An electron and a proton are each accelerated from rest through a potential difference of 100 V. Afterward, which particle has the larger de Broglie wavelength? Afterward, which particle has the larger de Broglie wavelength? A. electron b. proton c. The electron and the proton have the same de Broglie wavelength.
Answers
An electron and a proton are each accelerated from rest through a potential difference of 100 V. Afterward, Electron particle has the larger de-Broglie wavelength. Hence, the option (a) is correct i.e. electron.
Matter waves are essential to the understanding of quantum mechanics because they provide as an example of wave-particle duality. Every substance has a wave-like behavior. Like a light beam or a water wave, an electron beam can also be diffracted. However, the wavelength is typically too small to truly have an impact on daily operations. The notion that matter acts like a wave was first proposed by French scientist Louis de Broglie in 1924. The de-Broglie wavelength hypothesis is another name for this concept. De Broglie waves are known as matter waves. Other elementary particles have supported the de Broglie theory's accuracy. In George Paget Thomson's cathode ray diffraction experiment and the Davisson-Germer experiment for electrons, the presence of matter waves was first empirically proven. It has also been shown that neutral atoms and even molecules exhibit wavelike behavior.
To know more about de-Broglie wavelength please refer: https://brainly.com/question/23017698
#SPJ4
Related Questions
What compound has 4 hydrogen atoms and one carbon
Answers
Carbon atoms may thus form bonds to as many as four other atoms. For example, in methane (CH 4start subscript, 4, end subscript), carbon forms covalent bonds with four hydrogen atoms
__________________________________________________________
what is the complex equation for copper sulfate and sodium hydroxide reaction?
Answers
CuSO4 + 2NaOH → Cu(OH)2 + Na2SO4
There you go, happy now? But I have a feeling that you don't actually understand what this equation means or how the reaction works. So, before you start pretending to be a chemist again, maybe actually learn some basic chemistry first.
Cuso4 + NaoH -》cu(oH)2 +Na2So4
Cuso4 + 2NaoH -》cu(oH)2 +Na2So4
Explanation:
this is balanced equation
What is the percentage by mass of chromium in sodium dichromate (VI) Na2
Cr2
O7
?
Answers
Answer:
39.7%
Explanation:
To obtain the percentage composition of chromium (Cr) in Na₂Cr₂O₇, do the following:
Molar mass of Na₂Cr₂O₇ = (23×2) + (52×2) + (16×7)
= 46 + 104 + 112
= 262 g/mol
Mass of Cr in Na₂Cr₂O₇ = 2 × 52 = 104 g
Percentage composition of Cr = mass of Cr / Molar mass of Na₂Cr₂O₇ × 100
= 104 / 262 × 100
= 39.7%
Thus, the percentage composition of chromium (Cr) in Na₂Cr₂O₇ is 39.7%
O Macmillan Learning
What is the IUPAC name for the compound shown?
IUPAC name:
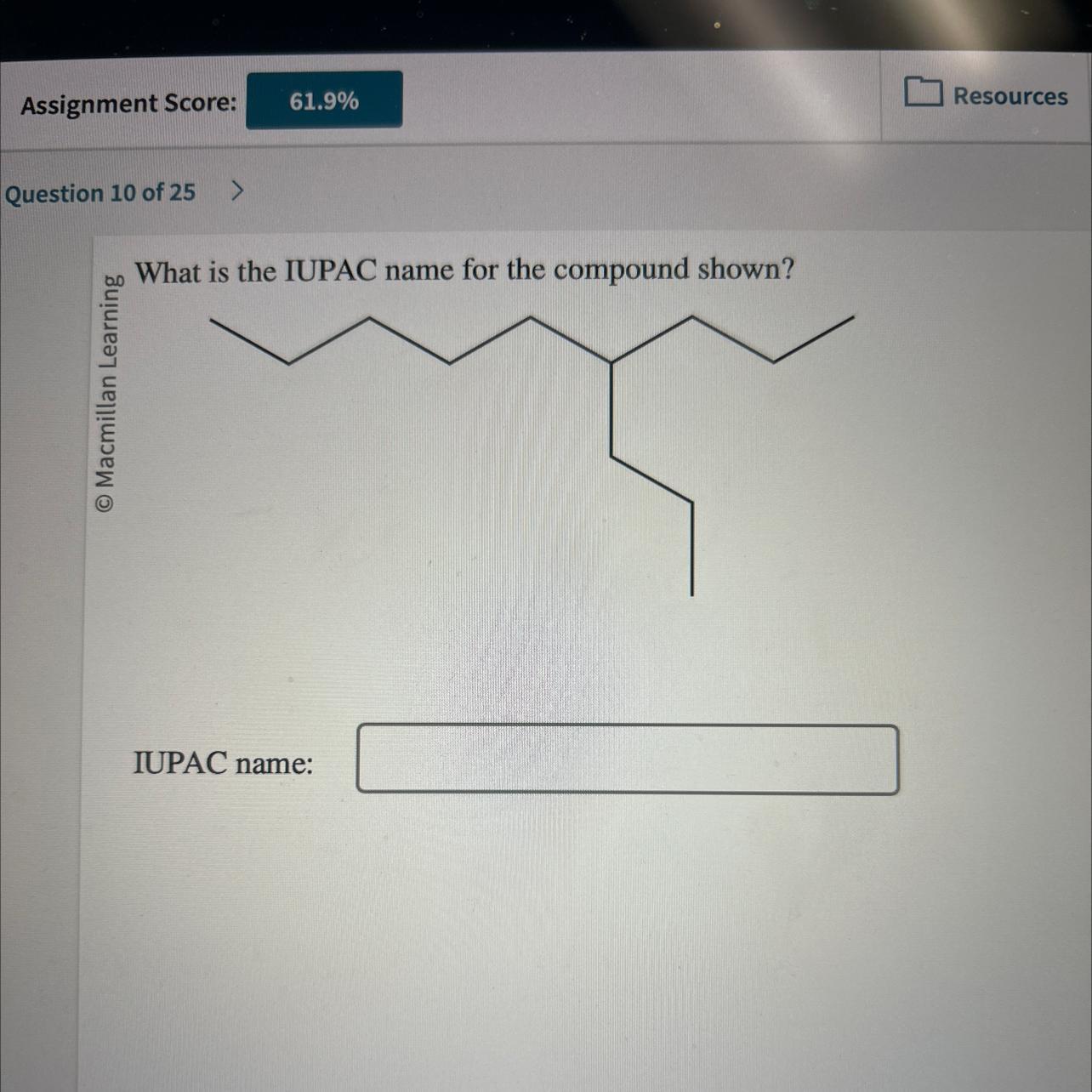
Answers
The compound shown has the IUPAC name O Macmillan Learning. -3-ethyl-2,2-dimethyl-hexane Incorrect.
Why was 1 6 dimethylhexane wrong?Explanation and response: Because it implies that there's methyl groups on carbons number one and sixth of the parent carbon chain, the name — appears-dimethylhexane is incorrect. Because the name "hexane" implies that a parent atom chain only has six molecules long, the methyl groups are located at the ends of every molecule.
Is hexane considered an organic chemical?Hexane, commonly referred to as sextane, is an organic compound that belongs to the alkane class. They are acyclic branched as well unbranched hydrocarbons with the standard structure CnH2n+2, and thus entirely composed of hydrogen and saturated oxygen atoms. Hexane is a colorless, clear liquid.
To know more about dimethylhexane visit :
https://brainly.com/question/30438544
#SPJ1
What chemicals need to make liquid bakelite ?
Answers
Reaction 1: Solid sodium hydroxide dissolves in water to form an aqueous solution of ions. ????????????H(????) → ????????+(????????) + ????H −(????????) + x1????????
Reaction 2: Solid sodium hydroxide reacts with an aqueous solution of hydrogen chloride to form water and an aqueous solution of sodium chloride. ????????????H(????) + H +(????????) + ????????−(????????) → H2????(????) + ????????+(????????) + ????????−(????????) + x2????????
Reaction 3: An aqueous solution of sodium hydroxide reacts with an aqueous solution of hydrogen chloride to form water and an aqueous solution of sodium chloride. ????????+(????????) + ????H −(????????) + H +(????????) + ????????−(????????) → H2????(????) + ????????+(????????) + ????????−(????????) + x3????J
Procedure Reaction
1 a. In the glassware menu, take out a 50 mL graduated cylinder and a foam cup. From the tools menu, take out the scale. From the solutions stockroom, move the distilled water and solid NaOH onto the workbench.
b. Transfer 50.0 mL of water to the foam cup. To do this, drag the carboy of water onto the graduated cylinder. (Before you release the mouse button, the cursor will show a "plus sign" to indicate that it is the recipient). A transfer textbar will appear, enter "50.0" mL and click on pour. (You will notice that the graduated cylinder now reads 50.0 mL).
c. Weigh about 1 gram of solid sodium hydroxide pellets, NaOH(s), directly into the foam cup and record its mass to the nearest 0.01 gram. To do this, place the foam cup on the balance so it registers a mass, then click the "Tare" button. Drag the NaOH bottle onto the foam cup. (When you release the mouse, the bottle will be tipped to show that it is in the pour mode). Next, type "1.00" grams into the transfer bar and then click pour. Note that the balance now reads the mass of the transferred NaOH. You may now take the cup off of the scale.
d. Click on the graduated cylinder, record its temperature and then drag it onto the foam cup. (When you release the mouse, the graduated cylinder will be tipped to show that it is in pour mode.) Enter "50.0" mL in the transfer bar and then click pour. Record the highest temperature. e. Remove the foam cup and graduated cylinder from the workbench. (Right click on the item and select "remove.")
Reaction 2
a. Take the 0.5 M HCl from the strong acids cabinet and a fresh foam cup and a fresh 50 mL graduated cylinder from the glassware menu and place them on the workbench. The procedure for Reaction 2 is the same as for Reaction 1 except that 50.0 mL of 0.50 M hydrochloric acid solution is used in place of the water. After measuring 50.0 mL of the HCl solution into the graduated cylinder, proceed as before with steps b-e of the procedure for Reaction 1.
Reaction 3
a. Take out a 25 mL graduated cylinder, a fresh foam cup, the 1.0 M HCl and the 1.0 M NaOH. (If you are running out of room on the workbench, you may remove the previously used chemicals.) Use the graduated cylinder to measure and transfer 25.0 mL of 1.0 M HCl into the foam cup. Pour an equal volume of 1.0 M sodium hydroxide solution into a clean graduated cylinder.
b. Record the temperature of each solution to the nearest 0.1 oC. Pour the sodium hydroxide solution into the foam cup and record the highest temperature obtained during the reaction.
Data and Analysis
Reaction 1Reaction 2Reaction 3
Mass of solution* (g) 1.03g 1.03g
Initial temperature(°C) 25oC 25OC 25OC
Maximum temperature (°C) 30.3oC 37oC 31.7oC
Temperature change (∆T)
Heat energy q (kJ)
Moles of NaOH
Molar heat of reaction (-q/mol) also known as Enthalpy change,
DH (kJ/mol)
Answers
Source https://www.physicsforums.com/threads/calorimetry-help-chemistry.399653/
The conversion of more than one substance reactant into one or more distinct substances, products, and subsequent discussion can be characterized as follows:
Reaction Calculation:Calculating the Reaction 1:
\(NaOH\ (s) \rightarrow Na^+ \ (aq) + OH^- \ (aq) + X_1\ \ KJ ......................... (1)\)
\(NaOH\) mass = \(1\ g\)
\(H_2O\) mass = \(50 \ mL = 50\ g\)
water heat of \(s_p\) = \(4.186\ \frac{ J}{ g\ ^{\circ}C}\)
\(\Delta T\) = final temp - initial temp \(= 30.3 - 25 = 5.3^{\circ} \ C\\\)
Therefore
Calculating the releasing heat
= mass × sp heat × \(\Delta T\)
= 50 × 4.186 × 5.3 J
= 1109.3 J
Calculating the \(NaOH\) mass \(= 1\ g = \frac{1}{ 40}\ mole= 0.025 \ mole\)
Calculating the releasing heat per mole:
\(\to NaOH = \frac{1109.3}{ 0.025} = 44372\ J = 44.4\ KJ\)
Thus
\(\to X_1 = 44.4\ KJ\)
Calculating the Reaction 2:
\(NaOH \ (s) + H^+\ (aq) + Cl^- \ (aq) \rightarrow Na^+ \ (aq) + Cl^- \ (aq) + H_2O + X_2 \ KJ\\\)
Calculating the net ionic from the equation:
\(NaOH\ (s) + H^+\ (aq) \rightarrow Na^+ \ (aq) + H_2O \ (l) + X_2 \ KJ ................................... (2)\)
Calculating the \(NaOH\) mass:
\(= 1\ g = \frac{1 }{ 40} = 0.025\ mole\)
Calculating the \(HCl\) mass:
\(= 50\ mL = 50\ g\) [ density = 1 approx]
sp heat of the solution \(= 4.186 \frac{J}{g\ ^{\circ}C}\) [ assume the sp heat same as water]
\(\Delta T\) = final temp - initial temp \(= 36.97 - 25 = 11.97^{\circ} \ C\)
Calculating the releasing heat:
= mass × sp heat × \(\Delta T\)
= 50 × 4.186 × 11.97 J
= 2505.3 J
Calculating the releasing heat per mole in \(NaOH\):
\(= \frac{ 2505.3 }{ 0.025} = 100212\ J = 100.2 KJ\)
Thus
\(X_2 = 100.2 \ KJ\)
Calculating the Reaction 3:
\(Na^+ \ (aq) + OH^-\ (aq) + H^+ \ (aq) + Cl^- \ (aq) \rightarrow Na^+\ (aq) + Cl^-\ (aq) + H_2O + X_3\ KJ\)
Calculating the net ionic in the given equation
\(H^+ + OH\rightarrow H_2O\ (l) + X_3\ KJ .............................................................. (3)\)
Calculating the volume of \(NaOH\):
\(= 25 \ mL\ of\ 1.0\ M = 25 \times \frac{1 }{ 1000} \ mole = 0.025 \ mole\)
Calculating the volume of HCl:
\(= 25 \ mL\ of\ 1.0\ M = 25 \times \frac{1 }{ 1000} \ mole = 0.025 \ mole\)
Calculating the total volume
\(= 50 \ mL = 50\ g\) { density = 1]
Calculating the sp heat in the solution
\(= 4.186 \frac{J}{ g \ ^{\circ} C}\) [ assumed the sp heat is the same as water]
\(\Delta T\) = final temp - initial temp \(= 31.7- 25 = 6.7^{\circ}\ C\)
Calculating the releasing heat
= mass × sp heat × \(\Delta T\)
= 50 × 4.186 × 6.7 J
= 1402.3 J
Calculating the releasing heat per mole in \(NaOH\):
\(=\frac{1402.3 }{ 0.025} \ J\\\\= 56092\ J\\\\= 56,09\ KJ\)
Therefore
\(X_3 = 56.09 \ KJ\\\\X_1 = 44.4\ KJ\\\\X_2 = 100.2\ KJ\\\\X_3 = 56.09\ KJ\\\\X_2 - [ X_1+ X_3 ] = 100.2 - [44.4 + 56.09]\ = 100.2 - 100.49= -0.29\)
So, the difference is equal to zero.
\(\to X_2 = X_1 + X_3\)
This is due to the fact that if we add the reaction (1) and (3) we get the reaction (2)
Calculating the difference percentage:
\(= [\frac{0.29 }{100.2} ] \times 100 = 0.29\%\)
The number of joules released in reaction 1 would be 4 times what is released in the calculation if we used 4 g of \(NaOH\).
\(\to 4 \times 1109.3\ J = 4437.2 \ J\\\\\)
Calculating the \(NaOH\) moles \(= \frac{4}{40} = 0.1\)
\(\to X_1 = \frac{4437.2}{ 0.1} = 44372 \ J = 44.4\ KJ\)
As a result, it has no bearing on the solution's molar heat.
Find out more about the reaction here:
brainly.com/question/17434463
Which metal ion is responsible for the red firework ?
Answers
Answer:
I'm pretty sure that's Strontium
Strontium ion is responsible for the red firework.
Strontium is the chemical element with the image Sr and atomic variety 38. An alkaline earth steel, strontium is a gentle silver-white yellowish steel detail this is fantastically chemically reactive.
What is a metal ion?A metal ion is a type of atomic compound that has an electrical charge. Such atoms willingly lose electrons to form positive ions called cations. Ions are essentially capped by delocalized electrons, which are responsible for processes such as conductivity.
What is a firework?Fireworks are a class of low-explosion fireworks equipment used for aesthetic and recreational purposes. They are most commonly used at fireworks festivals (also known as fireworks shows or fireworks) and combine numerous devices in outdoor settings. Such exhibitions are the focus of many cultural and religious celebrations.
Learn more about metal ion here https://brainly.com/question/11185179
#SPJ2
Literally struggling with this concept.(problem set included below)
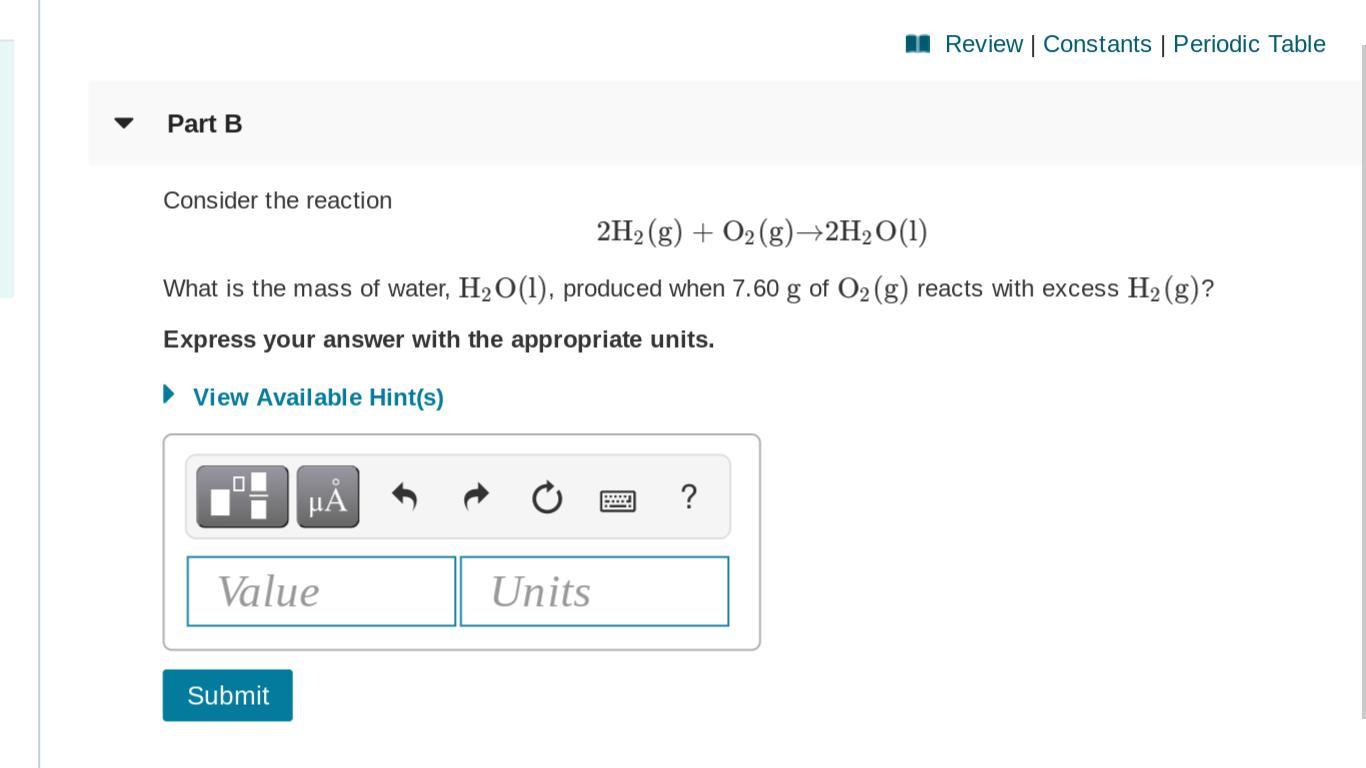
Answers
2. Do a mole to mole ratio of the limiting reactant to the product you want to find
3. Change the result from step 2 from moles to grams.
I know this is broad. If you have trouble with a specific step in this process I will go more in depth. Just let me know :)
In the electron dot diagram for sulfur monoxide, each atom of sulfur will have how many unshared pairs of electrons an each atom of oxygen will have how many unshared pairs of electrons.
Answers
Answer:
Both sulphur and oxygen have two unshared pairs of electrons
Explanation:
The compound sulphur monoxide has the formula S=O. It is quite analogous to the diatomic molecules of the group 16 elements.
We must remember that each group element has six electrons in their outermost shell. Two of these are lone pairs leaving only two electrons available for bond formation.
These two electrons are used to form the sigma and pi bonds in S=O, leaving two lone pairs on each of sulphur and oxygen atoms.
The volume of a sample of oxygen is 580.3 mL when
the pressure is 0.376 atm and the temperature is 43 °C.
At what temperature is the volume 907.1 L and the
pressure 0.415 atm?
a. 545
O b. 74.2
O c. -89.9
O d. 272
Answers
Answer:
545
Explanation:
If we let the unknown temperature in Kelvin be x,
\( \frac{0.376 \times 580.3}{43+273} = \frac{0.415 \times 907.1}{x} \\ \\ x = 545\)

write the structural formula for 2-bromo-3-chloro-4,4-dimethylpentanal
Answers
Answer:
Br-CH2-CH(CH3)2-C(Cl)H-CH(CH3)2-CHO
Explanation:
The molecule has a total of 14 carbon atoms, 13 hydrogen atoms, and 1 bromine atom. The carbon atoms are arranged in a chain with a methyl group attached to the second carbon atom, a chlorine atom attached to the third carbon atom, and two methyl groups attached to the fourth carbon atom. The fifth carbon atom has a carbonyl group attached to it.
The molecule is an aldehyde, which means that it has a carbonyl group (C=O) at the end of the chain. The carbonyl group is polar, and the oxygen atom has a partial negative charge. The hydrogen atom has a partial positive charge. This polarity makes the aldehyde group susceptible to nucleophilic attack.
The bromine and chlorine atoms are both electrophilic, which means that they have a partial positive charge. This makes them susceptible to nucleophilic attack.
The methyl groups are non-polar and do not have any significant reactivity.
The molecule is a chiral molecule, which means that it has a mirror image that is not superimposable on itself. This is because the carbon atom with the carbonyl group is attached to four different groups.
The molecule is a liquid at room temperature and has a strong odor. It is used in a variety of products, including perfumes, flavorings, and plastics.
the average shot piece is a cylinder with a diameter of 7.5 mm, a height of 2.0 mm, and a mass of .40 g. The average zinc granule is a sphere with a diameter of .84 mm. Using these formulas calculate the total surface area for .40 g of each form of zinc.
Surface are of a cylinder= 2(pi)r2 +2(pi)rh Volume of a sphere = 4/3(pi)r3
Surface area of a sphere= 4(pi)r2 Volume of a cylinder= (pi)r2h
Density of zinc= 7.141g/ml
Answers
Answer:
The surface area of a shot piece of zinc would be 4.01 cm2, while the surface area of a zinc granule would be 0.30 cm2. To calculate the surface area of a cylinder, you would use the formula 2(pi)r2 + 2(pi)rh, where r is the radius of the cylinder and h is its height. For a shot piece of zinc, with a diameter of 7.5 mm and a height of 2.0 mm, the radius is 3.75 mm and the surface area is 4.01 cm2. For a zinc granule, with a diameter of .84 mm, the radius is .42 mm and the surface area is 0.30 cm2.
How many known elements have atoms, in their ground-state, with valence electrons whose quantum numbers are n = 5 and l = 1?a. 36..
b. 37.
c. 6.
d. 8.
Answers
Answer:
c. 6.
Explanation:
Looking at the description given in the question, the elements involved must belong to the p- block of the periodic table and must be in period 5. They also must possess valence electrons in the 5p- orbital.
Now if we look at the p- block of period 5, the following elements satisfy these requirements; Sr, In, Sn, Sb, Te and I.
Hence there are six of such elements.
The chemical elements that have atoms, in their ground-state, with valence electrons whose quantum numbers are n = 5 and l = 1 are: C. 6.
What is a valence electron?A valence electron can be defined as the number of electrons that is present in the outermost shell of an atom.
In Chemistry, valence electrons are primarily used to determine whether an atom or group of chemical elements that are found on the periodic table can bond with others.
Based on electronic configuration, quantum numbers of n = 5 and l = 1 simply represents a 6p orbital and there a total of six (6) chemical elements with this orbital, and these include;
ThalliumLeadRadonAstatinePoloniumBismuthRead more on valence electrons here: https://brainly.com/question/16554158
Which amphibian organ has a high blood supply and many folds to increase surface area?
a. heart
b. stomach
c. lungs
d. brain
Answers
Answer:
lungs
Explanation:
What are the products of photosynthesis
Answers
Answer:Glucose, Oxygen and Water
Explanation:
Answer:
Hello i have don this... : )
Explanation:
It is oxygen and glucose
Have a good day im 100% sure this is... i forgot but it is before or after the asexual and sexual lessons
I added a meme for no reason you will only get it if you have read heroes of Olympus

Identify the Lewis acid and the Lewis base in each the following reactions. (Omit states of matter.) a. B(OH)2(aq) + H2O(l) + B(OH)4 - (aq) + H+ (aq) Acid: Base: b. H2O(1) + CN- (aq) + HCN(aq) + OH- (aq) Acid: Base: C. HgI,(s) +21+ (aq) → Hg1,2(aq) Acid: Base:
Answers
Base: Water, b. \(HCN\) Acid Base \(OH-\) , c. Base: I- Acid:\(HgI2\) . Chemical substances known as acids have the ability to donate a proton \((H+)\) to a base or another molecule.
Chemical substances known as acids have the ability to donate a proton \((H+)\) to a base or another molecule. They have a sour flavour, have the power to dissolve metals, and can make litmus paper turn red. On the pH scale, where 7 is neutral and lower numbers indicate higher acidity, acids have a pH below 7. Hydrochloric acid, sulfuric acid, and acetic acid are a few typical examples of acids. Acids are essential for many chemical processes, such as digestion, the creation of energy, and the synthesis of numerous significant chemicals. Also, they are employed in a number of sectors, such as industry, food production, and agriculture.
Learn more about Acid Base here:
https://brainly.com/question/15717190
#SPJ4
Understanding resonance is a key concept to understanding the different ways a molecule can exist, simultaneously. How many resonance structures does the following molecule have: NO2
Answers
1) We have to write the Lewis structure of the molecule.




23
A solid is insoluble in water but dissolves in aqueous acid and in aqueous alkali without
evolution of a gas in either case. What could the solid be?
A
B.
с
D
copper(II) carbonate
copper(II) hydroxide
zinc carbonate
zinc hydroxide
Answers
Answer:
copper(II) carbonate
Draw the structure of the peptide DTLH, showing the backbone and side-chain atoms, at its isoelectric point. Draw the molecule on the canvas by choosing buttons from the Tools (for bonds and charges), Atoms, and Templates toolbars. Draw peptide molecule as zwitter ion.
Answers
Answer:
Explanation:
A peptide is any class of organic compounds composed of different numbers of amino acids in which the amine of one is reacted with the carboxylic acid of the next to form an amine bond. The peptide of DTLH is composed of the following amino acids:
Asp-Thr-Leu-His
Their structures are first drawn out in the image attached below. This is followed by the isoelectric structure of the peptide DTLH

A small coffee cup calorimeter contains 28.0 g of H2O at 19.73 oC. A 2.05 g sample of a metal alloy is heated to 98.88 oC and then placed in the water. The contents of the calorimeter come to a temperature of 21.23 oC. What is the specific heat of lead
Answers
Answer:
1.104 J/g°C
Explanation:
Using Q = m × c × ∆T
Where;
m = mass of substance (g)
c = specific hear capacity (J/g°C)
∆T = change in temperature (°C)
For a colorimeter,
Q(water) = - Q(metal)
m. c. ∆T (water) = - m. c. ∆T (metal)
According to the information provided;
For water:
m = 28.0g
c = 4.184 J/g°C
∆T = (21.23 - 19.73°C)
For the metal:
m = 2.05g
c = ?
∆T = (21.23 - 98.88°C)
m. c. ∆T (water) = - m. c. ∆T (metal)
[28 × 4.184 × (21.23 - 19.73°C)] = -[2.05 × c × (21.23 - 98.88°C)]
[117.152 × 1.5] = -[2.05 × c × (-77.65)]
175.728 = -[-159.1825c]
175.728 = 159.1825c
c = 175.728 ÷ 159.1825
c = 1.104
c = 1.104 J/g°C
The following properties are either physical or chemical. Which one is different from the rest based on those two categories? a) Boiling point,b) flammability,c) Magnetism,d) thermal conductivity
Answers
Answer:
Not D.
Explanation:
I saw the answer somewhere else on brainly and i took the test and it was wrong
Two reactants combine to form a product in the reaction A + BC. The rate of the
reaction depends on the concentrations of both reactants squared (rate = K[A]²[B]²).
What's the total reaction order of this reaction?
OA) 3
OB) 4
OC) 2
OD) 1
Answers
To calculate the total reaction order, we add up the individual orders: 2 + 2 = 4.
Therefore, the total reaction order of the given reaction is 4. Option (OB) is the correct answer.
Given: S03(g) + H20(1) -> H2SO4(1); AH° = -130. kJ
determine AH° for the following thermochemical equation.
5H2S04(1) -> 5S03(8) + 5H20(1)
Answers
Considering the definition of enthalpy of reaction, the enthalpy change for the reaction is 650 kJ.
Enthalpy of reactionThe enthalpy of a chemical reaction as the heat absorbed or released in a chemical reaction when it occurs at constant pressure. That is, the heat of reaction is the energy that is released or absorbed when chemicals are transformed into a chemical reaction.
Enthalpy in this caseIn this case you want to calculate the enthalpy change of:
5 H₂SO₄ → 5 SO₃ + 5 H₂O
You know the following reaction, with his corresponding enthalpie:
SO₃ + H₂O → H₂SO₄ ΔH = –130 kJ
To obtain the enthalpy of the desired chemical reaction you need 5 moles of H₂SO₄ on reactant side. The given equation has 1 mole of H₂SO₄ on the product side, soit is necessary to locate it on the reactant side (invert it) and multiply it by 5.
When an equation is inverted, the sign of delta H also changes. And since enthalpy is an extensive property, that is, it depends on the amount of matter present, since the equation is multiply by 2, the variation of enthalpy also.
In summary, you know that the enthalpy change is 650 kJ.
Learn more about enthalpy of a chemical reaction:
brainly.com/question/5976752
brainly.com/question/13707449
#SPJ1
Is a Animal and Plant cell living
Answers
A balloon has a volume of 7.00 liters at a pressure of 740 mm Hg. If the temperature remains constant, at what pressure will the volume decrease to 2.00 liters?
a. 749 mm Hg
b. 52.9 mm Hg
c. 211 mm Hg
d. 2590 mm Hg
Answers
Answer:
D
Explanation:
P1 x V1 = P2 x V2
7.00(740) = x(2.00)
2590 mm Hg
took me a while because i dont know this, hope its right good luck
Balance the entire chemical
reaction using an atom inventory.
What is the correct whole
number coefficient for propane,
C3H8?
[?]C3H8+ [ 0₂
]CO2+[ ]H2O
Answers
The balanced chemical equation for the combustion of propane with oxygen is: C₃H₈ + 5O₂ → 3CO₂ + 4H₂O
To balance the equation, first balance the carbon atoms on both sides of the equation. There are three carbon atoms in the propane molecule and three in the carbon dioxide molecule, so balance the carbon atoms by putting a coefficient of 3 in front of the CO₂ molecule.
C3H8 + 5O2 → 3CO₂
Next, balance the hydrogen atoms. There are eight hydrogen atoms in the propane molecule and four in the water molecule, so balance the hydrogen atoms by putting a coefficient of 4 in front of the H₂O molecule.
C₃H₈ + 5O₂ → 3CO₂ + 4H₂O
Finally, balance the oxygen atoms. There are five oxygen atoms on the left side and 10 on the right side, so balance the oxygen atoms by putting a coefficient of 5 in front of the O₂ molecule.
Therefore, the correct whole number coefficient for propane, C3H8, is 1.
To learn more about the combustion, follow the link:
https://brainly.com/question/15117038
#SPJ1
7.0 x 10 -3 mol of I2 in 100.00ml of solution
Answers
Given:
- Moles of I2: 7.0 x 10^(-3) mol
- Volume of solution: 100.00 mL (which is equal to 0.1000 L)
Molarity (M) = Moles of solute / Volume of solution in liters
Molarity = (7.0 x 10^(-3) mol) / (0.1000 L)
Molarity = 0.070 M
Therefore, the concentration of the I2 solution is 0.070 M.
What is true about asteroids
Answers
Answer:
They are big rocks that fly through space and are made of most commonly chondrite. When they collide, they collide with such force that they create craters on places like the moon.
The activation energy, Ea, for a particular reaction is 37.8 kJ/mol. If the rate constant at 280 K is 0.178 M/s, then what is the value of the rate constant at 381 K? (R = 8.314 J/mol • K)
Answers
The rate constant that we have at 381 K will be 2.19 M/s.
What is the Arrhenius equation?The Arrhenius equation suggests that the rate of a reaction increases with temperature, because higher temperatures provide more kinetic energy to the reactant molecules, making them more likely to react.
By the use of the Arrhenius equation, we have that;
ln k2/k1 = -Ea/R(1/T2 - 1/T1)
ln k2/0.178 = -37.8 * 10^3/8.314 (1/381 - 1/280)
ln k2/0.178 = - 4647 * (2.62 - 3.57) * 10^-3
lnK2 = 0.786
k2 =e^0.786
k2 = 2.19 M/s
Learn more about Arrhenius equation:https://brainly.com/question/12907018
#SPJ1
Each sketch below shows three objects with an electric charge. In each case, decide whether there is a net force acting on the object outlined in green. If there
is a net force, decide whether it pushes the green-ring object to the left or right. Then select the appropriate button under the sketch.
For example, if there is a net force pushing the green-ring object in the first sketch to the left, select the left button under the first sketch. If there is no net
force on the green-ring object in the second sketch, select the middle button under the second sketch. And so on.

Answers
1. The net force on the -2 Charge is +2; acts to the right
2. The net force on the -1 charge is -1; acts to the left
3. There is no net force on the +3.
What is a net force?The net force is the vector sum of all the force acting at a point taking into consideration the magnitude and direction of each of the individual forces.
Electric charges experience electrical forces due to the electric field.
The two types of electric charges are positive and negative charges.
Like charges repel each other while opposite charges attract each other.
Considering the charges highlighted green;
The net force on the -2 Charge is: +3 - (+ 1) = +2; the net force on the +2 charge acts to the rightThe net force on the -1 charge is: -3 - (-2) = -1; the net force on the -1 charge acts to the leftThe net force on the +3 Charge is: +1 + (-1) = 0; there is no net force on the +3.In conclusion, the net force obtained by vector addition.
Learn more about net force at: https://brainly.com/question/14361879
#SPJ1