A Tesla roadster electric vehicle has a MPGe (Miles per Gallon equivalent) of 102 miles per gallon. A Ford F-150 has a rating of 21 MPG. The weight of the electric battery in the Tesla is 1062 lb. The volume of the gas tank in the F-150 is 83.2791 L. The density of gasoline is 748.90002 kg/m³. Which weighs more, the battery in the Tesla or the gasoline in the F-150.?
Answers
Answer:
The battery in the Tesla weighs more
Explanation:
Mass = Density × volume
Density of gasoline = 748.90002 Kg/m³
Volume of tank in m³ = 83.2791/1000 = 0.0832791 m³
Mass of gasoline in F-150 = 748.90002 × 0.0832791 = 62.3771 Kg
Converting mass in Kg to lb
Mass of gasoline (lb) = 62.3771 × 2.204623
Mass of gasoline (lb) = 137.51 lb
Weight of battery in Tesla = 1062 lb
Comparing the two shows that the battery of the Tesla weighs more.
Related Questions
pOH of the 0.001M NaOH solution is
Answers
The pOH of the 0.001 M NaOH solution is approximately 3.
To determine the pOH of a solution, we need to know the concentration of hydroxide ions (OH-) in the solution.
In the case of a 0.001 M NaOH solution, we can assume that all of the NaOH dissociates completely in water to form Na+ and OH- ions. Therefore, the concentration of hydroxide ions in the solution is also 0.001 M.
The pOH is calculated using the equation:
pOH = -log[OH-]
Substituting the concentration of hydroxide ions, we have:
pOH = -log(0.001)
Using a calculator, we can evaluate the logarithm:
pOH ≈ 3
Therefore, the pOH of the 0.001 M NaOH solution is approximately 3.
Know more about hydroxide ions here:
https://brainly.com/question/28464162
#SPJ8
High-pressure liquid chromatography (HPLC) is a method used in chemistry and biochemistry to purify chemical substances. The pressures used in this procedure range from around 500 kilopascals (500,000 Pa) to about 60,000 kPa (60,000,000 Pa). It is often convenient to know the pressure in torr. If an HPLC procedure is running at a pressure of 5.05×10^8 Pa , what is its running pressure in torr?
Answers
Answer:
3787500 Torr
Explanation:
1 Pascal = 0.0075 Torr
So:
5.05x10^8 Pa --- x
1 Pa --- 0.0075 Torr
x = 5.05x10^8 × 0.0075
x = 3787500 Torr
how much of a 400 Gram sample of rubidium-87 would remain after 1 half life
Answers
Answer:
200 g.
Explanation:
What is given?
Initial quantity (N₀) = 400 g.
Half-life of rubidium-87 = 4.8 x 10¹⁰ years.
Step-by-step solution:
Let's see the formula to calculate the quantity remaining:
\(N(t)=N_0\cdot(\frac{1}{2})^{t\text{ /t}_{\frac{1}{2}}}.{}\)Where N₀ is the initial quantity, t is the time, and t (1/2) is the half-life of the substance, in this case, Rb-87. Based on this, our formula will be:
\(N(t)=400\cdot(\frac{1}{2})^{\text{t/\lparen4.8}\cdot10^{10})}\)If we want to find the amount of Rb-87 after 1 half-life, our t would be equal to 4.8 x 10¹⁰ years, so replacing this value in the formula we obtain:
\(\begin{gathered} N(4.8\cdot10^{10})=400\cdot(\frac{1}{2})^{4.8\cdot10^{10}\text{/4.8}\cdot10^{10}}, \\ N(4.8\cdot10^{10})=400\cdot(\frac{1}{2})^1, \\ N(4.8\cdot10^{10})=400\cdot(\frac{1}{2}), \\ N(4.8\cdot10^{10})=200\text{ g.} \end{gathered}\)The answer is that after 1 half-life of a 400 g sample of Rb-87, the remaining quantity is 200 g.
the work function of magnesium metal is 5 86/10J
a, calculate the minimum frequency of required to release elections from the metal.
b, calculate the kinetic energy of the ejected electronic light of frequency 2.00/10 s is used to irradiating the metal.
Answers
a) To calculate the minimum frequency of electromagnetic radiation required to release electrons from the metal, you can use the following formula:
f = W / h
where f is the minimum frequency of electromagnetic radiation required, W is the work function of the metal in joules, and h is the Planck constant in joules per second.
Plugging in the values for W and h, you get:
f = (5.86 x 10^-19 J) / (6.626 x 10^-34 J/s) = 8.9 x 10^14 Hz
This is the minimum frequency of electromagnetic radiation required to release electrons from the magnesium metal.
b) To calculate the kinetic energy of the ejected electronic light of frequency 2.00 x 10^14 Hz, you can use the following formula:
KE = hf - W
where KE is the kinetic energy of the ejected electron, h is the Planck constant in joules per second, f is the frequency of the electromagnetic radiation in hertz, and W is the work function of the metal in joules.
Plugging in the values for h, f, and W, you get:
KE = (6.626 x 10^-34 J/s) * (2.00 x 10^14 Hz) - (5.86 x 10^-19 J) = 1.32 x 10^-19 J - 5.86 x 10^-19 J = -4.54 x 10^-20 J
This is the kinetic energy of the ejected electron when light of frequency 2.00 x 10^14 Hz is used to irradiate the magnesium metal. Since the kinetic energy is negative, this means that the electron is not released from the metal when irradiated with this frequency. The frequency of the electromagnetic radiation needs to be higher than the minimum frequency required to release the electron in order for the electron to be ejected from the metal.
An ionic compound is created, and heat is released in an exothermic reaction. What is the best explanation for why that reaction was exothermic?
a. Anions carry extra heat that is released when they react
b. Cations carry extra heat that is released when they react
c. Energy was required to create the bond
d. Energy was released in creating the bond
Answers
Answer:
energy was release in creating the bond
Explanation:
According to the VSEPR theory, a molecule or ion of CO2 will have a _______ shape. A. flat linear B. flat trigonal C. bent D. pyramidal E. None of the Above
Answers
According to the VSEPR theory, a molecule or ion of CO2 will have a flat linear shape. Option A
In CO2, the carbon atom forms double bonds with each oxygen atom. The carbon-oxygen double bonds consist of two pairs of electrons, which are arranged linearly, leading to a linear molecular shape.
The VSEPR theory suggests that electron pairs in the valence shell of the central atom repel each other and try to position themselves as far apart as possible, resulting in the linear shape.
The VSEPR theory allows us to predict the molecular geometry based on the arrangement of bonding and non-bonding electron pairs around the central atom. In the case of CO2, there are no lone pairs of electrons on the carbon atom, and the molecule has a symmetrical arrangement, leading to a linear shape. Option A
For more such questions on VSEPR theory visit:
https://brainly.com/question/14225705
#SPJ8
Given the following equation: Mg + 2HCI → MgCl₂ + H₂
How many moles of H₂ can be produced by reacting 2 moles
of HCI?
Answers
Taking into account the reaction stoichiometry, 1 mole of H₂ can be produced by reacting 2 moles of HCI.
Reaction stoichiometryIn first place, the balanced reaction is:
Mg + 2 HCl → MgCl₂ + H₂
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
Mg: 1 moleHCl: 2 molesMgCl₂: 1 moleH₂: 1 moleMoles of H₂ producedBy reaction stoichiometry 2 moles of HCl form 1 mole of H₂.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
brainly.com/question/24653699
#SPJ1
Assume that 0.531 g of diborane is combusted in a calorimeter whose heat capacity (Ccalorimeter) is 7.854 kJ/°C at 23.93°C. What is the final temperature of the calorimeter?
Answers
The final temperature of the calorimeter, given that it has initial temperature of 23.93 °C is 19.19 °C
How do I determine the final temeperature?We'll begin by obtaining the heat of combustion of 0.531 g of diborane. This is shown below:
Mass of diborane = 0.531 gMolar mass of diborane = 27.66 g/molMole of diborane = 0.531 / 27.66 = 0.0192 mole1 mole of diborane combust at -1941 KJ
Therefore,
0.0192 mole of diborane will combust at = 0.0192 × -1941 = -37.2672 KJ
Finally, we shall determine the final temperature of the calorimeter. Details below:
Heat of combustion (H) = -37.2672 KJInitial temperature (T₁) = 23.93 °CHeat capacity ofcalorimeter (C) = 7.854 kJ/°CFinal temperature (T₂) = ?H = C(T₂ - T₁)
-37.2672 = 7.854 × (T₂ - 23.93)
Clear bracket
-37.2672 = 7.854T₂ - 187.94622
Collect like terms
-37.2672 + 187.94622 = 7.854T₂
150.67902 = 7.854T₂
Divide both sides by 7.854
T₂ = 150.67902 / 7.854
T₂ = 19.19 °C
Thus, the final temperature is 19.19 °C
Learn more about temperature:
https://brainly.com/question/14281142
#SPJ1
A 40.4 g sample of a protein contains 17.16 g of carbon, 3.17 g of hydrogen, 13.71 g of oxygen, and the rest being nitrogen. This 40.4-gram sample is known to be 0.07141 moles. Determine the molecular formula of this protein.
Answers
The empirical formula is the smallest comparison of atoms of compound forming elements.
A molecular formula is a formula that shows the number of atomic elements that make up a compound.
(empirical formula) n = molecular formula
The principle of determining empirical formula and molecular formula
Determine the mass ratio of the constituent elements of the compound. Determine the mole ratio by dividing the elemental mass with the relative atomic mass obtained by the empirical formula Determine molecular formulas by looking for values of nFind mol ratio for every component :
C (Ar=12 g/mol):\(\tt \dfrac{17.16}{12}=1.43\)
H(Ar=1 g/mol) :\(\tt \dfrac{3.17}{1}=3.17\)
O(Ar=16 g/mol) :\(\tt \dfrac{13.71}{16}=0.857\)
N (r=14 g/mol) :
Mass of Nitrogen :
40.4-(17.16+3.17+13.71)=6.36 g
N :\(\tt \dfrac{6.36}{14}=0.454\)
C : H : O : N = 1.43 : 3.17 : 0.857 : 0.454 = 3.15 : 7 : 1.89 : 1=3:7:2:1
Empirical formula : C₃H₇O₂N
Molecular mass of protein :
\(\tt \dfrac{40.4}{0.07141}=565.75\)
(C₃H₇O₂N)n=565.75
(12.3+1.7+2.16+14)n=565.75
(89)n=565.75
n=6.4≈6
so the molecular formula : C₁₈H₄₂O₁₂N₆
how many ions of aluminum oxide (Al²O³) are there in 200 g of Al²O³??
Answers
= 6.022 × 1020
Explanation;
Mole of aluminium oxide (Al2O3) is
⇒ 2 x 27 + 3 x 16
Mole of aluminium oxide = 102 g
i.e., 102 g of Al2O3= 6.022 x 1023 molecules of Al2O3
Then, 0.051 g of Al2O3 contains = 6.022 x 1023 / (102 x 0.051 molecules)
= 3.011 x 1020 molecules of Al2O3
The number of aluminium ions (Al3+) present in one molecule of aluminium oxide is 2.
Therefore, the number of aluminium ions (Al3+) present in 3.11 × 1020 molecules (0.051g) of aluminium oxide (Al2O3)
= 2 × 3.011 × 1020
= 6.022 × 1020
hope it helps_
Can H2 be broken down? (Not H)
Answers
Hello, this is Bing. I can help you with your question. Based on the information I found on the web, **H2** can be broken down into its two atoms of hydrogen (H) by supplying enough energy to overcome the bond that holds them together⁴. This process is called **dissociation** and requires an energy equal to or greater than the **dissociation energy** of H2, which is about 436 kJ/mol⁴.
One way to break down H2 is by using **electricity** to split water (H2O) into hydrogen (H2) and oxygen (O2) through a process called **electrolysis**¹. In this process, water is decomposed into its elements by passing an electric current through it. The electric current is provided by a battery or another source of electricity and the water needs to have an **electrolyte**, such as salt or acid, added to it to make it conductive¹. Two electrodes, usually made of metal or other conductive material, are inserted into the water and connected to the battery. The electrode connected to the positive terminal of the battery is called the **anode** and the one connected to the negative terminal is called the **cathode**¹. When the electric current flows through the water, hydrogen gas bubbles form at the cathode and oxygen gas bubbles form at the anode¹. The overall chemical reaction for electrolysis of water is:
2 H2O → 2 H2 + O2
Another way to break down H2 is by using **heat** to cause a reaction between hydrogen and oxygen that produces water and releases a large amount of energy. This reaction is called **combustion** or **oxidation** and can be ignited by a spark or a flame³. The reaction is very fast and explosive and can be dangerous if not controlled. The overall chemical reaction for combustion of hydrogen is:
2 H2 + O2 → 2 H2O
I hope this helps you understand how H2 can be broken down and what methods are used to do so.
2. Write the Ksp of Ca(OH)2 in terms of its
(a) molar solubility s
(b) [OH-]
(c) [Ca²+].
Answers
The expression of the Ksp is Ksp = [Ca²+] [2OH-]^2
What is the Ksp?In the balanced chemical equation for the solute's dissolution, Ksp is defined as the product of the ion concentrations in a saturated solution, each concentration being raised to the power of its stoichiometric coefficient.
Ksp is temperature-dependent and varies with different compounds. It is used to predict the maximum amount of a compound that can dissolve in a given solvent under specific conditions.
We know that we can be able to use the expression that has been given in the problem to arrive at the fact that;
Ksp = [Ca²+] [2OH-]^2
Learn more about Ksp:https://brainly.com/question/31384943
#SPJ1
The strength of mild steel is found to be 232.9 MPa when the grain size is 17.43 pm. and 874.2 MPa when the grain size is 0.80 um. 1. Determine the constants in the Hall-Petch equation. (Express your answer to three significant figures.) K =_____MPa um 0o =______MPa 2. Determine the strength of the mild steel when the grain size is reduced to 0.160 pm. (Express your answer to three significant figures.) 0 = MPa
Answers
When the grain size is reduced to 0.160 pm, the strength of mild steel is 1851 MPa.There are a number of variables that can affect mild steel's strength, including the grain size, which is controllable.
We may use the above information to determine the constants in the Hall-Petch equation: The strength of mild steel at grain size d1 = 17.43 pm is 1 = 232.9 MPa. Mild steel has a strength of 2 = 874.2 MPa when the grain size is d2 = 0.80 um (or 800 nm). This is the formula for the Hall-Petch equation: = o + Kd(-1/2). We can use the following equation to determine K: K = (σ2 - σ1)(d2^(1/2) - d1^(1/2))^(-1) (-1) When the values are plugged in, we get the following result: K = (874.2 - 232.9)(800(1/2) - 17.43(1/2))(-1) = 276.3 MPa um(1/2) We can use the following equation to determine o: σo = σ1 - Kd1^(-1/2) Plugging in the numbers yields the following result: o = 232.9 - 276.3(17.43(-1/2)) = 62.28 MPa. The Hall-Petch equation's constants are K = 276.3 MPa um(1/2) and o = 62.28 MPa. Hence, the power of the yield strength of mild steel at 0.160 pm grain size is 1851 MPa.
Learn more about mild steel here:
https://brainly.com/question/30036139
#SPJ4
how many calories are in a snack with 175 kj of energy
Answers
Answer:
41826.05 Calories
Explanation:
1 J = 0.239006 Calories
175 KJ
= 175 x 1000 J
= 175000 J
175000 J to Calories
= 175000 x 0.239006
= 41826.05 Calories
Answer: 41.8
Explanation:
Acellus verified ✅
A chemical adds 1.10 L of a 0.384mol/L barium chloride
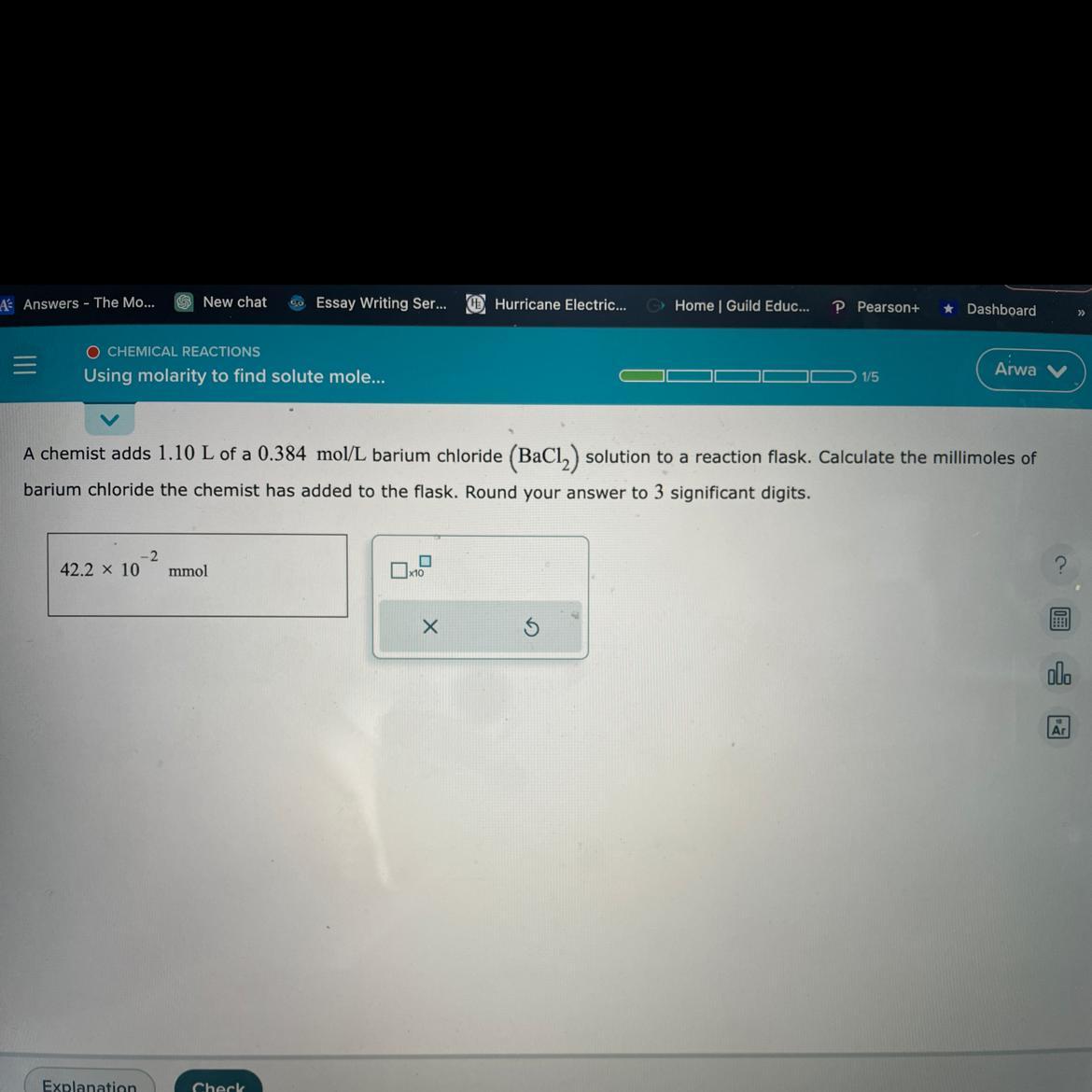
Answers
The number of millimoles of barium chloride added to the flask to 3 significant figures would be 422 mmol.
Number of moles calculationTo calculate the millimoles of barium chloride added to the flask, we need to use the following formula:
moles = concentration x volume
where concentration is in units of mol/L, and volume is in units of L. We are given that the volume is 1.10 L and the concentration is 0.384 mol/L, so:
moles = 0.384 mol/L x 1.10 L
moles = 0.4224 mol
Now, to convert moles to millimoles, we multiply by 1000:
millimoles = 0.4224 mol x 1000
millimoles = 422.4 mmol
Therefore, the millimoles of barium chloride added to the flask is 422 mmol (rounded to 3 significant figures).
More on number of moles can be found here: https://brainly.com/question/12513822
#SPJ1
Vinegar is sold at the grocery store with a concentration of 5.0 % acetic acid. How many grams of acetic acid are in 28 g of Vinegar?
Answers
White vinegar typically consists of 93%–96% water and 4–7% acetic acid. It can be used to cooking, bake, cleaning, and get rid of weeds. It can also help you lose weight and lower your blood sugar and cholesterol. Consumption is safe in moderation, but excessive consumption or when combined with certain medications could be harmful.
Apple cider vinegar is widely used in cooking and as a salad dressing because it contains acetic acid and nutrients like vitamins C and B vitamins. But at the same time, it's been utilized customarily as medication. It helps in losing weight.
Learn more about vinegar, here:
https://brainly.com/question/23700611
#SPJ1
What is a solute?
substance in which another substance dissolves and mixes evenly A
a solution unable to be separated by any means B
a mixture that can easily be separated with simple tools C
the substance that dissolves into another substance D
Answers
\(▪▪▪▪▪▪▪▪▪▪▪▪▪ {\huge\mathfrak{Answer}}▪▪▪▪▪▪▪▪▪▪▪▪▪▪\)
The Correct choice is ~ D
Solute is the substance that dissolves into another substance
Aqueous solution of two salts Na2CO3 and Na2SO4 is given. How to prove the simultaneous occurrence of both carbonate and sulphate anions?
Answers
Answer:
See the answer below.
Explanation:
If an aqueous solution of two salts contains both Na2CO3 and Na2SO4, the following steps will prove the occurrence of both carbonate and sulphate ions:
1. Add a dilute acid (such as HCl) to the solution. The presence of carbonate ion will result in the release of carbon dioxide gas which will be shown by formation of effervescent bubbles. The gas can be proven to be carbon dioxide by channeling it into a lime water which usually turns milky with the presence of the gas.
\(CO^{2-}_3(aq) + 2H^+(aq) ==> H_2O(l) + CO_2(g)\)
2. Add barium chloride to an acidified portion of the aqueous solution. The presence of sulphate ion will be indicated by the formation of white barium sulphate precipitate. Initial acidification is done to disperse off any carbonate ion that might be present in the solution and give a false-positive white precipitate result.
\(Ba^{2+}(aq) + SO^{2-}_4(aq) --> BaSO_4(s)\)
Which type of chemical reaction is condensation?
Match the words in the left column to the appropriate blanks in the sentences on the right.

Answers
Condensation is a chemical reaction of synthesis. It represents two organic molecules producing one organic molecule and a water molecule.
What are condensation reactions?Condensation reactions are reactions in which two or more substances combine together chemically to produce a larger molecule with the elimination of a small molecule such as water, ammonia, or HCl.
Condensation reactions are important reactions in the formation of polymers.
Polymers are large organic molecules that are composed of smaller repeating units known as monomers.
An example of a condensation reaction is the reaction in which two amino acids are linked together by a peptide bond to form a dipeptide. A water molecule is also eliminated.
Learn more about condensation reaction at: https://brainly.com/question/4043946
#SPJ1
Writing and balancing complex half-reactions in acidic solution.
Answers
Writing and balancing complex half-reactions in acidic solutions involves a systematic approach. Here are the general steps to follow:
Identify the oxidation and reduction half-reactions: Determine which species is being oxidized (losing electrons) and which is being reduced (gaining electrons).
Balance the atoms: Begin by balancing all atoms except hydrogen and oxygen in each half-reaction.
Balance the charges: Add electrons (e-) to one side of each half-reaction to balance the charges.
Balance the oxygen atoms: Add water (H2O) to the side of the equation that lacks oxygen atoms.
Balance the hydrogen atoms: Add hydrogen ions (H+) to the side of the equation that lacks hydrogen atoms. Keep in mind that the solution is acidic.
Balance the charges: Adjust the number of electrons (e-) on each side of the equation to ensure that the charges are balanced.
Multiply the half-reactions: Multiply each half-reaction by an appropriate factor so that the number of electrons gained and lost are equal.
Combine the half-reactions: Add the two balanced half-reactions together and cancel out common terms on both sides of the equation.
By following these steps, you can effectively write and balance complex half-reactions in acidic solutions, ensuring that the overall reaction is balanced in terms of both atoms and charges.
Learn more about Writing and balancing here:
https://brainly.com/question/27192586
#SPJ11
HCl(50ml) + NaOH(50ml) --> NaCl+H2O
Calculate the value of heat released (Q = mcT) and the Delta H
Time | Temperature
0s 22C
10s 27C
20s 29C
30s 30C
Answers
Answer:
To calculate the heat released in this reaction, we need to use the formula:
Q = mcΔT
where Q is the heat released, m is the mass of the solution, c is the specific heat capacity of the solution, and ΔT is the change in temperature.
Assuming the density of the solution is 1 g/mL, the mass of the solution is 100 g (50 mL HCl + 50 mL NaOH). The specific heat capacity of the solution can be assumed to be the same as that of water, which is 4.18 J/g°C.
The change in temperature can be calculated as the final temperature minus the initial temperature:
ΔT = 30°C - 22°C = 8°C
Therefore, we have:
Q = (100 g) * (4.18 J/g°C) * (8°C) = 3344 J
The heat released in the reaction is 3344 J.
The value of ΔH for the reaction can be calculated using the formula:
ΔH = -Q/n
where Q is the heat released, and n is the number of moles of limiting reactant used in the reaction. In this case, the limiting reactant is NaOH, and we can calculate the number of moles of NaOH from its concentration and volume:
n(NaOH) = (0.1 L) * (1 mol/L) = 0.1 mol
Therefore, we have:
ΔH = -(3344 J) / (0.1 mol) = -33,440 J/mol
The value of ΔH for the reaction is -33,440 J/mol, which is negative because the reaction is exothermic (heat is released).
If you mixed three cups of orange juice together containing 1.5 L of .75M orange juice, 2.5 L of 2.5M orange juice and .25 L of 0.50M orange juice, what would the molarity be of the resulting solution?
Answers
3.75M
Explanations;Molarity is the ratio of the moles of solute to volume of solution. It is measured in moles/Litre
From the given question, the molarity of the cups of juice is given as;
molarity of first solution = 0.75M
molarity of second solution = 2.5M
molarity of third solution = 0.50M
Determine the molarity of the resulting solution
Molarity = 0.75 M + 2.5M + 0.50M
Molarity = 3.75M
Hence the molarity be of the resulting solution is 3.75M
What is the coefficient for oxygen in the balanced equation? C 5H 12 + ? O2 → ? CO2 + ? H2O. 2 4 5 6 8
Answers
Answer:
8
Explanation:
When you balance the entire equation, you should get:
C5H12 + 8O2 ---> 5CO2 + 6H2O
4
A chemist encounters an unknown metal. They drop the metal into a
graduated cylinder containing water, and find the volume change is
6.1 mL. If the metal weighs 8.1 g, what is the density of the metal in
g/mL?
Answers
The Density of metal is 1.32 g/mL.
density is the mass of a material substance per unit volume. d = M/V, where d is density, M is mass, and V is volume, is the formula for density. Grams per cubic centimetre are a typical unit of measurement for density.Volume (V) is defined as mass divided by density (M/d). By multiplying a body's mass by the acceleration of gravity, one can get the weight of the object, which is typically more important practically than its mass.Despite the fact that the SI unit of density is kg/m3, we instead use g/cm3 for solids, g/ml for liquids, and g/L for gases out of convenience.Because various substances have varying densities, they weigh differently for a given volume.Given,
volume change observed by chemist when metal is dropped into the cylinder filled with water is 6.1 mL.
weight of metal is 8.1 g
we know that,
Density = mass / Volume
density = 8.1 / 6.1
Density = 1.32 g/mL
Hence, density of metal of mass 8.1 g is 1.32 g/mL
Learn more about Density here:
https://brainly.com/question/1354972
#SPJ9
Chrysanthenone is an unsaturated ketone. If Chrysanthenone has M+ = 150 and contains 2 double bond(s) and 2 ring(s); what is its molecular formula? Enter the formula in the form CH first, then all other atoms in alphabetical order; do not use subscripts. The formula is case-sensitive.
Answers
Answer:
the Molecular formula will be; C10H14O
Explanation:
Given the data in the question;
Chrysanthenone is an unsaturated ketone,
it has M+ = 150 and contains 2 double bond(s) and 2 ring(s).
molecular formula = ?
we know that ketone contain 1 oxygen and mass of oxygen is 16
so mass of the C and H remaining will be;
⇒ 150 - 16 = 134
Now we determine the number of C atoms;
⇒ 134 / 13 = 10
hydrocarbon with 10 hydrogen atom have CnH2n+2 means
⇒ ( 10 × 2 ) +2 = 22 hydrogens
But then we have 3 unsaturation meaning 6 hydrogens less and also we have ring meaning 2 more hydrogens
⇒ 22 - 6 - 2 = 14
Hence the Molecular formula will be; C10H14O
A sample of 2.05 g of polystyrene plastic was dissolved in enough toluene to form 100 mL of solution. The osmotic pressure of this solution was found to be 1.21 kPa at 25°C. Calculate the molar mass of polystyrene.
Answers
The molar mass of the polystyrene is 41750 g/mol.
What is the molar mass?We know that the molar mass is the mass of one mole of the substance. We need to obtain the concentration of the solution in order to obtain the molar mass.
Number of moles = 2.05 /M moles
Volume of the solution = 100 mL or 0.1 L
Concentration = 2.05 /M/0.1 or 2.05/0.1 M
π = iCRT
π = Osmotic pressure
i = Van't Hoff factor
C = concentration
R = gas constant
T = temperature
0.012 = 1 * 2.05/0.1 M * 0.082 * 298
0.012 = 50.1/0.1 M
0.012 * 0.1 M = 50.1
M = 50.1/0.012 * 0.1
M = 41750 g/mol
Learn more about osmotic pressure:https://brainly.com/question/15501096
#SPJ1
Consider a 75.0-g sample of H2O(g) at 1258C. What phase or phases are present when 215 kJ of energy is removed from this sample
Answers
Answer:
Explanation:
The first process is to draw out the heating curve to have a look at the transition possible which we've shown in the file below.
Now;
To find the energy removed to convert 75g steam → ice then comparing it with 215 kJ.
So conversion of steam at 125 °C → 100 °C
\(\Delta H = (75 \ g) (2.0 \ J/g^0C ) (125 - 100)^0 \ C\)
\(\Delta H = 3750 \ J\)
\(\Delta H = 3.75 \ kJ\)
Since this is lesser than the energy given (215 kJ), we then have to find the energy removed in the next phase.
For condensation of steam
Find the energy removed to change the steam from 100° C to liquid at 100° C
\(\Delta H_2 = ( \dfrac{75 \ g}{18.02 \ g/mol}) (40.7 \ kJ/mol)\)
\(\Delta H_2 =1.690* 10^ 2 \ J\)
\(\Delta H_2 =169 \ kJ\)
The total energy that is now removed from the system is:
\(\Delta H_1+ \Delta H_2 = (169 + 3.75) \ kJ\)
\(\Delta H_1+ \Delta H_2 \simeq 173 \ kJ\) which is still lesser than 215 kJ
To the third step; which is the conversion of water at 100° C → water at 0° C
\(\Delta H_3 = ( 75 \ g) (4.2 \ J/g^0 C)(100-0)^0C\)
\(\Delta H_3 = 31500 \ J\)
\(\Delta H_3 = 31.5 \ kJ\)
Thus;
\(\Delta H_1+\Delta H_2+\Delta H_3 = (169 + 3.75 +31.5) \ kJ\)
\(\Delta H_1+\Delta H_2+\Delta H_3 =204 \ kJ\)
To the fourth step;
For freezing of water; we need to find the energy removed to change the water at 0° C → ice at 0° C
\(\Delta H_4 = (\dfrac{75 \ g}{18.02 \ g/mol})(6.02 \ kJ/mol)\)
\(\Delta H_4 = 25.1 \ kJ\)
∴
To change to the solid phase at 0° C; the total energy that is being removed from the steam is
\(\Delta H_1+\Delta H_2+\Delta H_3 +\Delta H_4 =(169 + 3.75 + 31.5 +25.1) \ kJ\)
\(\Delta H_1+\Delta H_2+\Delta H_3 +\Delta H_4 =229 \ kJ\)
Here, the calculated total energy is more than the given energy 215 kJ.
This implies that the water is not fully converted to ice when 215 kJ of energy removal occurs, Hence the two phases that exist are liquid and solid.

Which could be the missing reason in Step 3?
alternate interior angles are congruent
alternate exterior angles are congruent
vertical angles are congruent
corresponding angles are congruent
Answers
Answer:
a) alternate interior angles are congruent
Explanation:
on edgen
How many mL of 0.626 M NaOH solution are needed to obtain 0.332 mol of NAOH?
Answers
Any time we are working with solutions and its molar concentration we will use the equation:
\(C=\frac{n_{\text{solute}}}{V_{\text{solution}}}\)Where C is the concentration, n is the number of moles of the solute and V is the volume of solution.
We can identify such situations when we are working with molarity, molar concentration, solutions with concentration in "M" or "mol/L" units and other cases.
In this case, we can see that we are working with these three quantities:
- We want the volume in mL os a solution, V.
- We have a molar concentration, C, 0.626 M NaOH.
- We have a number of moles of solute we want to get, n, 0.332 mol of NaOH.
So, we have:
\(\begin{gathered} V_{\text{solution}}=\; \text{?} \\ C=0.626M=0.626mol/L \\ n_{\text{solute}}=0.332mol \end{gathered}\)So:
\(\begin{gathered} C=\frac{n_{\text{solute}}}{V_{\text{solution}}} \\ V_{\text{solution}}=\frac{n_{\text{solute}}}{C}=\frac{0.332mol}{0.626mol/L}=0.530351\ldots L=530.351\ldots mL\approx530mL \end{gathered}\)So, the volume we need is approximately 530 mL.
Is Water and kerosine a mixture
Answers
Answer:
No.Kerosene oil and water do not mix with each other and form two separate layers.
Answer:
No
Explanation:
They cannot be mixed together they will form upper and lower layer