A 5-column table with 2 rows. Column 1 is labeled number of protons, with entries A and D; column 2 is number of neutrons, with entries 7 and E; Column 3 is atomic number, with entries B and 26; Column 4 is Mass Number, with entries 15 and 56, and Column 5 is Element (symbol) with entries C and F. Using the periodic table, complete the table to describe each atom. Type in your answers.
Answers
Answer:
A = 8
B = 8
C = Oxygen (O)
D = 26
E = 30
F = Iron (Fe)
Explanation:
protons neutrons atomic number mass number element
A 7 B 15 C
D E 26 56 F
mass number = protons + neutrons
E = 56 - 26 = 30
A = 15 - 7 = 8
protons = atomic number
B = 8
D = 26
From atomic number:
C = Oxygen (O)
F = Iron (Fe)
Answer:
8
8
o
26
30
fe
Explanation:
edge 21
Related Questions
e Conversions
Mar 26 at 12:30pm
- Instructions
Question 1
How many grams are present in 2.93 x 10^23 atoms of Manganese (Mn)?
Answers
To determine the number of grams present in 2.93 x 10²³ atoms of manganese (Mn), we need to use the atomic weight of manganese and Avogadro's number.
The atomic weight of manganese is 54.94 g/mol. Avogadro's number is 6.022 x 10²³ particles per mole.
First, we need to convert the given number of atoms to moles using Avogadro's number.
2.93 x 10²³ atoms Mn x (1 mol Mn/6.022 x 10²³ atoms Mn) = 0.0485 moles Mn
Next, we can use the atomic weight of manganese to convert moles to grams.
0.0485 moles Mn x (54.94 g Mn/mol) = 2.67 grams Mn
Therefore, 2.93 x 10²³ atoms of manganese (Mn) weigh approximately 2.67 grams.
To know more about Avogadro's number, visit :
https://brainly.com/question/11907018
#SPJ1
If the volume of a ping pong ball is approximately 100. 0 cm ³, how many ping pong balls could you put in an empty science laboratory whose dimensions are 15. 2 m, 8. 2 m, 3. 1 m?
Answers
The volume of the science laboratory can be calculated by multiplying its dimensions: 15.2 m * 8.2 m * 3.1 m = 398.608 m³. To determine the number of ping pong balls that can fit in the laboratory, we need to convert the volume of the laboratory to cubic centimeters and then divide it by the volume of a ping pong ball. Therefore, the laboratory can accommodate approximately 3,986,080 ping pong balls.
To find the volume of the science laboratory, we multiply its dimensions: 15.2 m * 8.2 m * 3.1 m = 398.608 m³. However, since the volume of the ping pong ball is given in cubic centimeters, we need to convert the volume of the laboratory to the same unit. Since 1 m³ is equal to 1,000,000 cm³, we can multiply the volume of the laboratory by 1,000,000 to convert it to cubic centimeters: 398.608 m³ * 1,000,000 cm³/m³ = 398,608,000 cm³.
Next, we need to determine how many ping pong balls can fit in this volume. Dividing the volume of the laboratory by the volume of a single ping pong ball, we get: 398,608,000 cm³ / 100.0 cm³ = 3,986,080 ping pong balls. Therefore, approximately 3,986,080 ping pong balls can fit in the empty science laboratory.
To learn more about Dimensions : brainly.com/question/32471530
#SPJ11
Which word identifies the shaking that results from movement under Earth's surface?
O earthquake
fault
O plate
O stress
Answers
Answer:
Earthquake...
Explanation:
I mean, its kinda the only one that makes sense aha
And took test!
The word earthquake identifies the shaking that results from movement under Earth's surface.
What is earthquake?An earthquake is an intense shaking of Earth’s surface. The shaking is caused by movements in Earth’s outermost layer.When tectonic plates move, it also causes movements at the faults. An earthquake is the sudden movement of Earth’s crust at a fault line.Hence, option 1 is the answer.
To learn more about earthquake here
https://brainly.com/question/17738430
#SPJ2
3. Ions in the same group tend to form ions with the same
Answers
Answer:
Same charge.
Explanation:
Ions in the same group tend to form ions with the same charge. Elements belong to the same group on the periodic table form ions with the same charge because they have the same number of valence electrons. For example, all members of alkali metals have different number of shells but same number of electrons in their outermost shell so due to same number of outermost shell these alkali metals will make bonding with halogens which is a seven group that have seven electrons and needs one electron for attaining stability and forming ions on both atoms.
Calculate the density of carbon dioxide at STP
Answers
Answer:
Density = mass/volume
= 44/22.4
= 1.96 gram/liter
The density of the Carbon Dioxide at S.T.P. (Standard Temperature and Volume) is 1.96 gram/liter.
They are made of mostly hydrogen and helium, held together by its Sun. What is being described? Odwarf planets moons stars
Answers
Stars are made of mostly hydrogen and helium, held together by the Sun.
Are stars mostly made of hydrogen and helium?
Massive celestial objects called stars, which are primarily composed of hydrogen and helium, generate light and heat from the churning nuclear forges inside their cores. The other flecks of light we see in the sky, besides our sun, are all light-years away from Earth.
What holds a star together?
The process responsible for a star's luminosity is known as nuclear fusion. The pull of gravity is resisted by the hot gas as it pushes outward. A star is created by this harmony of forces. It maintains the star's structural integrity and maintains its temperature throughout the majority of its lifespan.
What causes stars to group together?
The majority of stars in the Milky Way are found in pairs or clusters of multiple stars, which is consistent with predictions made by three-dimensional computer models of star formation that the collapsing spinning clouds of gas and dust may split into two or three blobs.
To know more about stars:
https://brainly.com/question/17870368
#SPJ1
in the following unbalanced reaction which atom is oxidized hno3 hbr no br2 h2o
Answers
N is being reduced and is the oxidizing agent.
The given unbalanced chemical equation is:HNO3 + HBr → NO + Br2 + H2O
To balance the above chemical reaction, we need to make sure that the number of atoms of all the elements present in the reactants is equal to the number of atoms of the same elements present in the products.
Thus, we can balance the above reaction as follows:
HNO3 + 5HBr → NO + 3Br2 + 3H2O
In the above chemical equation, the oxidation state of N changes from +5 to +2.
Therefore, nitrogen (N) is being reduced and is the oxidizing agent.
Thus, the answer is as follows:Atom oxidized: Nitrogen (N).
Given: HNO3 + HBr → NO + Br2 + H2O
The oxidation state of H is +1, N is +5, Br is -1, O is -2, and that of NO is +2.
Now, let's find out the oxidation states of each element on both sides of the equation:
HNO3 + HBr → NO + Br2 + H2O+5
-1 0 -1 -2→+2 0 +2 0 -2
Oxidation states of N change from +5 to +2, and the oxidation states of Br change from -1 to 0.
Thus, N is being reduced and is the oxidizing agent. Therefore, the answer is Nitrogen (N).
Learn more about chemical equation
brainly.com/question/28792948
#SPJ11
How are molecules formed?
Answers
Answer:
When two or more atoms chemically bond together, they form a molecule. Sometimes the atoms are all from the same element. ... In a covalent bond, electrons are shared between atoms. The bonds between the two hydrogen atoms and the oxygen atom in a molecule of water are covalent bonds
Explanation:
Molecules are made up of atoms that are held together by chemical bonds. These bonds form as a result of the sharing or exchange of electrons among atoms. The atoms of certain elements readily bond with other atoms to form molecules.
Answer:
Molecules are formed when one or more atom of an element/elements are chemically joined together to create a new type of matter.
Explanation:
Hope it helped!
can there be an ionic bonding between 2 metals or 2 non-metals?
Answers
Answer:
No
Explanation:
Ionic bonding is where an one atom wants to gain e- while the other wants to lose e-
Such as NaCl.
The photons move in straight lines, but every once in a while, a photon's path is bent after passing through the box. These photons are mostly UV photons, but there were some other colors of photons as well. The photons' paths are bent when they collide into an electron. The electron absorbs energy from the photon, causing the photons to move in a different path than it was before. UV photons are more likely to change paths because they contain more energy than the other colors.
Answers
Photons move in straight lines but sometimes, a photon's path is bent after passing through the box. These photons are usually UV photons, but there were some other colors of photons as well.
The photons' paths are bent when they collide with an electron. The electron absorbs energy from the photon, causing the photons to move in a different path than it was before. UV photons are more likely to change paths because they contain more energy than the other colors. What is happening here is a process called Compton scattering. This is when an incoming photon collides with an electron, which causes the photon to scatter at a different angle and lose energy. This process is more likely to occur with photons that have higher energy, such as UV photons. When this happens, the photon's path is bent, and it moves in a different direction than it was before.
In summary, photons usually move in straight lines, but they can be bent when they collide with an electron. This is known as Compton scattering, and it causes the photon to scatter at a different angle and lose energy. UV photons are more likely to change paths because they contain more energy than other colors.
Learn more about Photons at https://brainly.com/question/15946945
#SPJ11
HELP PLEASE!
The more bonds an atom can make, the more likely it is to combie with other atoms in different ways.
Which element is most likely able to make the greatest variety of bonds?
a. nitrogen
b. hydrogen
c. oxygen
d.carbon
Answers
Answer: a. nitrogen
Explanation:
Nitrogen comprises of five valence electrons in its outermost shell which enables it to form triple, double and single bonds with other atoms. It can share three electrons with other nitrogen atom and form a covalent bond. Moreover, nitrogen can form multiple bonds with itself and with other elements due to its small size and high electronegativity. Nitrogen, carbon, fluorine, and oxygen are the elements with which nitrogen can form bonding.
explain how you would find the number of moles that are rpresented by a certaib nunver if reoresnetatuce oartuckes
Answers
To find the number of moles represented by a certain number of particles, you can use Avogadro's number and the concept of molar mass.
Avogadro's number (symbolized as N<sub>A</sub>) is a fundamental constant that represents the number of particles (atoms, molecules, ions, etc.) in one mole of a substance. It is approximately equal to 6.022 × 10²³ particles/mole.
Molar mass is the mass of one mole of a substance and is expressed in grams/mole. It represents the sum of the atomic masses or molecular masses of the constituent particles in a substance.
To calculate the number of moles, you can follow these steps;
Determine the number of particles you have (atoms, molecules, ions, etc.).
Identify the molar mass of the substance or the average molar mass if it's a mixture.
Divide the number of particles by Avogadro's number to convert them into moles. The formula is:
Moles = Number of Particles / Avogadro's number
Moles = Number of Particles / (6.022 ×10²³ particles/mole)
The result will give you the number of moles represented by the given number of particles.
To know more about Avogadro's number here
https://brainly.com/question/24175158
#SPJ4
--The given question is incorrect, the correct question is
"Explain how you would find the number of moles that are represented by a certain number of representative particles."--
How many moles are there in 135g of aluminum ?
Answers
Answer:
The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles Aluminium, or 26.981538 grams.
A second container of NaOH of unknown concentration shows up at your lab station. This time you are wary of the concentration and decide to titrate with 45.0 mL of 3.5MHCl. You that it takes only 16.57 mL of NaOH to reach the endpoint, yeesh. What is the concentration of the unknown NaOH in M ? (Hint: find your sig figs at the end)
Answers
The concentration of the unknown sodium hydroxide in M is 9.5.
On titrating the solution of sodium hydroxide and hydrochloric acid, we will be using the following relation for molarity and concentration -
Molarity × volume of HCl = Molarity × volume of NaOH
Keep the values in stated equation to find the concentration of sodium hydroxide-
3.5 × 45 = M × 16.57
Rewriting the equation according to molarity of sodium hydroxide
M = 3.5 × 45/16.57
Performing multiplication and division
M = 9.5 M
Hence, the concentration is 9.5 M.
Learn more about Molarity -
https://brainly.com/question/30404105
#SPJ4
when enzyme action stops due to a buildup of end product this control is called A. negative feedback. B. competitive inhibition. C. enzyme induction. D. enzyme repression.
Answers
The control mechanism you're referring to, where enzyme action stops due to a buildup of end product, is called A. negative feedback.
Negative feedback occurs when the accumulation of an end product inhibits the initial enzyme responsible for its production. This process helps maintain the optimal levels of substances within the cell and prevents overproduction. Here's a step-by-step explanation:
1. An enzyme catalyzes a reaction, leading to the formation of a product.
2. As the product accumulates, it reaches an optimal level within the cell.
3. When the optimal level is reached, the end product binds to the enzyme or its regulatory site, decreasing the enzyme's activity.
4. As a result, the production of the end product slows down, maintaining a balance within the cell.
This process ensures that resources and energy are not wasted in producing excess product and helps maintain homeostasis within the cell.
Learn more about Negative feedback here:
brainly.com/question/31105398
#SPJ11
How many protons z and how many neutrons n are there in a nucleus of the most common isotope of silicon?
Answers
The number of protons and the number of neutrons is 14.
The most common isotope of silicon is silicon 28 with the mass number=28 and atomic number=14.
An atomic number is defined as the number of protons or the number of electrons present in the given element. The atomic number is used to define the chemical properties of the elements. The atomic number of silicon is 14. Hence the number of protons is 14.
The mass number is defined as the combined total of the number of protons and the number of neutrons present in the given element. The mass number of silicon is 28. Hence the number of neutrons=28-14=14.
To know more about isotopes, Visit
https://brainly.com/question/14220416
#SJP4
A 22. 5 mL solution of H2SO4 solution is titrated with a 0. 105 M NaOH solution. If 43. 2 mL of the titrant is required to reach the equivalence point, determine the molarity of the H2SO4 solution. 0. 0273 M 0. 101 M 0. 403 M 0. 202 M What is the enthalpy of formation of SO3(g)? 2 SO2(g) + O2(g) → 2 SO3(g) ΔH°rxn = -198 kJ ΔH°f, kJ/mol SO2(g) = −297 −396 kJ/mol −495 kJ/mol −792 kJ/mol −575 kJ/mol
Answers
The molarity of the H₂SO₄ solution is 0.101 M. The enthalpy of formation of SO₃(g) is 396 kJ/mol.
To determine the molarity of the H₂SO₄ solution, we can use the concept of stoichiometry and the volume and concentration of the NaOH solution used in the titration.
Given:
Volume of H₂SO₄ solution = 22.5 mL
Volume of NaOH solution used = 43.2 mL
Concentration of NaOH solution = 0.105 M
First, we need to determine the moles of NaOH used in the titration. We can do this by multiplying the volume of NaOH solution used by its concentration:
Moles of NaOH = (43.2 mL) x (0.105 mol/L) = 4.536 mol
According to the balanced equation for the reaction between H₂SO₄ and NaOH, the stoichiometric ratio is 1:2. This means that 1 mole of H₂SO₄ reacts with 2 moles of NaOH.
Therefore, the moles of H₂SO₄ in the solution would be half of the moles of NaOH used:
Moles of H₂SO₄ = (1/2) x (4.536 mol) = 2.268 mol
Now, we can calculate the molarity of the H₂SO₄ solution by dividing the moles of H₂SO₄ by the volume of the solution in liters:
Molarity of H₂SO₄ = (2.268 mol) / (22.5 mL / 1000 mL/L) = 0.101 M
Therefore, the molarity of the H₂SO₄ solution is 0.101 M.
Regarding the enthalpy of formation of SO₃(g), the given enthalpy of reaction (ΔH°rxn) for the reaction 2 SO₂(g) + O₂(g) → 2 SO₃(g) is -198 kJ.
The enthalpy of formation (ΔH°f) of a compound is the enthalpy change when one mole of the compound is formed from its constituent elements in their standard states.
Given:
ΔH°f, kJ/mol SO₂(g) = -297 kJ/mol
To determine the enthalpy of formation of SO₃(g), we can use the following equation:
ΔH°rxn = ΣΔH°f (products) - ΣΔH°f (reactants)
Since we have the ΔH°rxn and the enthalpy of formation of SO₂(g), we can rearrange the equation and solve for ΔH°f of SO₃(g):
ΔH°f (SO₃) = ΣΔH°f (products) - ΣΔH°f (reactants) + ΔH°rxn
ΔH°f (SO₃) = 0 kJ/mol (since it is a product) - 2 x (-297 kJ/mol) + (-198 kJ/mol)
ΔH°f (SO₃) = 594 kJ/mol + (-198 kJ/mol) = 396 kJ/mol
Therefore, the enthalpy of formation is 396 kJ/mol.
Learn more about The enthalpy of formation here:
https://brainly.com/question/29888663
#SPJ11
Chemistry is study of:
A. Living systems
B. The stars and planets
C. All matter
D. Reactions in a test tube
Answers
Answer:
C
Explanation:
Living systems is the study of biology. Not A
The stars and planets is the study of astronomy. Not B
The answer is C
D is too confining. Chemistry does not always take place in a test tube.
beeans beans beans beans beans!!
Answers
Answer:
points go brrrrrr
Explanation:
Answer:
Beans.
Explanation:
Which of the following reactions would have a decrease in entropy?
A. 2NO2(g) → N₂04(9)
B. 2SO3(g) →2SO₂(g) + O₂(g)
C. 2NH3(g) → N₂(g) + 3H₂(g)
OD. 2H₂O(g) → 2H₂(g) + O₂(g)
Answers
the reactions would have a decrease in entropy is 2NO2(g) → N₂04(9).
What is entropy's straightforward definition?The quantity of thermal energy each unit of temperature in a system that cannot be used to carry out beneficial work is known as entropy. While work is produced by ordered molecular motion, entropy also serves as a proxy for a system's molecular disorder or unpredictability.
In the actual world, what is entropy?All parts of our daily life are impacted by entropy, which is merely a measure of disorder. In actuality, it can be considered nature's tax. Disorder spreads over time if left uncontrolled. Systems collapse into chaos when energy disperses. Something is considered to be more entropic the more disorganized it is.
To know more about entropy visit:
https://brainly.com/question/13999732
#SPJ1
Determine the partial pressure and number of moles of each gas in a 16.75L vessel at 30 degree C containing a mixture of xenon and neon gases only. The total pressure in the vessel is 7.10 atm, and the mole fraction of xenon is 0.721.
What is the partial pressure of xenon?
What is the partial pressure of neon?
What is the number of moles of xenon?
What is the number of moles of neon?
Answers
First, we will calculate the number of moles of mixture of Xenon and Neon gases.Number of moles of mixture of Xenon and Neon gases:
Let x be the mole fraction of Neon.
Therefore, (1 - x) is the mole fraction of Xenon
.Mole fraction of Neon + Mole fraction of Xenon = 1x + (1 - x) = 1x = 1 - (1 -
x = 0 + x
x = 0.279
Mole fraction of Neon = 0.279
Mole fraction of Xenon = 0.721
Number of moles of gas = (Total Pressure * Volume)/(Gas Constant * Temperature)
Number of moles of Xenon = (7.10 atm * 16.75L * 0.721)/(0.08206 * (273 + 30))
Number of moles of Xenon = 8.44 moles
Number of moles of Neon = (7.10 atm * 16.75L * 0.279)/(0.08206 * (273 + 30))
Number of moles of Neon = 3.29 moles
Now, we can calculate the partial pressure of Xenon and Neon.
Partial pressure of Xenon:
Partial Pressure of Xenon = Mole fraction of Xenon * Total Pressure
Partial Pressure of Xenon = 0.721 * 7.10 atm
Partial Pressure of Xenon = 5.12 atm
Partial pressure of Neon
Partial Pressure of Neon = Mole fraction of Neon * Total Pressure
Partial Pressure of Neon = 0.279 * 7.10 atm
Partial Pressure of Neon = 1.98 atm
Learn more about atoms at
https://brainly.com/question/33049833
#SPJ11
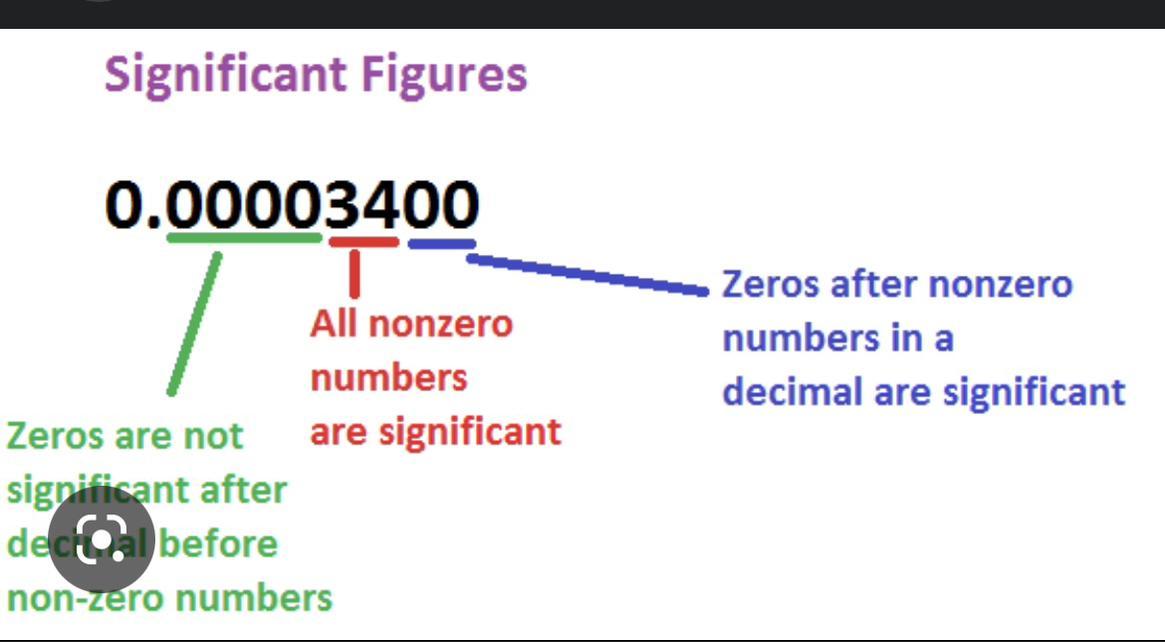
how would you round 34.9279 if there was 3 sig figs and 4 sig figs
Answers
Consider the Zn(s) + Cu*2(aq) ➜>> Zn+2(aq) + Cu(s) system.
The electron donor is the__
agent.
Choices:reducing, oxidizing
Answers
The electron donor in the Zn(s) + Cu^2+(aq) —>Zn^2+(aq) + Cu(s) system is zinc (Zn) which is being oxidized. Therefore, the electron donor in this system is the reducing agent.
CALCULATE THE RMM OF , CA(OH)2, AL2SO4.
Answers
Answer:
The RMM of Ca (OH)2 is
40+ (16+1)2
40+(17)2
40+34
74
The RMM of Al2SO4 is
(27×2)+32+(16×4)
54+32+64
=150
Explanation:
Note that the RAM of:
Ca=40
O=16
H=1
Al=27
S=32
Which is a diatomic molecule?
Na-CI
o=O
H-F
Si-O
which one
Answers
(C)
C21H44 can be cracked to produce ethene.
Cz1H44 — 3C2H4 + C15H32
Relative formula mass (M) of C21H44 = 296
Calculate the mass of C21H44 needed to produce 50.4 kg of ethene.
Answers
Answer:
177.3kg C₂₁H₄₄
Explanation:
Based on the chemical reaction:
C₂₁H₄₄ → 3C₂H₄ + C₁₅H₃₂
Where 1 mole of C₂₁H₄₄ produce 3 moles of ethene, C₂H₄.
To solve this question we need to determine the moles of ethene in 50.4kg. 1/3 these moles are the moles of C₂₁H₄₄ that must be added:
Moles Ethene -Molar mass: 28.05g/mol-
50.4kg = 50400g * (1mol / 28.05g) = 1796.8 moles of ethene
Moles C₂₁H₄₄:
1796.8 moles of ethene * (1 mol C₂₁H₄₄ / 3 mol C₂H₄) = 589.93 moles C₂₁H₄₄
Mass C₂₁H₄₄:
589.93 moles C₂₁H₄₄ * (296g / mol) = 177283g =
177.3kg C₂₁H₄₄If 1.0 mole of C8H18 are available to completely react
with enough O2, how many moles of H20 can be produced?
Answers
Answer: 1 mol C8H18 produces 9 mol H2O
Explanation: reaction : C8H18 + 12.5 O2 ⇒ 8 CO2 + 9 H2O
Compare the ability to hold on to / attract electrons between metals and nonmetals.
(Use the concepts and trends about atomic radius and electronegativity.)
Answers
Nonmetals have the ability to attract electrons better than metals because they have a higher electron affinity or electronegativity than metals.
What is electronegativity?Electronegativity is the tendency, or a measure of the ability, of an atom or molecule to attract electrons and thus form bonds.
An element in the periodic table with a high electronegativity will automatically have a high electron affinity.
Metals (low electronegativity) are known to lose electrons to non-metals (high electronegativity), hence, nonmetals have the ability to attract electrons better than metals because they have a higher electron affinity or electronegativity than metals.
Learn more about electronegativity at: https://brainly.com/question/2060520
#SPJ1
2. Many calculations in chemistry will involve certain symbols, such as the symbol triangle or delta
What does this symbol stand for or mean?
A. "The total"
B. "The change in"
C. "The unit"
D. "The formula"

Answers
B. "The change in"
I saw that symbol a lot in chemistry and physics so I immediately recognized it.
B. The change in
The change in speed that could occur in 4 seconds in an object with an acceleration of 6 m/s/s.
Answers
Answer:
24m/s
Explanation:
a=change of v/change of t
6m/s^2=v/4s
multiply both sides by 4s
v=24m/s