x10^8 Pa --- x
1 Pa --- 0.0075 Torr
x = 5.05x10^8 × 0.0075
x = 3787500 Torr
How many moles of calcium chloride are needed to produce 6.50 moles of sodium chloride?
Answers
Answer:
This question appear incomplete
Explanation:
This question appear incomplete because an equation to show the production of sodium chloride from calcium chloride should have been illustrated. However, if the balanced chemical equation showing sodium chloride (NaCl) been a product of a reaction involving calcium chloride (CaCl₂) as a reactant (shown below) is to be used, then we start by writing a complete balanced chemical equation
CaCl₂ + Na₂CO₃ ⇒ CaCO₃ + 2NaCl
From the equation above, it can be deduced that 1 mole of CaCl₂ is required to produce 2 moles of NaCl, thus how many moles of CaCl₂ will be required to produce 6.5 moles of NaCl.
1 mole of CaCl₂ = 2 moles of NaCl
? moles of CaCl₂ = 6.5 moles of NaCl
cross multiply
? moles of CaCl₂ = 6.5 × 1/2
? moles of CaCl₂ = 3.25 moles of CaCl₂
3.25 moles of CaCl₂ will be needed to produce 6.5 moles of NaCl