ANSWER ASAP FOR 90 POINTS Question: Air pressure is...
A.high and low at the bottom
B. same at the top same at the bottom
C.low at the top low at the bottom
D.low at the top hight at the bottom
Answers
Air pressure is D.low at the top hight at the bottom.
How much air pressure is typical?Pressure is the force that the air applies to the ground when gravity acts on it. At sea level, there is typically a pressure of 1013.25 millibars, or 14.7 pounds per square inch.
When the air pressure is high, what happens?High pressure frequently heralds sunny, dry weather. Clouds and precipitation are frequently indicative of low pressure. Air that is sinking is related with high pressure. Because it is pushing DOWN on the ground, air pressure is higher.
How do we react to air pressure?High barometric pressure exerts additional pressure against our bodies, which restricts how much tissue can expand. On the other hand, when air pressure is low, our body's tissues can expand more, which results in more pressure.
Learn more about air pressure here:
brainly.com/question/7381033
#SPJ1
Related Questions
What is the charge on the potassium ion?Select one:a.1 +b.1 -c.2 -d.2 +
Answers
The charge on the potassium ion is 1+. So, Option A is the correct answer from the given options.
What does ionic charge mean?A positive or negative charge on an atom is known as an ionic charge. The atom's electron arrangement and the quantity of valence electrons affect the charge. Elements in the same group on the periodic table have an equal amount of valence electrons. Therefore, they often have the same ionic charge.We must ascertain the charge carried by the potassium ion.
Being a group 1 element, potassium will produce an ion with a positive charge.
Since potassium achieves an extremely stable noble gas electrical structure after losing one electron, this element possesses a steady +1 valency.
As a result, the potassium ion has a 1+ charge. The right response from the available alternatives is thus Option A.
Learn more about valence electrons here:
https://brainly.com/question/371590
#SPJ9
4.
A container holds 500 mL of Co, at 742 torr. What will be the volume of the Co, if the
pressure is increased to 795 torr?
GIVEN
WORK
Answers
Answer:
V2 = 0.465
Explanation:
To solve this question, we need to follow the equation below:
P1V1 = P2V2
We know that:
V1 = 500ml = 0.5L (must be convert to L)
P1 = 742 torr (must be convert to atm by divided by 760) = 0.976 atm
P2 = 795 torr = 1.05 atm
V2 = ?
P1V1 = P2V2
0.976 x 0.5 = 1.05 x V2
0.488 = 1.05 x V2
(Divided by side by 1.05 to get rid of it)
V2 = 0.465
Element Y has two naturally occurring isotopes. The most dominant isotope has a mass of 114.3789 amu and a percent abundance of 64.23%. What is
the mass of the second isoptope if the average atomic mass is 128.4359 amu? Remember that the two percentages will have to sum to 100%.
Answer to the correct number of sigtigs including units in your answer.
(to receive full credit, work must be submitted for this question)
Answers
Answer:
153.6771 amu
Explanation:
From the question given above, the following data were:
Isotope A:
Mass of A = 114.3789 amu
Abundance (A%) = 64.23%
Isotope B:
Mass of B =.?
Abundance (B%) = 100 – A%
Abundance (B%) = 100 – 64.23
Abundance (B%) = 35.77%
average atomic mass of Element Y = 128.4359 amu
The mass of the 2nd isotope (i.e isotope B) can be obtained as follow:
Average atomic mass = [(Mass of A × A%)/100] + [(Mass of B × B%)/100]
128.4359 = [(114.3789 × 64.23)/100] + [(Mass of B × 35.77) /100]
128.4359 = 73.4656 + (Mass of B × 0.3577)
Collect like terms
128.4359 – 73.4656 = Mass of B × 0.3577
54.9703 = Mass of B × 0.3577
Divide both side by 0.3577
Mass of B = 54.9703 / 0.3577
Mass of B = 153.6771 amu
Therefore, the mass of the 2nd isotope is 153.6771 amu
100 POINTS - is air trapped in a jar a compound, element, homogeneous mixture or heterogeneous mixture
Answers
Answer:
homogenous mixture
Explanation:
homogenous mixtures appear in one phase and have even particle distribution.
Answer:
Air is indeed a homogeneous mixture of dinitrogen, dioxygen, carbon dioxide and a few other gases. All of the gases are in the same phase, and thus constitute a homogeneous mixture.
Explanation:
hope it helps youWhat is the average speed of a bicyclist who rides 75 km in 2.5 hours? please show how you worked it out if you can bc my teacher is very specific
Answers
Answer:
30
Explanation:
I’m pretty sure it is

classify each of the following heterocyclic compounds as aromatic, anti-aromatic, or non-aromatic.
Answers
The classification of heterocyclic compounds as aromatic, anti-aromatic, or non-aromatic depends on their molecular structure and electron configuration.
Aromatic compounds are characterized by having a planar, cyclic arrangement of alternating double and single bonds, which results in an electron delocalization known as aromaticity. The most well-known example of an aromatic compound is benzene.
Anti-aromatic compounds have a similar arrangement of alternating double and single bonds, but the resulting electron configuration is not favorable for aromaticity, and the molecule is destabilized.
Non-aromatic compounds do not have the planar, cyclic arrangement of alternating double and single bonds required for aromaticity. Instead, they have a linear or non-planar arrangement of bonds that does not result in significant electron delocalization. It is necessary to specify the heterocyclic compounds in order to classify them as aromatic, anti-aromatic, or non-aromatic.
Learn more about heterocyclic compounds:
brainly.com/question/17164215
#SPJ4
What element is found in Group 16 and Period 3?
Answers
Answer:
sulfur is found in group 16 period 3
!!DUE TODAY, NEED HELP ASAP!!
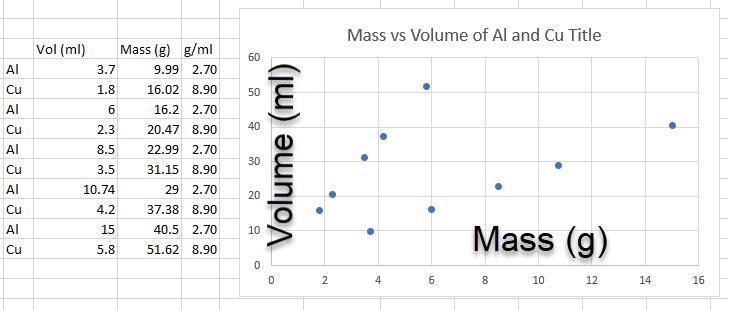
A student measured the masses of some aluminum and copper cylinders of different volumes. The data is displayed below:
Aluminum: Copper:
Volume (mL)
Mass (g)
Volume (mL)
Mass (g)
3.7
9.99
1.8
16.02
6
16.2
2.3
20.47
8.5
22.99
3.5
31.15
10.74
29
4.2
37.38
15
40.5
5.8
51.62
On graph paper graph the data above on ONE graph. Use the graph paper so the longer side is your y-axis.
Use up the majority of the graph paper. Do not make a small graph!
Use a ruler/straight edge so you graph is neat.
You will need to plot the points for each substance and create a double line graph. When connecting your points for each substance, continue the lines past the plotted points.
Be sure to include a key to identify the substances.
Make your X-axis volume(mL) and your Y-axis mass(g)
Look at the numbers for both substances when creating your scales. You will most likely have a different scale for mass and volume. What is the lowest number? What is the highest number? What does it make sense to go by?
Calculate the density of each metal.
Aluminum:_________________________ Copper:________________________
What happens to the mass of the copper at the volume increases from 6 to 8mL?
Which sample is more dense? How does the graph show you this?
Answers
Answer: The data are not arranged in a useful pattern. I arranged them as best I could and made a graph in Excel for demonstration.
Explanation: The data are plotted with mass on the x axis and volume on the y. Add a key to identify the top line as CU and the bottom as Al.
The density of Cu is 8.9 g/ml and 2.70 for Al.
The mass of copper increases by 17.8 grams going from 6 to 8 ml. (8.9 g/ml)*(2 ml) = 17.8 grams.
Copper is more dense. c The graph shows that for the same volumes, copper has the higher mass.
which of the following is the primary cause of corrosion in ferrous piping systems? select one: a. calcium salts b. vinegar c. alkalinity d. oxygen
Answers
Oxygen is the primary cause of corrosion in ferrous piping systems.
Rust is created when iron (or an iron alloy) is exposed to oxygen while being in the presence of moisture. This reaction often takes place over a considerable amount of time and is not instantaneous. Iron oxides are created when oxygen atoms join forces with iron atoms. The iron atoms in the object or structure lose their bonding power as a result.
Iron is a reducing agent, but oxygen is a particularly effective oxidizing agent. As a result, when exposed to oxygen, the iron atom rapidly gives off electrons. What causes the chemical reaction?
Fe → Fe2+ + 2e-
When water is present, the oxygen atom further raises the oxidation state of iron.
O2 + 4Fe2+ = 4Fe3+ + 2O2-
The iron cations and water molecules now engage in the following acid-base processes.
Fe(OH)2 + 2H+ = Fe2+ + 2H2O
Fe(OH)3 + 3H+ = Fe3+ + 3H2O
The direct interaction of the iron cations and hydroxide ions results in the formation of the iron hydroxides as well.
4e- + O2 + H2O = 4OH-
2OH- + Fe2+ Fe(OH)2
Fe3+ + 3OH- yields Fe(OH)3.
For more information on corrosion kindly visit to
https://brainly.com/question/28487650
What makes the element oxygen different from the element hydrogen?
Answers
Answer: the difference is due to the fact that one water molecule has two hydrogen atoms to one oxygen this means it takes two water molecules (2 H2O) to make one oxygen molecule (02) .At the same time however two molecules of water (2 H20) can make two molecules of hydrogen (2 H20)
Explanation: hope this helped and mark me as brainliest please =)
which part of the human body is more complex your heart of the circulatory system
Answers
Answer:
The circulatory system
Explanation:
The human body has two circulatory systems, the pulmonary system, and the systemic system.
Quick
Choch
Place each description in the correct category.
represented by symbols
Elements
Compounds
made of one type of atom
represented by formulas
cannot be broken down
can be chemically broken down
made of two or more types of atoms
Answers
Answer:
a i believe
Explanation:
a
2C2H6 + 7O2 → __CO2 + 6H2O
What coefficient will need to be placed in the blank in order for the following equation to be correctly balanced?
A.
1
B.
3
C.
4
D.
7
Answers
Answer:
From the Combustion formula of Organic Compounds....
Exactly 4 Moles of CO2 would make the equation balanced
So
OPTION C
4MOLES
In the ground state, which atom has a completely filled valence electron shell?
Answers
Answer:
The noble gases(neon, helium, argon)
Explanation:
In the ground state, noble gas atom has a completely filled valence electron shell. Space makes up the majority of an atom.
What is atom?The smallest unit of matter that may be split without producing electrically charged particles is the atom. It is also the smallest piece of substance with chemical element-like characteristics. Electric forces, which link electrons towards the nucleus of atoms, cause them to be drawn to any positive charge.
Space makes up the majority of an atom. The remainder is made up of a cloud of negatively charged electrons around a positively charged nucleus made up of protons and neutrons. Compared to electrons, that are the smallest charged particles in nature, the nucleus is tiny and dense. In the ground state, noble gas atom has a completely filled valence electron shell.
Therefore, in the ground state, noble gas atom has a completely filled valence electron shell.
To learn more about atom, here:
https://brainly.com/question/29712157
#SPJ6
Common indoor air contaminants include all of the following EXCEPT:
a)formaldehyde
b)ozone
c)sulfur oxides
d)carbon monoxide
e)radon
Answers
Answer:
Formaldehyde, ozone, carbon monoxide, and radon are common indoor air contaminants. Formaldehyde can come from building materials and household products, ozone can be produced by electronic devices, carbon monoxide can be produced by gas appliances, and radon can seep into homes from the ground. Sulfur oxides, on the other hand, are more commonly found in outdoor air pollution, particularly from industrial emissions and fossil fuel combustion.
Mauren and Esteban have been investigating various reactions to find out if they are exothermic or endothermic
Which of these reactions are endothermic and which are exothermic
Answers
Which of the following processes have a ΔS that is negative? Select all that applyGroup of answer choicesa. CO2 (s) → CO2 (g)b. N2 (g) + 3 H2 (g) → 2 NH3 (g)c. Al (s) → Al (l)d. 2 N2O5 (g) → 4 NO2 (g) + O2 (g)e. N2 (g) → N2 (l)
Answers
The processes have a negative ΔS are N₂ (g) + 3 H₂ (g) → 2 NH₃ and N₂ (g) → N₂ (l)
So the correct answer is B and E
In the given processes, those with a negative ΔS (change in entropy) involve a decrease in disorder.
Process (b) N₂ (g) + 3 H₂ (g) → 2 NH₃ (g) has a negative ΔS, as the number of gas particles decreases from 4 moles to 2 moles, reducing the disorder.
Process (e) N₂ (g) → N₂ (l) also has a negative ΔS, as the transition from gas to liquid represents a decrease in entropy due to the more structured arrangement of particles in the liquid state.
The other processes increase the disorder and have a positive ΔS.
Hence,the answer if the question is B and E.
Learn more about entropy at https://brainly.com/question/16676779
#SPJ11
Which substance would NOT normally be expected in urine? A. sodium B. protein C. water D. potassium E. uric acid
Answers
Protein is not normally expected in urine in significant amounts. So, the correct option is B. protein.
The kidneys play a crucial role in filtering waste products and excess fluids from the blood, while retaining essential substances such as sodium, potassium, and water. Uric acid is a byproduct of purine metabolism and is normally excreted in urine.
However, when the kidneys are not functioning properly, uric acid levels can build up and lead to conditions like gout. Sodium and potassium levels in urine can also provide important information about a person's overall health, particularly in relation to hydration status and kidney function. Elevated levels of protein in urine, known as proteinuria, can be a sign of kidney damage or disease and require further evaluation.
For more such questions on Protein
https://brainly.com/question/30245761
#SPJ11
How many moles are in 3.27 x 1027 atoms of carbon (C)?
Answers
Answer:
Try:
1. How many atoms are in 6.5 moles of zinc?
6.5 moles 6.02 x 1023 atoms = 3.9 x 1024 atoms
1 mole
2. How many moles of argon are in a sample containing 2.4 x 1024 atoms of argon?
2.4 x 1024 atoms of argon 1 mole = 4.0 mol
6.02 x 1023 atoms
3. How many moles are in 2.5g of lithium?
2.5 grams Li 1 mole = 0.36 mol
6.9 g
4. Find the mass of 4.8moles of iron.
4.8 moles 55.8 g = 267.84 g = 270g
1 mole
Explanation:
i think i did it about right..?? :-(
WILL GIVE BRAINLY
What are three facts about the biosphere that are in depth
Answers
Answer:
Facts About Biosphere 1: The Evolution Of Biosphere
Biosphere has evolved. It started from the non living matter or simple organic compound which created the life. This process is called bioprocess which conducted around 3.5 billion years ago.
Facts about Biosphere 2: the life of earth
It is not easy for the scientists to determine the life of earth. However, they can estimate it after the discovery of the metasedimentary rocks from Western Greenland. The scientists believed that it was dated back around 3.7 billion years old.
Facts About Biosphere 3: Another Evidence For The Earth’s Life
Evidence was found in Western Australia. The discovery of microbial mat fossils made the scientists estimated that the earth was dated around 3.48 billion years ago.
1. Consider NH3.If it dissolves in water(i) NH3 + H20 + NHẤ4+ H2O(ii)NH3 + H2O → NH+3 + OH-(iii) NH3 + H2O + NH+4+ OH-(iv) NH3 + H2O → NH+4+ OH-Which represents the dissolution of NH3 in water(a) i(b) ii (c) iii (d) iv (e) iii and iv2. HOA2+H20 . → H3O+ + OA-CIn this reaction:(i) OA c is the conjugate base of H2O(ii)OA-c is the conjugate base of HOAc (iii) H3O+ is theсconjugate base of HOA.(iv) H3O+ is the conjugate acid of H2O(a) i(b) ii (c) iii (d) iv (e) none3. Arrange the following according to increasing acid strength(i) Ka= 2.5 + 10-15(ii) Ka= 9.0 + 10-9(iii) pKa= 7.5(iv) % dissociation =100(a) iv, iii, ii, i2(b) ii, I, iii, iv(c) i, iii, iv, ii(d) i, ii, iii, iv(e) iii, iv, ii, i2
Answers
1. Ammonia is a colorless gas with a chemical formula of NH3, when it comes in contact with water, it will be transformed into Ammonium ion and it will produce one hydroxide ion, and this is why Ammonia will present a more basic (pH) behavior, the reaction that represents this behavior is:
NH3 + H2O -> NH4+ + OH-
Number 4 is the only one that represents it well
Number 3 has the same reaction but since there is a plus sign instead of an arrow, I consider it wrong.
What is the volume of 5.07 grams of copper? The density of copper is 8.96g/mL
Answers
Explanation
Given:
Mass of copper = 5.07 g
Density of copper = 8.96 g/mL = 8960 g/L
Requested: Volume of copper
Solution
p = m/V where p is the density, m is the mass and V is the volume
m = p x V
V = m/p
V = 5.07 g/8.96 g/mL
V = 0.566 mL
Answer
Volume of copper = 0.566 mL
Two jars are shown. Jar A is filled with air. Jar B is filled with water and air.
Compare the volume of the air in jar A with the volume of the water in jar B. Which statement is supported by the evidence shown?
A. If the lids are removed, the volume of the air in Jar A will change, but the volume of water in Jar B will not change.
B. If lids are removed, the volume of the air in Jar A will not change, but the volume of water in Jar B will change.
C. The volume of the air in Jar A is less than the volume of water in Jar B.
D. The volume of the air in Jar A is greater than the volume of water in Jar B.
Answers
Answer:
D for answer
Explanation:
The air column determines the frequency of the sound produced, less air column produces increased frequency. Jar B has lesser air column above water and it will produce higher pitch.
the molality of hydrochloric acid, hcl, in an aqueous solution is 8.56 mol/kg.what is the mole fraction of hydrochloric acid in the solution?
Answers
The mole fraction of hydrochloric acid (HCl) in the solution is approximately 0.460.
Molality (m) is defined as the number of moles of solute per kilogram of solvent. In this case, the molality of HCl is given as 8.56 mol/kg. Mole fraction (X) is defined as the ratio of the moles of a component to the total moles of all components in the solution.
To calculate the mole fraction of HCl, we need to know the total number of moles in the solution. However, the information provided only gives the molality of HCl, which provides the moles of HCl per kilogram of solvent, but not the total moles of the solution. Without the total moles of the solution, it is not possible to directly calculate the mole fraction of HCl. Therefore, based on the given information, it is not possible to determine the mole fraction of HCl in the solution accurately.
Learn more about mole fraction: https://brainly.com/question/14783710
#SPJ11
which of the following explains why salt water is considered a mixture
Answers
Answer:
sal5 water = kaka
Explanation:
because i need points ty man
1. Which of the following parts allow different activities of the cell to happen? A cytoplasm C vacuole B. lysosome D. vesicle 2. If the chloroplasts of a plant cell are damaged, which will it be unable to do? A protect the cell B. make food for the cell C. excrete waste materials D. give instruction for cell to reproduce
Answers
Answer: A. Cytoplasm
Explanation: Cytoplasm is a jelly, like material that fills up the remaining space in the cell. It is also responsible in making the shape of the cell. Without it, other parts of the cells or the organelles cannot make its function because it serves as the hallway of the transportation of the nutrients needed by each cell.
Learn more: https://brainly.ph/question/73078
a process in which a covalent or ionic bond is broken or formed is generally called a ______ reaction.
Answers
A process in which a covalent or the ionic bond is broken or it formed is generally called a chemical reaction.
A chemical reaction is a process in which the bond break and the formation of the bond take place. In a chemical reaction two more more species called as the reactant will react and form the new substance called as the product. The chemical is the process in the chemical transformation takes place. The examples of the chemical reaction is as follows :
CH₄ + 2O₂ -----> CO₂ + 2H₂O
Thus, in a chemical reaction the covalent bond and the ionic bond broke or it formed.
To learn more about bond here
https://brainly.com/question/30359258
#spj4
How many grams of potassium nitride will be produced if you start with 555g of potassium sulfide?
Answers
226.11 g of potassium nitride will be produced if you start with 555 g of potassium sulfide.
How to solve the problem?The reaction between potassium sulfide and nitrogen gas to produce potassium nitride can be represented as:
2 K2S + N2 -> 2 K2N
The amount of potassium nitride produced depends on the stoichiometry of the reaction, meaning the ratio of the reactants and products. In this case, 2 moles of potassium sulfide react with 1 mole of nitrogen gas to produce 2 moles of potassium nitride.
To calculate the amount of potassium nitride produced, you need to first determine the number of moles of potassium sulfide that you have:
555 g K2S / 119.18 g/mol = 4.66 moles K2S
Since 2 moles of K2S react with 1 mole of N2, you have 4.66 moles / 2 = 2.33 moles of N2.
Since the reaction produces 2 moles of K2N for every 2 moles of K2S and 1 mole of N2, you would produce 2.33 moles of K2N.
The mass of 2.33 moles of K2N can be calculated as:
2.33 moles * 97.10 g/mol = 226.11 g
Therefore, 226.11 g of potassium nitride will be produced if you start with 555 g of potassium sulfide.
Learn more about potassium in brainly.com/question/13321031
#SPJ1
(05.04 hc) the temperature of a chemical reaction ranges between 40 degrees celsius and 180 degrees celsius. the temperature is at its lowest point when t = 0, and the reaction completes 1 cycle during a 12-hour period. what is a cosine function that models this reaction? (6 points) a f(t) = 70 cos 12t 110 b f(t) = 110 cos 12t 70 c f(t) = −70 cos pi over 6 t 110 d f(t) = −110 cos pi over 6 t 70
Answers
The cosine function of a chemical reaction that occurs at temperatures between 40 and 180 degrees Celsius is \(f (t) = -70\cos \left(\frac{\pi}{6}\right)t+110\). Option c is the correct expression.
A cosine function's amplitude is equal to half the vertical distance between its highest and lowest points. The distance between the tallest point and the center line is equal to this. This is of the form f (t) = A cos (bt)+k where A is the wave amplitude, t is the time period, and k is the vertical displacement.
In this problem, the temperature is measured by f(t), which ranges from 40 to 180 degrees Celsius. Then the temperature is not negative. The amplitude will be,
\(A=\frac{|180-40|}{2}=70\)
Given the time period is 12 hours.
\(\begin{aligned}\text{Period}&= \frac{2\pi}{b}\\12&= \frac{2\pi}{b}\\b&= \frac{2\pi}{12}\\b&=\frac{\pi}{6}\end{aligned}\)
When, t = 0, the wave must be at its lowest point. Then, f(t)=40. Find k using this.
\(\begin{aligned}f(t=0)&=40\\40&=-70\cos\left(\frac{\pi}{6}\times 0\right)+k\\40&=-70+k\\k&=110\end{aligned}\)
The required cosine function will be of the form, \(f (t) = -70\cos \left(\frac{\pi}{6}\right)t+110\)
To know more about cosine function:
https://brainly.com/question/4599903
#SPJ4
Use the mass and volume data to calculate the density of an unknown metal to the nearest hundredth.
Mass of unknown metal = 222.50 g
Volume of unknown metal = 25.00
What is the density of the unknown metal?
0.11
0.89
8.90
5,562.50
Answers
Answer:
8.90
Explanation:
Density = mass ÷ volume
D = 222.50 g ÷ 25.00
= 8.9
The density of the unknown metal is 8.90.
Hope that helps.
Answer:
C: 8.90
Explanation: