A balloon occupies a volume of 44 L at 200K, what will the temperature of the balloon become if it is changed to 500K?
Answers
Answer:
110L
Explanation:
The following data were obtained from the question:
Initial volume (V1) = 44L
Initial temperature (T1) = 200K
Final temperature (T2) = 500K
Final volume (V2) =..?
The new volume of the balloon can be obtained by using Charles' law equation as shown below:
V1/T1 = V2/T2
44/200 = V2/500
Cross multiply
200 x V2 = 44 x 500
Divide both side by 200
V2 = 44 x 500/200
V2 = 110L
Therefore, the new volume of the balloon is 110L
Related Questions
Plz answer fast , (it’s a picture

Answers
Answer:
Explanation:
1.4193.61
2.1.69205e7
3.1.57611e14
4.46270.5
What does it mean when a solution is saturated?
O The container is full.
O No more solute can dissolve in solvent.
O The solute has turned into a solid.
O The solvent has turned into a solid.
Answers
Answer:
A solution in which the maximum amount of solvent has been dissolved. Any more solute added will sit as crystals on the bottom of the container.
Explanation:
Which actions are functions of the circulatory system? Select four options.
delivers nutrients to cells
brings carbon dioxide to cells for use
protects the body from pathogens
removes waste materials from cells
helps cuts and wounds to heal
just giving random points to people who answer this
Answers
Answer:
carries oxygen, nutrients, and hormones to cells, and removes waste products, like carbon dioxide.
Explanation:
Why might changes to an environment cause an organism’s population to decrease?
Answers
A lump of zinc is tossed into a beaker of 500L of 14M hydrochloric acid. this reaction produces Hydrogen Gas and zinc (II) chloride. If the hydrogen gas is combusted and produces 645L of water vapor at 400 kelvin and 1.75 atm, what is the mass of the zinc?
Answers
If the hydrogen gas is combusted and produces 645L of water vapor at 400 kelvin and 1.75 atm, 2796.96 g mass of the zinc is produced .
Using the ideal gas law equation:
PV = nRT
n = (PV) / (RT)
= (1.75 atm * 645 L) / (0.0821 atm·L/(mol·K) * 400 K)
= 42.71 moles
the balanced equation for the reaction between zinc and hydrochloric acid:
Zn + 2HCl -> \(ZnCl_{2}\) + \(H_{2}\)
1 mole of zinc produces 1 mole of hydrogen gas. Therefore, the moles of zinc are also 42.71.
The molar mass of zinc is 65.38 g/mol.
Mass of zinc = moles of zinc * molar mass of zinc
= 42.71 moles * 65.38 g/mol
= 2796.96 g
Therefore, the mass of the zinc is 2796.96 grams.
learn more about hydrogen gas :
https://brainly.com/question/30829657
Argon is a noble gas. How many electrons would you expect an atom of argon to have in its outer
shell?
Answers
fire extinguishers that can be used on any type of fire, a, b, or c generally contain a to extinguish the fire. a. water b. carbon dioxide c. nitrogen d. dry chemical powder
Answers
Answer:
b? im pretty sure
Explanation:
Write a balanced equation for the following nuclear decay reaction.
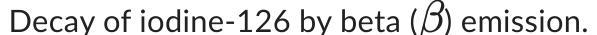
Answers
The balanced equation for the beta emission decay of iodine-126 is as follows:
Iodine-126 (126I) → Xenon-126 (126Xe) + Beta particle (β-)What is decay process in nuclear reaction?Decay process in nuclear reactions refers to the spontaneous transformation of an unstable nucleus into a more stable one, emitting one or more particles or radiation in the process.
The types of decay include
alpha decay, beta decay, and gamma decay.This process results in a decrease in the total number of protons and neutrons in the nucleus and a decrease in the total atomic number and mass number of the nucleus.
In the equation, the beta particle is an electron emitted from the nucleus during the decay process.
Learn more about beta emission at:
https://brainly.com/question/16409346
#SPJ1
what is the equilibrium constant for the following reaction? be sure your answer has the correct number of significant digits.
Answers
The equilibrium constant for the reaction 2SO2(g) + O2(g) → 2SO3(g) is Kc = 0.113.
This value is determined using the reaction quotient equation, which calculates the ratio of product concentrations to reactant concentrations at equilibrium.
The number of significant digits in the equilibrium constant is dependent on the number of significant digits in the concentrations of the reactants and products.
In this case, the concentration for each reactant and product is only known to two significant digits, so the equilibrium constant is also only known to two significant digits. As a result, the equilibrium constant for this reaction is Kc = 0.113.
Know more about significant digits here
https://brainly.com/question/1658998#
#SPJ11
Al agregar 150g de una sustancia X en un recipiente que sostiene que contiene agua hasta 50, el nivel del agua aumenta hasta 120ml. Calcula la densidad de la sustancia X
Answers
Answer:
2,14 g / ml
Explanation:
Sabemos que el volumen de una sustancia es igual al cambio de volumen del agua cuando el objeto en cuestión se sumerge en el agua.
Dado que el volumen original del agua = 50 ml
Volumen de agua después de sumergir el objeto = 120 ml
Masa del objeto = 150 g
Ahora,
Densidad = masa / volumen
Densidad = 150g / 120-50 ml
Densidad = 150/70 ml
Densidad = 2,14 g / ml
Argon has density of 1.78 g/L at STP. Which of the following gases have density at STP greater than that of argon?1. ${F_2}$2. ${H_2}$3. $N{H_3}$4. $C{O_2}$A. 1 onlyB. 2 onlyC. 4 onlyD. 1 and 3 only
Answers
Option (c) is correct. CO2 gas have density at STP greater than that of Argon. This is determined by ideal gas law, PV = n RT.
According to the Ideal gas law, the pressure times the volume of a gas divided by the number of moles and temperature of the gas is always equal to a constant number. The numerical value of the constant depends on which units the pressure volume and temperature are in
PV = n RT
at STP, P is constant and T is constant.
so, V = km / M
so, the density is greater with the molar mass. so, density of CO2 is greater than argon as it has the molar mass of 44.01 g/ mole and argon has 39.148 g/ mole. Density is defined as the mass per unit volume of a material substance. Density is commonly expressed in units of grams per cubic centimeter.
To learn more about Density please visit:
https://brainly.com/question/1354972
#SPJ4
I need help please this is so confusing

Answers
The genotype probability is :
PP = 25 %
Pp = 50 %
pp = 25 %
Phenotype probability:
Purple color) = 75 %
white color = 25 %
What is the genotype and phenotype probability in a cross of parents with the genotype PP and Pp?The genotype of an offspring is the sum total of all the genes inherited from the parents.
The phenotype is the physical expression of that genotype.
Given that purple color P is dominant over white color, p in flowers, the cross between two heterozygous purple-colored flower plant, Pp will produce the following genotype and phenotype probabilities:
Pp x Pp = PP, Pp, Pp, pp
Genotype ratio will:
PP = 25 %
Pp = 50 %
pp = 25 %
Phenotype ratio:
Purple color (PP, Pp, Pp) = 75 %
white color (pp) = 25 %
Learn more about phenotype and genotype at: https://brainly.com/question/902712
#SPJ1
A Cell is B.00 un in diameter' and has a cell width of 60.0 nm thrck. If densty x (mass druided by volome) of the wall is the Same as thent of pure water (1000kym
−3
). What ts the mass (in my) of the cell wall cossuming cell is splowicul and the wall is thin sphericul slell?
Answers
The mass of the cell wall, assuming the cell is spherical and the wall is a thin spherical shell, is approximately 0.91 milligrams.
To calculate the mass of the cell wall, we first need to determine the volume of the wall.
The given diameter of the cell is 0.00 μm, which means the radius (r) of the cell is half of that, so r = 0.00/2 = 0.00 μm = 0.00 nm.Now, we need to find the volume of the cell wall, which can be approximated as a thin spherical shell. The volume of a thin spherical shell can be calculated using the formula:
V = 4/3 * π * (r_outer^3 - r_inner^3)
Since the cell is spherical, the inner radius of the shell is the same as the radius of the cell (r), and the outer radius of the shell is the sum of the radius of the cell (r) and the thickness of the wall (60.0 nm). Thus, the outer radius (r_outer) of the shell is:
r_outer = r + thickness = 0.00 + 60.0 = 60.0 nm
Substituting the values into the formula, we have:
V = 4/3 * π * (60.0^3 - 0.00^3)
= 4/3 * π * (216,000 nm^3)
= 288,000 π nm^3
Next, we need to calculate the mass of the cell wall using the density of pure water. The density (ρ) is given as 1000 kg/m^3, which is equivalent to 1000 kg/1,000,000,000 nm^3 since 1 m = 1,000,000,000 nm. Thus, the mass (m) of the cell wall is:
m = ρ * V
= 1000 kg/1,000,000,000 nm^3 * 288,000 π nm^3
= 0.000288 π kg
Now, we can calculate the mass of the cell wall by substituting the value of π (pi) as 3.14159:
m = 0.000288 * 3.14159 kg
= 0.000905 kg
≈ 0.91 mg
For more such questions on mass visit:
https://brainly.com/question/24191825
#SPJ8
How many grams of aluminum burned if 200.0 grams of aluminum oxide formed?
Answers
Answer:
approximately 106 grams
Explanation:
The molecular mass of Aluminum Oxide is approximately 102 grams per mole.
2
×
A
l
=
2
×
27
=
54
3
×
O
=
3
×
16
=
48
54
+
48
=
102
200
102
=
1.96
moles
There are two atoms of Aluminum in one mole and each atom has a mass of approximately 27 grams so
Mass of Al =
1.96
×
2
×
27
=
106
grams
issued this? watch kcv: atomic theory; read section 2.3. you can click on the review link to access the section in your etext. carbon and oxygen form both carbon monoxide and carbon dioxide. when samples of these are decomposed, the carbon monoxide produces 3.36 g of oxygen and 2.52 g of carbon, while the carbon dioxide produces 9.92 g of oxygen and 3.72 g of carbon.
Answers
The atomic ratio of carbon to oxygen in carbon monoxide (CO) is 1:1, and the atomic ratio of carbon to oxygen in carbon dioxide (CO₂) is 2:1.
Firstly, we can analyze the decomposition of carbon monoxide (CO) and carbon dioxide (CO₂) to determine the atomic ratios involved.
Let's denote the atomic ratio of carbon to oxygen in carbon monoxide as x, and the atomic ratio of carbon to oxygen in carbon dioxide as y.
According to the given data;
Decomposition of carbon monoxide (CO);
Oxygen produced = 3.36 g
Carbon produced = 2.52 g
We know that the atomic mass of carbon is 12 g/mol, and the atomic mass of oxygen is 16 g/mol. Using these values, we can calculate the number of moles for each element;
Number of moles of oxygen = mass / atomic mass = 3.36 g / 16 g/mol = 0.21 mol
Number of moles of carbon = mass / atomic mass = 2.52 g / 12 g/mol = 0.21 mol
Since the atomic ratio of carbon to oxygen in carbon monoxide is x, we can write the following equation;
0.21 mol C / (0.21 mol O) = x
Simplifying the equation, we have;
x = 1
Therefore, the atomic ratio of carbon to oxygen in carbon monoxide is 1:1.
Decomposition of carbon dioxide (CO₂);
Oxygen produced = 9.92 g
Carbon produced = 3.72 g
Following the same calculations as before;
Number of moles of oxygen = mass / atomic mass = 9.92 g / 16 g/mol = 0.62 mol
Number of moles of carbon = mass / atomic mass = 3.72 g / 12 g/mol = 0.31 mol
Since the atomic ratio of carbon to oxygen in carbon dioxide is y, we can write the following equation;
0.31 mol C / (0.62 mol O) = y
Simplifying the equation, we have;
y = 0.5
Therefore, the atomic ratio of carbon to oxygen in carbon dioxide is 1:0.5, which can be simplified to 2:1.
To know more about decomposition here
https://brainly.com/question/20418092
#SPJ4
--The given question is incomplete, the complete question is
"Missed this? watch kcv: atomic theory; read section 2.3. you can click on the review link to access the section in your text. carbon and oxygen form both carbon monoxide and carbon dioxide. when samples of these are decomposed, the carbon monoxide produces 3.36 g of oxygen and 2.52 g of carbon, while the carbon dioxide produces 9.92 g of oxygen and 3.72 g of carbon. Calculate the atomic ratio of carbon to oxygen in carbon monoxide, and carbon dioxide."--
[b] Potassium-40 has a half-life of 1.25 billion years. If a rock sample contains W Potassium-40 atoms for every 1000 its daughter atoms, then how old is this rock sample? Your answer should be significant to three digits. Remember to show all your calculations,
Answers
The rock sample is approximately 1.992 billion years old.
Potassium-40 (K-40) has a half-life of 1.25 billion years, which means that after 1.25 billion years, half of the original K-40 atoms would have decayed into daughter atoms. In this particular rock sample, we are given that there are W Potassium-40 atoms for every 1000 daughter atoms.
To determine the age of the rock sample, we need to find the value of W. Since the half-life of K-40 is 1.25 billion years, after each half-life, the ratio of K-40 to daughter atoms will be halved. So, after one half-life, the ratio would be 1:2000 (W:1000).
To calculate the number of half-lives, we can use the equation:
(number of half-lives) = (log(W/1000)) / (log(1/2))
Since we are given W Potassium-40 atoms for every 1000 daughter atoms, we can substitute the ratio into the equation:
(number of half-lives) = (log(W/1000)) / (log(1/2))
(number of half-lives) = (log(W/1000)) / (-0.301)
Simplifying the equation, we find:
(number of half-lives) = -3.32 * log(W/1000)
Since we want to find the age of the rock sample, we multiply the number of half-lives by the half-life of K-40:
Age = (number of half-lives) * (half-life of K-40)
Age = -3.32 * log(W/1000) * 1.25 billion years
By substituting the given value of W and performing the calculations, we can determine the age of the rock sample to be approximately 1.992 billion years.
Learn more about Rock
brainly.com/question/29898401
#SPJ11
1. 7.74 x 1026 molecules of Cesium nitrate to moles.
2. 58.0 grams of magnesium nitrate to moles
Answers
Answer:
Explanation:
1.To convert 7.74 x 10^26 molecules of cesium nitrate to moles, we need to use Avogadro’s number, which is 6.022 x 10^23 molecules per mole. We can set up the following conversion factor:
1 mole / (6.022 x 10^23 molecules)
This conversion factor allows us to cancel out the units of molecules and convert to moles. Multiplying the given quantity by this conversion factor, we get:
7.74 x 10^26 molecules x (1 mole / 6.022 x 10^23 molecules)
= 128.5 moles (rounded to three significant figures)
Therefore, 7.74 x 10^26 molecules of cesium nitrate is equal to 128.5 moles of cesium nitrate.
2.To convert 58.0 grams of magnesium nitrate to moles, we need to use the molar mass of magnesium nitrate.
The molar mass of magnesium nitrate can be calculated by summing the atomic masses of its constituent elements, which are:
Magnesium (Mg): 24.31 g/mol
Nitrogen (N): 14.01 g/mol
Oxygen (O) (3 atoms): 3 x 16.00 g/mol = 48.00 g/mol
So the molar mass of magnesium nitrate (Mg(NO3)2) is:
24.31 g/mol (Mg) + 2 x (14.01 g/mol (N) + 3 x 16.00 g/mol (O)) = 148.31 g/mol
We can use this molar mass as a conversion factor to convert grams of magnesium nitrate to moles. The conversion factor is:
1 mole / 148.31 grams
So, we can calculate the number of moles of magnesium nitrate as follows:
58.0 grams x (1 mole / 148.31 grams) = 0.391 moles
Therefore, 58.0 grams of magnesium nitrate is equal to 0.391 moles of magnesium nitrate.
How many 4d electrons would be predicted in the ground state for the following elements?a. zirconiumb. cadmiumc. iridiumd. iron
Answers
In order to answer the question first we must write the atomic number of each element:
Zirconium (Zr): 40
Cadmium (Cd): 48
Iridium (Ir): 77
Iron (Fe): 26
Then, we have to complete the distribution of electrons in each orbital for each atom:
The first 4 levels have the following distribution:
Level1: 1s
Number of electrones: 2
Level 2: 2s, 2p
Number of electrones 8 (2 in the s orbital and 6 in the p orbitals).
Level3: 3s, 3p, 3d
Number of electrones 18 (2 in the s orbital, 6 in the p orbital and 10 in the d orbitals)
Level 4: 4s, 4p, 4d, 4f
Number of electrones 32 (2 in the s orbital, 6 in the p orbitals, 10 in the d orbitals and 14 in the f orbitals)
The order in which the orbitlas are completed depends on the energy of each level. For example the 4s orbitals will be completed before the 3d orbitals because their energy is lower.
The order is as follows:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p...
Now, knowing the atomic number we can answer the question:
For Zirconium (total 40 electrones):
\(1s^2,2s^2,2p^6,3s^2,3p^6,4s^2,3d^{10},4p^6,5s^2,4d^2\)2 electrones are predicted in the 4d orbital
For Cadmium (total 48 electrones):
\(1s^2,2s^2,2p^6,3s^2,3p^6,4s^2,3d^{10},4p^6,5s^2,4d^{10}^{}\)10 electrones are predicted in the 4d orbital
For iridium, as it has an atomic number higher than Cadmium we can predict tha it also complets the 4d orbital, then it has also 10 electrones in it.
For iron (total 26 electrones)
\(1s^2,2s^2,2p^6,3s^2,3p^64s^2,3d^6\)Iron has no electrones in the 4d orbitals
A mixture of nitrogen, oxygen, fluorine, and neon are in a container which has a tiny, tiny pinhole leak. Which element leaks the slowest from the container?.
Answers
A mixture of nitrogen, oxygen, fluorine, and neon are in a container which has a tiny, tiny pinhole leak. The element leaks the slowest from the container is fluorine.
The effusion of gas is expressed as follows :
rate ∝ 1 / √M
rate of effusion is inversely proportional to the square root of molecular weight.
molecular weight of nitrogen = 14amu
molecular weight of oxygen = 16 amu
molecular weight of fluorine = 19 amu
the rate of effusion with increase of molecular weight. so the elements leaks the slowest from the container is fluorine.
Thus, A mixture of nitrogen, oxygen, fluorine, and neon are in a container which has a tiny, tiny pinhole leak. The element leaks the slowest from the container is
To learn more about effusion here
https://brainly.com/question/5272713
#SPJ1
During a chemical reaction, calcium carbonate (CaCO3)
breaks down to form calcium oxide (Cao) and carbon
dioxide (CO2).
Which equation represents this chemical reaction?
O A. CaO + CO2- CaCO3
B. CaCO3 + CaO + CO2
C. CaCO3 + CaO + CO2
D. CaCO3 + CO2 + Cao
Answers
The equation represents calcium carbonate (CaCO3) breaks down to form calcium oxide (CaO) and carbon dioxide (CO2) chemical reaction will be
CaO + CO2 → CaCO3.
What is chemical reaction?A chemical reaction occurs that results in the chemical change of one number of chemical components into another set of organic compounds.
What is equation?A chemical equation is just a description of a chemical reaction in symbols that indicates the products, reactants, but also reaction direction.
Calcium carbonate dissociate into calcium oxide and carbon dioxide.
It can be expressed as CaO + CO2 → CaCO3.
Therefore, the correct answer will be option (A).
To know more about chemical reaction.
https://brainly.com/question/22817140
#SPJ2
in a titration, 14.5cm3 of nitric acid, HNO3 neutralised exactly 25cm3 of 0.05 mol/dm3 sodium hydroxide, NaOH
calculate the concentration of the nitric acid solution in mol/dm3
Calculate the concentration of the nitric acid in g/dm3
Answers
The concentration of the nitric acid solution is 0.0862 mol/\(dm^3\) and the concentration of the nitric acid in g/\(dm^3\) is 5.431 g/\(dm^3\).
What is titration?
A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution.
According to the neutralization law,
\(n_1M_1V_1 = n_2M_2V_2\)
\(n_1\)is the basicity of \(HNO_3\) =1
\(M_1\) is the molarity of \(HNO_3\) =0.05 mol/\(dm^3\)
\(V_1\) is the volume of \(HNO_3\) solution = 25\(cm^3\)
\(n_2\) is the acidity of NaOH =1
\(M_2\) is the molarity of NaOH =?
\(V_2\) is the volume of NaOH solution =14.5\(cm^3\)
Putting in the values we get:
\(n_1M_1V_1 = n_2M_2V_2\)
1 x 0.05 mol/\(dm^3\) x 25cm^3 = 1 x \(M_2\) x 14.5\(cm^3\)
\(M_2\) = 0.0862 mol/\(dm^3\)
Hence. the concentration of the nitric acid solution in 0.0862 mol/\(dm^3\) and the concentration of the nitric acid in g/dm3 is 0.0862 x63.01 g/\(dm^3\)= 5.431 g/\(dm^3\).
Learn more about the titration here:
https://brainly.com/question/22138555
#SPJ1
Given : 4.50 molte
How many moles of ammonia would you get if 4.50 moles of hydrogen gas
reacted
Answers
Which types of atomic orbitals of the central atom mix to form hybrid orbitals in:
(d) NF₃?
Answers
Hybrid orbitals form on the central atom and can either be sp, sp2, sp3, sp3d, or sp3d2. The type of hybrid orbitals that are used is dictated by the electron domain geometry.
Sigma bonds are formed by the end-to-end overlap of bonding orbitals. Pi bonds are formed by the side-to-side overlap of p orbitals.
How are hybrid orbitals formed?Hybrid orbitals are the result of a model which combines atomic orbitals on a single atom in ways that lead to a new set of orbitals that have geometries appropariate to form bonds in the directions predicted by the VSEPR model. The VSEPR model predicts geometries that are very close to those seen in real molecules.
What is the hybridization of nitrogen in NF3?Nitrogen trifluoride (NF3) lewis structure contains three sigma bonds and one lone pair around nitrogen atom. Therefore, there are total of four electrons regions around nitrogen atom. So, hybridization of center atom, nitrogen is sp3.
Learn more about NF3 here:
https://brainly.com/question/15351545#SPJ4What is an ion? What are the different properties of an ion? There is more than one answer choose all that apply

Answers
Answer:
An ion is an atom or molecule that has a different number of electrons than protons, so it has a charge.
Explanation:
Trust me please
What is the total pressure inside a flask that contains 2.15 atm helium, 4.62 atm xenon, and 0.65 atm argon?
Answers
Answer:
7.42 atm
Explanation:
The total pressure is just the sum of partial pressures:
2.15 + 4.62 + 0.65 = 7.42 atm
Highlight the claim.
A common type of asexual reproduction found in nature is called Mitosis. Mitosis requires less energy than sexual reproduction does. Mitosis can
occur in seconds and does not require a mate to reproduce. Sexual reproduction requires two compatible parents. It also requires time to produce
the egg and sperm cells and then for fertilization to occur. Energy is required to find a compatible mate, produce sex cells, and for fertilization
Therefore Mitosis requires less energy than sexual reproduction does.
Answers
Answer:
The claim is: Therefore Mitosis requires less energy than sexual reproduction does.
Explanation:
What can air pollutants do?
Answers
Answer:
Air pollution can also cause headaches, dizziness, and nausea. ... Long-term health effects from air pollution include heart disease, lung cancer, and respiratory diseases such as emphysema. Air pollution can also cause long-term damage to people's nerves, brain, kidneys, liver, and other organs.
Explanation:
A student proposes the following step of a mechanism. Why would an expert question this mechanism step? 3A+B→2C A) The number of reactants and products must be the same. B) The number of products must always exceed the reactants. C) This would require 4 molecules to collide and react simultaneously.
Answers
Option C. An expert would question the proposed mechanism step 3A+B→2C due to the requirement of four molecules to collide and react simultaneously.
The expert would question this mechanism step for several reasons. Firstly, according to the law of conservation of mass, the number of atoms of each element must be the same on both sides of a chemical equation. In the proposed step, there are three reactant molecules (3A and B) but only two product molecules (2C), violating the principle that the number of reactants and products must be the same.
Secondly, the statement that the number of products must always exceed the number of reactants is incorrect. While it is possible for the number of products to exceed the number of reactants in some chemical reactions, it is not a universal rule. There are reactions where the number of products is equal to or even less than the number of reactants.
Finally, the mechanism step suggests that four molecules (3A and B) would need to collide and react simultaneously, which is highly unlikely. In most chemical reactions, collisions between molecules occur randomly, and it is rare for four molecules to collide at the exact same time and in the correct orientation.
Learn more about reactants here:
https://brainly.com/question/30129541
#SPJ11
Consider the following reaction in a gas phase:C(s) + H2O(g) ⇄ CO(g) + H2(g) KC = 0. 2 at 1000 °CCalculate the concentration of CO in an equilibrium mixture (in mol/L) if a reaction mixture initially contains only reactants, and the equilibrium concentration of H2O(g) is [H2O] = 0. 500 M at 1000 °C
Answers
The concentration of CO (g) in the equilibrium mixture is 0.020 M. In other words, only a small amount of CO (g) is produced in this reaction at 1000°C. T 0.020 M.
The concentration of CO in an equilibrium mixture (in mol/L) is 5.8 M.
Given that the concentration of H2O (g) is [H2O] = 0.500 M at 1000°C, and the reaction is:
C(s) + H2O(g) ⇄ CO(g) + H2(g) KC = 0.2 at 1000°C
We need to determine the concentration of CO in an equilibrium mixture (in mol/L)
if a reaction mixture initially contains only reactants.
We can solve this problem using the ICE table method as follows:
Let x be the change in concentration of H2O (g) and CO (g) when they reach equilibrium.
Then the equilibrium concentrations of CO (g) and H2 (g) are equal to x. Hence, the equilibrium concentration of H2O (g) is (0.500 - x) M. Substitute these values in the expression for Kc and solve for x.
Kc = [CO (g)] [H2 (g)] / [H2O (g)] [C (s)]
= 0.2[CO (g)] = Kc [H2O (g)] [C (s)] / [H2 (g)]
= 0.2 × (0.500 - x) / x
We can simplify this expression by cross-multiplication to get:
5x = 0.1 - 0.2xx = 0.02 M
Substituting x = 0.02 M in the expression for [CO (g)], we get:
[CO (g)] = 0.2 × (0.500 - 0.02) / 0.02 = 5.8 M (approx.)
Therefore, the concentration of CO (g) in an equilibrium mixture (in mol/L) is 5.8 M. The problem requires us to find the equilibrium concentration of CO (g) in a mixture that initially contains only reactants.
To solve this problem, we need to use the expression for the equilibrium constant Kc, which is given by:
Kc = [CO (g)] [H2 (g)] / [H2O (g)] [C (s)]
We can also use the ICE table method to solve this problem. In this method, we start with the initial concentration of the reactants and calculate the change in concentration of each species as they reach equilibrium.
We then use the equilibrium concentrations to calculate the value of Kc and solve for the unknowns. Here is how we can set up the ICE table for this problem: Reaction:
C(s) + H2O(g) ⇄ CO(g) + H2(g)
Initial: [C] = [H2]
= 0 M,
[H2O] = 0.500 M
Equilibrium: [C] = [H2] = x,
[H2O] = 0.500 - x,
[CO] = [H2] = x
Change: +x +x -x -x
Substituting the equilibrium concentrations into the expression for Kc, we get:
Kc = [CO] [H2] / [H2O] [C]
= x² / (0.500 - x)
= 0.2
Solving for x, we get: x = 0.020 M
Substituting this value of x into the expression for [CO], we get:
[CO] = x = 0.020 M
Therefore, the concentration of CO (g) in the equilibrium mixture is 0.020 M.
In other words, only a small amount of CO (g) is produced in this reaction at 1000°C. T 0.020 M.
The concentration of CO in an equilibrium mixture (in mol/L) is 5.8 M.
To know more about reactants visit:
brainly.com/question/32459503
#SPJ11
Show your work and the correct number of sig figs.
1. N2 + 3H2 ----> 2NH3
How many grams of nitrogen, H2, is necessary to react completely with 50.0g of nitrogen, N2?
Please help, I need real answer only
Answers
To react completely with 50.0g of nitrogen gas (N2), we would need approximately 10.820 grams of hydrogen gas (H2).The correct number of significant figures, is 10.8 g H2.
To determine the amount of hydrogen gas (H2) required to react completely with 50.0g of nitrogen gas (N2), we need to use stoichiometry.
The balanced equation is:
N2 + 3H2 → 2NH3
From the equation, we can see that the molar ratio between nitrogen gas and hydrogen gas is 1:3. This means that for every 1 mole of N2, we need 3 moles of H2 to react completely.To find the moles of nitrogen gas (N2) in 50.0g, we need to divide the mass by the molar mass of nitrogen (28.02 g/mol):
moles of N2 = 50.0g / 28.02 g/mol = 1.784 mol
Since the molar ratio between N2 and H2 is 1:3, we can multiply the moles of N2 by the ratio to find the moles of H2 required:
moles of H2 = 1.784 mol N2 × (3 mol H2 / 1 mol N2) = 5.352 mol H2
Now, we can convert the moles of H2 to grams by multiplying by the molar mass of hydrogen (2.02 g/mol):
grams of H2 = 5.352 mol H2 × 2.02 g/mol = 10.820 g H2
for such more questions on nitrogen
https://brainly.com/question/1380063
#SPJ8