A 55kg skier accelerated at 3 m/s^2. How much force must the skier exert?
Answers
Answer:
The answer is 165 NExplanation:
The force acting on an object given it's mass and acceleration can be found by using the formula
force = mass × accelerationFrom the question
mass = 55 kg
acceleration = 3 m/s²
We have
force = 55 × 3
We have the final answer as
165 NHope this helps you
Related Questions
The figure represents three identical containers connected by valves that can be closed or opened to allow gas movement between the containers. At the beginning of a student's investigation, the valves are closed and the two outer containers are completely empty. The middle container holds particles of an ideal gas at a pressure of 9 atm. After the valves are opened and enough time has passed for net movement of particles between the containers to stop, the pressure of gas in the middle container will be closest to which of the following?
1.5 atm
2 atm
3 atm
9 atm

Answers
After valves are opened and enough time has passed for net movement of particles between containers to stop, pressure of gas in middle container will be closest to 3 atmospheres as pressure will be halved after opening valves.
What is pressure ?Pressure is defined as the force applied on an object perpendicular to it's surface per unit area over which it is distributed.Gauge pressure is a pressure which is related with the ambient pressure.
There are various units by which pressure is expressed most of which are derived units which are obtained from unit of force divided by unit of area . The SI unit of pressure is pascal .
Pressure will be halved after opening valves as it will be bifurcated ,thus, 9/2=4.5 which is closest 3 atmospheres.
Thus,the pressure of gas in the middle container will be closest to 3 atmospheres.
Learn more about pressure,here:
https://brainly.com/question/14760196
#SPJ1
Molecule 1 has the following sequences of bases: TCAAGT.Which set of bases in Molecule 2 will bond to this sequence in a complementary way?answer choicesUCAAGUAGTACAAGUUCAUCAATA
Answers
AGTTCA is the set of bases in Molecule 2 that will bond to this sequence in a complementary way.
From the rules of base pairing, we know that Adenine always pairs with Thymine, and Cytosine always pairs with Guanine.
Adenine is denoted as A, Thymine is denoted as T, Cytosine is denoted as C, and Guanine is denoted as G. Therefore if the base sequence is TCAAGT, then following the rule of base pairing, the complementary sequence to this will be AGTTCA.
This strict arrangement is because only with this arrangement can hydrogen bonds form between these base pairs. Any other arrangement cannot sustain the integrity of the molecule.
To know more about the sequence in a complementary:
https://brainly.com/question/787144
#SPJ4
write briefly on the the characteristics of
transition elements,halogens ,aclivider
and artificial elements
Answers
Transition elements have incomplete d or f orbitals, enabling them to form complex compounds, exhibit various oxidation states, and display properties such as conductivity, high melting points, and colorful compounds. Halogens are highly reactive nonmetals that readily form salts, have high electronegativity, and exhibit distinct colors and odors, finding applications in disinfectants and pharmaceuticals. Actinides are radioactive elements with large atomic sizes, capable of forming complex compounds and undergoing radioactive decay. Artificial elements, created artificially, are highly unstable with short half-lives and limited practical applications but contribute to our understanding of atomic structure.
Transition elements, also known as transition metals, exhibit several characteristic properties. Firstly, they have incomplete d or f orbitals in their atomic structure, enabling them to form complex compounds and exhibit a wide range of oxidation states. Transition metals are typically good conductors of heat and electricity, and many display high melting and boiling points. They often exhibit colorful compounds due to the presence of unpaired d electrons that can absorb and emit visible light. Additionally, transition metals are known for their catalytic properties, allowing them to accelerate chemical reactions.
Halogens, such as fluorine, chlorine, bromine, iodine, and astatine, share similar characteristics. They are highly reactive nonmetals found in Group 17 of the periodic table. Halogens readily form salts by accepting electrons from other elements, making them powerful oxidizing agents. They have high electronegativity and tend to gain one electron to achieve a stable electronic configuration. Halogens exist in various states, from gases like fluorine and chlorine to solids like iodine. They also display distinct colors and strong odors, and their compounds find applications in disinfectants, bleach, and pharmaceuticals.
Actinides are a group of elements in the periodic table, including actinium and the 15 elements from thorium to lawrencium. They are all radioactive and have large atomic sizes. Actinides share some properties with transition metals, such as the ability to form complex compounds and exhibit variable oxidation states. Due to their unstable nuclei, they undergo radioactive decay, releasing energy in the process. Actinides are important in nuclear technology and have applications in nuclear power generation and weaponry.
Artificial elements, also known as synthetic elements or transuranium elements, are elements that are not naturally occurring but are created through artificial means, typically in particle accelerators or nuclear reactors. These elements, such as technetium, promethium, and all the elements with atomic numbers higher than 92, have very short half-lives and are highly unstable. They are typically produced in small quantities and have primarily been studied for scientific purposes. Synthetic elements are crucial for expanding our understanding of atomic structure and the periodic table, but they have limited practical applications due to their instability.
For more question on elements
https://brainly.com/question/28376204
#SPJ8
5. What mass of water can be heated from 15.0°C to 40.0" with 925 Joules 2 points of heat?
Answers
Mass of water = 8.84 g
Further explanationHeat can be calculated using the formula:
Q = mc∆T
Q = heat, J
m = mass, g
c = specific heat, joules / g ° C
∆T = temperature difference, ° C / K
c of water = 4.182 J / g ° C
Q = 925 J
mass of water :
\(\tt Q=m.c.\Delta T\\\\925=m\times 4.182\times (40-15)\\\\m=8.84~g\)
The main hazard associated with using centrifuges is
- Broken tubes
- Aerosol formation from spinning the sample too rapidly
- Unbalanced samples leading to excessive vibration and rotor destruction
- Spilling samples since centrifuge tubes have round bottoms
Answers
The main hazards associated with using centrifuges include broken tubes, aerosol formation, unbalanced samples, and spilling samples.
Broken tubes can occur when the centrifuge tubes are damaged or overfilled, leading to leakage or breakage during operation. This can result in sample loss, contamination, and damage to the centrifuge rotor and other tubes.
The aerosol formation is another hazard, that occurs when the sample is spun too rapidly. High-speed centrifugation can cause the release of tiny liquid droplets, forming an aerosol. This can lead to the spread of hazardous materials or infectious agents, posing a risk to the user and environment.
Unbalanced samples pose a significant hazard as they can cause excessive vibration during centrifugation. This imbalance can lead to rotor destruction, which may damage the centrifuge and result in costly repairs or replacement. To prevent this, ensure equal sample volumes and masses are loaded symmetrically across the rotor.
Lastly, spilling samples is a risk since centrifuge tubes have round bottoms. Spilt samples can contaminate other samples, the rotor, and the centrifuge chamber, affecting the integrity of the experiment. To minimize this risk, securely cap the tubes and handle them with care when loading and unloading the centrifuge.
In conclusion, to ensure safety and accurate results when using a centrifuge, be mindful of potential hazards such as broken tubes, aerosol formation, unbalanced samples, and spilling samples. By taking necessary precautions and following proper procedures, these risks can be mitigated.
To learn more about aerosol formation, refer:-
https://brainly.com/question/31491688
#SPJ11
How many grams of iron (III) oxide can be produced from 2.50 g of oxygen reacting with iron, according to the
following equation?
4 Fe (s) + 3 02 (g) -->2 Fe₂O3(s)
Answers
3 moles of oxygen gives 2 moles of the product. Then, 2.50 g or 0.07 moles of oxygen gas will give, 0.04 moles or 14.8 g of Fe₂O₃.
What is Fe₂O₃ ?Metals are easily reactive towards atmospheric oxygen and they form their oxides. Fe reacts with oxygen to form one of its oxide in the + 3 oxidation state that is Fe₂O₃.
Here, 3 moles of oxygen gives 2 moles of the oxide.
molar mass of oxygen gas = 32 g/mol
no.of moles in 2.5 g = 2.5 /32 = 0.07 moles.
0.07 moles produce, 0.07 × 2 /3 = 0.04 moles.
molar mass of Fe₂O₃ = 319.2 g.
then, mass of 0.04 moles = 0.04 × 319.2 = 14.8 g.
Therefore, 2.5 g of oxygen gas will give 14.8 g of the product.
Find more on Fe₂O₃:
https://brainly.com/question/24236942
#SPJ1
When lithium metal reacts with fluorine gas it forms the ionic compound lithium fluoride (LiF). What is the correct electron configurations of the ions formed
Answers
Answer:
The electronic configuration of ions formed are:
Li+ (2) => 1s2
Cl- (18) => 1s2 2s2 2p6 3s2 3p6
Explanation:
Lithium metal, Li will lose 1 electron to lithium ion Li+. Chlorine atom, Cl will receive the 1 electron to form the chloride ion Cl- as shown by the following equation below:
Li —> Li+ + e
Cl+ e —> Cl-
Combine both equation
Li + Cl + e —> Li+ + Cl- + e
Cancel out 'e'
Li + Cl —> Li+ Cl-
Thus, we can write the electronic configuration for the reaction as follow:
Before reaction:
Li (3) => 1s2 2s1
Cl (17) => 1s2 2s2 2p6 3s2 3p5
After reaction
Li+ (2) => 1s2
Cl- (18) => 1s2 2s2 2p6 3s2 3p6
Therefore, the electronic configuration of the ions formed are:
Li+ (2) => 1s2
Cl- (18) => 1s2 2s2 2p6 3s2 3p6
Hydrogen is in column 1 of the periodic table. What information does this give us? (2 points)
There is one other element that hydrogen will bond with.
There is one neutron in the nucleus.
The mass of the atom is one.
There is one electron in the outer energy level.
Answers
Answer:
the last one
Explanation:
ydyeusueueueueudhs
Because hydrogen forms compounds with oxidation numbers of both +1 and -1, many periodic tables include this element in both Group IA (with Li, Na, K, Rb, Cs, and Fr) and Group VIIA (with F, Cl, Br, I, and At).
There are many reasons for including hydrogen among the elements in Group IA. It forms compounds (such as HCl and HNO3) that are analogs of alkali metal compounds (such as NaCl and KNO3). Under conditions of very high pressure, it has the properties of a metal. (It has been argued, for example, that any hydrogen present at the center of the planet Jupiter is likely to be a metallic solid.) Finally, hydrogen combines with a handful of metals, such as scandium, titanium, chromium, nickel, or palladium, to form materials that behave as if they were alloys of two metals.
There are equally valid arguments for placing hydrogen in Group VIIA. It forms compounds (such as NaH and CaH2) that are analogs of halogen compounds (such as NaF and CaCl2). It also combines with other nonmetals to form covalent compounds (such as H2O, CH4, and NH3), the way a nonmetal should. Finally, the element is a gas at room temperature and atmospheric pressure, like other nonmetals (such as O2 and N2).
It is difficult to decide where hydrogen belongs in the periodic table because of the physical properties of the element. The first ionization energy of hydrogen (1312 kJ/mol), for example, is roughly halfway between the elements with the largest (2372 kJ/mol) and smallest (376 kJ/mol) ionization energies. Hydrogen also has an electronegativity (EN = 2.20) halfway between the extremes of the most electronegative (EN = 3.98) and least electronegative (EN = 0.7) elements. On the basis of electronegativity, it is tempting to classify hydrogen as a semimeta
How are prepare Tolu Balsam syrup by percolation?
Answers
Tolu Balsam syrup prepared by percolation is commonly used as an expectorant and cough syrup.
Tolu Balsam syrup can be prepared by percolation as follows:
To know more about the Syrup, here
https://brainly.com/question/31543537
#SPJ4
As atomic radius decreases, electronegativity __________. This is mostly because a smaller atomic radius represents a ________ hold on the valence ________.
Answers
As atomic radius decreases, electronegativity increases. This is mostly because a smaller atomic radius represents a stronger hold on the valence electrons.
Based on the lewis structure, the number of electron domains in the valence shell of the boron atom in the bf3 molecule is.
Answers
With no lone pairs of electrons on the core boron atom, the BF3 molecule's Lewis structure reveals that the boron atom is surrounded by three fluoride atoms.
A shared pair of electrons between boron and one of the three fluorine atoms forms each covalent bond in the compound BF3. As a result, the number of shared electron pairs and the number of electron domains in the valence shell of boron are equal. The VSEPR theory states that in order to minimize repulsion, the electron domains surrounding the boron atom organize themselves as widely apart as possible. As a result, the molecule of BF3 has a trigonal planar molecular geometry with 120° bond angles between B-F links.
To know more about VSEPR theory, here
brainly.com/question/17177984
#SPJ1
what was acceleration of a car that took off with 175000 Nforce and weighs 2500kg
Answers
Acceleration of the car is the force divided by its mass. The acceleration of the car with 2500 kg with a force of 175000 N is 70 m/s².
What is acceleration ?Acceleration is a physical quantity measuring the rate of change in velocity. It is a vector quantity having both magnitude and acceleration. Acceleration has the unit of m/s².
According to Newton's second law of motion, the force acting on a body is the product of its mass and acceleration.
F = ma
Given that, mass of the car = 2500 kg
force applied = 175000 N
acceleration = force/mass
a = 175000 N/2500 kg = 70m/s²
Therefore, the acceleration of the car is 70 m/s².
Find more on acceleration:
brainly.com/question/3046924
#SPJ9
Which notation represents the largest atomic radius?
Cl
Cl^−
F
F^−
Answers
Answer:
Cl⁻ Or A
Explanation:
A stretched rubber band is an example of:
A. Kinetic energy
B. Potential energy
C. Gravitational energy
D. Light energy
Answers
Answer:
Potential Energy
Explanation:
Not sure how to explain it but C and D could be crossed off as answers, kinetic energy would be if it was released (?)
sorry if im wrong
Phospholipid molecules that prevent the alveoli from collapsing are known as ______. A) laryngitis. B) surfactant. C) mucus. D) plasma.
Answers
B) Surfactant is a phospholipid molecule that prevents alveoli from collapsing. It reduces surface tension in the lungs, allowing the alveoli to remain open and facilitating efficient breathing.
Phospholipid molecules that prevent the alveoli from collapsing are known as surfactants. Surfactant is a substance composed of phospholipids, proteins, and other components. It is produced by specialized cells in the lungs called type II alveolar cells.
The primary function of surfactant is to reduce the surface tension within the alveoli, which are tiny air sacs in the lungs where gas exchange takes place. Without surfactant, the surface tension would be too high, causing the alveoli to collapse during exhalation. Surfactant molecules disrupt the cohesive forces between water molecules on the alveolar surface, allowing the alveoli to remain open and preventing them from sticking together. The presence of surfactant is crucial for efficient breathing and maintaining lung function. In conditions where surfactant production is reduced or absent, such as in premature infants or certain lung diseases, respiratory distress syndrome and other breathing difficulties can occur.
learn more about phospholipid here:
https://brainly.com/question/20561742
#SPJ11
What is a poverty line?
Answers
Description
The poverty threshold, poverty limit, poverty line or breadline, is the minimum level of income deemed adequate in a particular country. Poverty line is usually calculated by finding the total cost of all the essential resources that an average human adult consumes in one year. Hop this help you
We wish to determine how many grams
of solid silver chromate will precipitate
when 150. mL of 0.500 M silver nitrate
solution is added to excess potassium
chromate.
2AgNO3(aq)
How many moles of AgNO3 are present
in 150. mL of 0.500 M AgNO3?
+ K₂ CrO4 (aq) → Ag₂ CrO4(s) + 2KNO3(aq)
Answers
Approximately 12.45 grams of solid silver chromate will precipitate when 150 mL of 0.500 M silver nitrate solution is added to excess potassium chromate.
To determine the number of moles of AgNO3 present in 150 mL of a 0.500 M AgNO3 solution, we can use the formula:
moles = concentration × volume
Given:
Concentration of AgNO3 solution = 0.500 M
Volume of AgNO3 solution = 150 mL
First, we need to convert the volume from milliliters (mL) to liters (L) since the concentration is given in moles per liter (M).
1 L = 1000 mL
Therefore, the volume of the AgNO3 solution in liters is:
150 mL × (1 L / 1000 mL) = 0.150 L
Now we can calculate the moles of AgNO3 using the formula:
moles = concentration × volume
moles = 0.500 M × 0.150 L
moles = 0.075 mol
So, there are 0.075 moles of AgNO3 present in 150 mL of the 0.500 M AgNO3 solution.
Now, let's proceed to determine how many grams of solid silver chromate (Ag2CrO4) will precipitate when the AgNO3 solution reacts with excess potassium chromate (K2CrO4).
From the balanced chemical equation:
2AgNO3(aq) + K2CrO4(aq) → Ag2CrO4(s) + 2KNO3(aq)
We can see that the molar ratio between AgNO3 and Ag2CrO4 is 2:1. Therefore, for every 2 moles of AgNO3, we will form 1 mole of Ag2CrO4.
Since we have 0.075 moles of AgNO3, we can calculate the moles of Ag2CrO4 formed:
moles of Ag2CrO4 = 0.075 mol / 2 = 0.0375 mol
To determine the mass of Ag2CrO4, we need to multiply the moles by its molar mass. The molar mass of Ag2CrO4 is calculated by summing the atomic masses of each element in the compound:
Ag2CrO4 = 2(Ag) + 1(Cr) + 4(O) = 2(107.87 g/mol) + 1(52.00 g/mol) + 4(16.00 g/mol) = 331.87 g/mol
mass of Ag2CrO4 = moles of Ag2CrO4 × molar mass of Ag2CrO4
mass of Ag2CrO4 = 0.0375 mol × 331.87 g/mol = 12.45 g
For more such questions on solid silver chromate visit:
https://brainly.com/question/32055228
#SPJ8
how do I write both conversion factors for converting L to ML
Answers
The capacity of a liquid is measured in metric units of volume called litres and millilitres.
There are four units of measurement for liquid volume: millilitres, centilitres, litres, and kiloliters.Liter is a fundamental metric unit that measures liquid volume and is equal to one cubic decimeter.The millilitre is a more compact metric unit that measures a liquid's volume or capacity. It is equivalent to one thousandth of a litre and is used to measure smaller amounts of liquid.By dividing the given amount by 1000, we may convert the given amount to millilitres. Let's, for illustration, convert 6 litres to millilitres. So, 6 × 1000 Equals 6000 ml. 6 litres hence equal 6000 millilitres.It should be remembered that we must divide the supplied amount by 1000 in order to convert from millilitres to litres. Let's convert 7000 millilitres to litres as an example. 7000 x 1000 equals 7 litres. So, 7000 millilitres equals 7 litres.A crucial component of measurement is unit conversion, which is done using the appropriate quantity conversion factor.
Learn more about unit measurement here:
https://brainly.com/question/141163
#SPJ9
What are the missing two words in this sentence? A polymer that retains its shape is known as a __________ __________ polymer.
Answers
According to the research, the correct words to fill the blank spaces in the sentence are shape, memory. A polymer that retains its shape is known as a shape memory polymer.
What is a shape memory polymer?These are capable polymers that typically have a permanent state, which is the state they always have before being stretched into what is known as a temporary state.
In this sense, they can maintain their temporary shape at or around room temperature, and return to their permanent state quickly, with only a slight increase in temperature.
Therefore, we can conclude that shape memory polymer returns to its original shape after the application of heat, that is, they can retain its shape.
Learn more about polymers here: https://brainly.com/question/27757193
#SPJ1
c) The amount of water on Earth changes every day. true or false?
Answers
Answer:
I think it's False
Explanation:
Explanation:
The amount of water in, on, and above our planet does not increase or decrease because of the water cycle
So it's false
How much energy is required to raise the temperature of 3 kg of lead from 15°C to 20°C? Use the table below and this equation: Q = MCAT.
The question is written right above the table given.
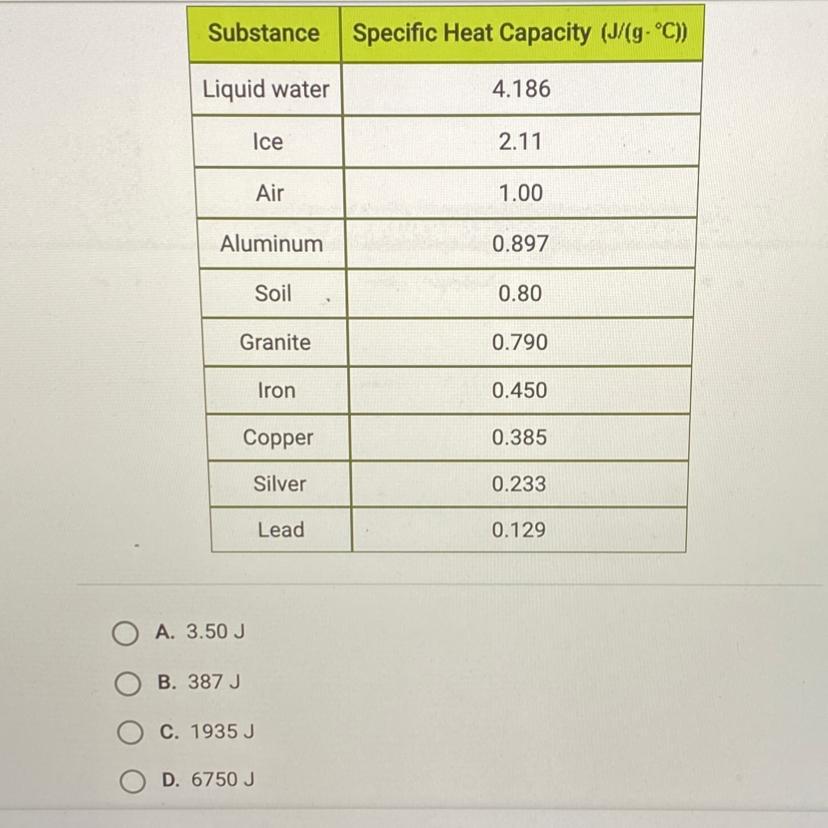
Answers
Answer:
1935J
Explanation:

Answer:
\(\boxed {\boxed {\sf C. \ 1935 \ J}}\)
Explanation:
The equation for this problem is:
\(q=mc\Delta T\)
where m is the mass, c is the specific heat capacity, and ΔT is the change in temperature.
The mass is 3 kilograms, but the specific heat capacity includes grams in the units. Convert kilograms to grams. There are 1000 grams in 1 kilogram.
\(\frac {1000 \ g}{1 \ kg}\)\(3 \ kg *\frac {1000 \ g}{1 \ kg}\)\(3 *1000 \ g = 3000 \ g\)The specific heat capacity for lead is found on the table. It is 0.129 J/g°C.
Let's find the change in temperature. It is raised from 15 °C to 20 °C.
\(\Delta T= final \ temperature - initial \ temperature \\\Delta T= 20 \textdegree C - 15 \textdegree C\\\Delta T= 5 \textdegree C\)Now we know every value.
m= 3000 g c= 0.129 J/g°CΔT= 5 °CSubstitute the values into the formula.
\(q= (3000 \ g)( 0.129 \ J/g \textdegree C)(5 \textdegree C)\)
Multiply the first 2 numbers together. The units of grams cancel.
\(q= (387 \ J/ \textdegree C )(5 \textdegree C)\)
Multiply again. This time the units of degrees Celsius cancel.
\(q= 1935 \ J\)
1935 Joules of energy are required and choice C is correct.
How much heat is required to raiset eh temperature of 50.0 g of water from 35°C to 55°C?
Answers
It requires 4180 joules of heat to raise the temperature of 50.0 grams of water from 35°C to 55°C.
To calculate the amount of heat required to raise the temperature of water, we can use the formula
q = m × c × ΔT
where; q will be the amount of heat (in joules),
m will be the mass of the water (in grams),
c will be the specific heat capacity of water (4.18 J/g°C),
ΔT will be the change in temperature (in °C).
Given; Mass of water (m) = 50.0 g
Specific heat capacity of water (c)= 4.18 J/g°C
Change in temperature (ΔT) = 55°C - 35°C = 20°C
Plugging in the values into the formula, we get
q = 50.0 g × 4.18 J/g°C × 20°C
Calculating this expression gives us
q = 4180 J
Therefore, it requires 4180 joules of heat.
To know more about amount of heat here
https://brainly.com/question/9588553
#SPJ4
Which statements about velocity are true?
Check all that apply.
A. The Sl units for velocity are miles per hour.
O B. For velocity, you must have a number, a unit, and a direction.
O C. To calculate velocity, divide the displacement by time.
D. The symbol for velocity is v.
Answers
What is the steps in converting moles liters and mass
Answers
To go from moles to liters, you can use the formula:
V = (nRT) / P
where n is the number of moles.
To go from moles to mass we can use:
mass = molar mass/Moles
What is the steps in converting moles liters and mass?To convert between moles, liters, and mass, you need to use the appropriate conversion factors and formulas based on the substance you are working with. Here are the general steps for converting between moles, liters, and mass:
Determine the substance and its molar mass: Find the molar mass of the substance you are working with. The molar mass represents the mass of one mole of that substance and is typically expressed in grams per mole (g/mol). You can find the molar mass on the periodic table or calculate it by adding up the atomic masses of the constituent atoms in the molecule.
Convert moles to mass: To convert moles to mass, you can use the formula:
Mass (in grams) = Number of moles × Molar mass
Multiply the number of moles by the molar mass of the substance to obtain the mass in grams.
Convert mass to moles: To convert mass to moles, use the formula:
Moles = Mass (in grams) / Molar mass
mass = molar mass/Moles
Divide the mass in grams by the molar mass to obtain the number of moles.
Convert moles to liters (for gases): If you are working with a gas, you can use the ideal gas law to convert moles to liters. The ideal gas law is expressed as:
PV = nRT
Where P is the pressure, V is the volume in liters, n is the number of moles, R is the ideal gas constant (0.0821 L·atm/(mol·K)), and T is the temperature in Kelvin.
Rearrange the equation to solve for V:
V = (nRT) / P
Substitute the values for n, R, T, and P to calculate the volume in liters.
These steps can be modified depending on the specific context and units you are working with, but they provide a general framework for converting between moles, liters, and mass.
Learn more about changes of units at:
https://brainly.com/question/141163
#SPJ1
Provide the elemental symbols for lead, iron, gallium, and tungsten respectively.
Answers

How
molecules of iron (iii) oxide
many
can be produced from 13.5 moles fe?
Answers
The number of molecules of iron (iii) oxide F₂O₃ that can be produced from 13.5 moles of Fe is 4.06×10²⁴ molecules
Balanced equation4Fe + 3O₂ —> 2F₂O₃
From the balanced equation above,
4 moles of Fe reacted to produce 2 moles of F₂O₃.
Therefore,
13.5 moles of Fe will react to produce = (13.5 × 2) / 4 = 6.75 moles of F₂O₃
How to determine the number of molecules of F₂O₃ produced
From Avogadro's hypothesis,
1 mole of F₂O₃ = 6.02×10²³ molecules
Therefore,
6.75 moles of F₂O₃ = 6.75 × 6.02×10²³
6.75 moles of F₂O₃ = 4.06×10²⁴ molecules
Thus, 4.06×10²⁴ molecules of F₂O₃ were obtained from the reaction
Learn more about Avogadro's number:
https://brainly.com/question/26141731
In the ground state, which atom has a completely filled valence electron shell?
Answers
Answer:
The noble gases(neon, helium, argon)
Explanation:
In the ground state, noble gas atom has a completely filled valence electron shell. Space makes up the majority of an atom.
What is atom?The smallest unit of matter that may be split without producing electrically charged particles is the atom. It is also the smallest piece of substance with chemical element-like characteristics. Electric forces, which link electrons towards the nucleus of atoms, cause them to be drawn to any positive charge.
Space makes up the majority of an atom. The remainder is made up of a cloud of negatively charged electrons around a positively charged nucleus made up of protons and neutrons. Compared to electrons, that are the smallest charged particles in nature, the nucleus is tiny and dense. In the ground state, noble gas atom has a completely filled valence electron shell.
Therefore, in the ground state, noble gas atom has a completely filled valence electron shell.
To learn more about atom, here:
https://brainly.com/question/29712157
#SPJ6
How plants adapt to temperature variation
Answers
Explanation: I found this answer from goggle
A gaseous product of a reaction is collected at 280K and 0.95 atm. Given
R= 0.0821L⋅atm/mol⋅K , what is the molar mass of the gas, in grams per mole, if 3.25 g of gas occupies 2.56 L?
Answers
The molar mass of the gas, given that 3.25 g of the gas occupied 2.56 L is 30.66g/mol
How do I determine the molar mass of the gas?To obtain the molar mass of the gas, we shall first obtain the number of mole of the gas. This can be obtained as follow:
Temperature (T) = 280 KPressure (P) = 0.95 atmVolume (V) = 2.56 L Gas constant (R) = 0.0821 atm.L/Kmol Number of mole (n) =?PV = nRT
0.95 × 2.56 = n × 0.0821 × 280
Divide both sides by (0.0821 × 280)
n = (0.95 × 2.56) / (0.0821 × 280)
n = 0.106 mole
Haven obtain the mole of the gas, we shall determine the molar mass of the gas as follow:
Mole of gas = 0.106 moleMass of gas = 3.25 gMolar mass of gas =?Molar mass = mass / mole
Molar mass of gas = 3.25 / 0.106
Molar mass of gas = 30.66g/mol
Thus, the molar mass of the gas is 30.66g/mol
Learn more about molar mass:
https://brainly.com/question/15874532
#SPJ1
After the HCl and NaOH react, Fernando measures the
mass again. Using the mass before the reaction in the
diagram, what is the mass after the reaction?
Remember, It is in a closed system.
A. 5.00 grams
OB. 10.00 grams
O C. 15.00 grams
OD. 20.00 grams
Answers
Answer:c
Explanation:
As the combined mass of the HCl and NaOH is 15 grams before the reaction. Therefore the mass after the reaction will be 15 grams according to the law of conservation of mass. Therefore, option (C) is correct.
What is law of conservation of matter?Matter can be transformed form via physical changes and chemical changes from one form to another form, during any of these changes, the total mass is conserved. The same quantity of matter exists before and after the chemical or physical as none of the matter is created or destroyed.
The balanced equation between the reaction of HCl and NaOH:
\(HCl +NaOH \longrightarrow H_2O +NaCl\)
According to the law of conservation of mass, the mass of HCl and NaOH will be equal to the mass of the products water and NaCl.
As mentioned in the question the combined mass of HCl and NaOH measured before the reaction is 15 grams. Therefore, the mass of the products in the closed container will be equal to 15 grams as well.
Learn more about law conservation of matter, here:
brainly.com/question/23910777
#SPJ5
Your question was incomplete, most probably the complete question was,
Fernando places 15 ml of HCl and 50 ml of NaOH in 100 ml of a beaker. He places them on a scale together and measures the combined mass of 15 grams.
After the HCl and NaOH react, Fernando measures the mass again. Using the mass before the reaction, what is the mass after the reaction? Remember, It is in a closed system.
A. 5.00 grams
B. 10.00 grams
C. 15.00 grams
D. 20.00 grams