8) 50 pounds is equal to 22,700 grams, would you be able to carry out that backpack filled with gold? How many pounds does this backpack full of gold weigh?
Answers
answer: 454
explanation: divide 22,700 and 50
Related Questions
Which of the following statements is related to a postulate (assumption) of the kinetic molecular theory of gases?
Gas particles are in constant motion because they are strongly attracted to each other.
O Gas particles are in constant motion and are very far apart compared to their size.
O Gas particles are in constant motion, giving them a large amount of potential energy.
Gas particles are in constant motion so they lose kinetic energy when they collide with the walls of their container.
Answers
The statements are related to a postulate of the kinetic molecular theory of gases is gas particles are in constant motion and are very far apart compared to their size. Therefore, option B is correct.
What is kinetic molecular theory ?According to kinetic molecular theory, gas particles are in constant motion and have perfectly elastic collisions. Both Charles' and Boyle's laws can be explained using kinetic molecular theory. The average kinetic energy of a gas particle collection is only proportional to absolute temperature.
The kinetic-molecular theory is a theory that explains matter states and is based on the idea that matter is made up of tiny particles that are constantly in motion. The theory contributes to the understanding of observable properties and behaviors of solids, liquids, and gases.
Gas particles are in constant motion and are very far apart in comparison to their size, according to a postulate of the kinetic molecular theory of gases.
Thus, option B is correct.
To learn more about the kinetic-molecular theory, follow the link;
https://brainly.com/question/17121882
#SPJ6
what determines the role of an organism in an ecosystem?

Answers
Answer:
An organism's role within an ecosystem depends on how it obtains its food. Plants and animals obtain their food in very different ways, so they have very different roles in an ecosystem. The way in which an organism obtains food also affects its interactions with other organisms in the ecosystem.
Explanation:
how many moles of h2o contain 4.02 × 1022 atoms of hydrogen?
Answers
0.0334 moles of H2O contain 4.02 × 1022 atoms of hydrogen.
To find out the number of moles of H2O that contain 4.02 × 1022 atoms of hydrogen, we will use Avogadro's constant and stoichiometry.
Avogadro's constant is a measure of the number of particles present in a mole of a substance. It has a value of 6.022 × 1023 particles/mol.
The stoichiometric ratio of hydrogen to water is 2:1. This means that 2 moles of hydrogen react with 1 mole of water. Water's molecular composition can be represented by the formula H2O.
Therefore, the number of moles of hydrogen atoms present in 4.02 × 1022 atoms of hydrogen is given by:
4.02 × 1022 atoms of hydrogen × 1 mol/6.022 × 1023 atoms = 0.0668 moles of hydrogen atoms
Since the stoichiometric ratio of hydrogen to water is 2:1, the number of moles of water that contains 0.0668 moles of hydrogen atoms is given by:
0.0668 moles of hydrogen atoms × 1 mol of water/2 moles of hydrogen atoms = 0.0334 moles of water
Therefore, 0.0334 moles of H2O contain 4.02 × 1022 atoms of hydrogen.
Learn more about hydrogen at: https://brainly.com/question/24433860
#SPJ11
This question is about methanol
Methanol is broken down in the body during digestion.
What type of substance acts as a catalyst in this process?
Amino acid
Enzyme
Ester
Nucleotide
Answers
Which of the following correctly completes the statement?
Cations are always _____ than the parent atom and anions are always _____ than the parent atom.
Answers
Answer: smaller, larger
Explanation:
Cations are always {smaller} than the parent atom. Anions are always {larger} than the parent atom.
Cations are always smaller than the parent atom, and anions are always greater than the parent atom.
What is parent atom ?The term "parent atom" refers to an atom that has not yet undergone any chemical transformation. A parent atom is one that is discovered in its initial state.
Because they contain fewer electrons while maintaining the same nuclear charge, cations are always smaller than their parent atoms. Because the protons in the nucleus are holding the remaining electrons more tightly, their radii are less than those of the parent atoms. In the case of anion, this is the opposite.
Due to variations in electron-electron repulsion, the ionic radii of cations and anions are always less or bigger than the parent atom, respectively. The trends in ionic radius follow those in atomic size.
Thus, Cations are always smaller than the parent atom, and anions are always greater than the parent atom.
To learn more about parent atom, follow the link;
https://brainly.com/question/13673451
#SPJ6
3. How many grams are in 9.015 x 1035 atoms of Cobalt?
Answers
Answer:
8.822 × 10¹³ g Co
General Formulas and Concepts:
Chemistry - Atomic Structure
Reading a Periodic TableUsing Dimensional AnalysisAvogadro's Number - 6.022 × 10²³ atoms, molecules, formula units, etc.Explanation:
Step 1: Define
9.015 × 10³⁵ atoms Co
Step 2: Identify Conversions
Avogadro's Number
Molar Mass of Co - 58.93 g/mol
Step 3: Convert
\(9.015 \cdot 10^{35} \ atoms \ Co(\frac{1 \ mol \ Co}{6.022 \cdot 10^{23} \ atoms \ Co} )(\frac{58.93 \ g \ Co}{1 \ mol \ Co} )\) = 8.82189 × 10¹³ g Co
Step 4: Check
We are given 4 sig figs. Follow sig fig rules and round.
8.82189 × 10¹³ g Co ≈ 8.822 × 10¹³ g Co
What is the main point of the reading? Group of answer choices Many elements combine to make a few compounds. Hawaii is made up of only a few types of compounds A few elements combine to make many compounds. Elements cannot be combined except by artificial means.
Answers
Answer:
A few elements combine to make many compounds.
Explanation:
The heading of the reading is 'Few elements, many Compounds'. The reading has focused on the process involved in the formation of elements and compounds. In the Hawaiian Islands the elements are found in abundance. These elements combine together to form compounds. The mountains, greenery, rocks and living matter are made up of many elements and compounds. The elements present helps in forming various compounds.
What element blackens when exposed to oxygen?
Answers
Answer:
i believe lithium
Explanation: i hope this is right.
X-ray crystallography can only be used for structure determination if the wavelength of the X-ray photon (λ) is on the order of the lattice constant (d). Selectone: True False
Answers
The statement "X-ray crystallography can only be used for structure determination if the wavelength of the X-ray photon (λ) is on the order of the lattice constant (d)" is false because X-ray crystallography is used to determine the atomic arrangement of crystals, and it does not depend on the wavelength of the X-ray photon.
X-ray crystallography is a technique used to determine the structure of crystals by shining X-rays through the crystal. The diffraction pattern generated by the X-rays is then analyzed to determine the atomic arrangement of the crystal. Therefore, X-ray crystallography is used to determine the atomic arrangement of crystals, and it does not depend on the wavelength of the X-ray photon. Rather, the resolution of the structure determination is affected by the wavelength of the X-ray radiation.
Learn more about X-ray crystallography: https://brainly.com/question/14617319
#SPJ11
Question 11
Which formula represents a hydrocarbon?
C₂H6
C₂H5OH
C₂H5Cl
C₂H6O
Answers
Answer:
C₂H6
Explanation:
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). Option A
A hydrocarbon is a compound that consists of only carbon and hydrogen atoms. It is important to identify the formula that represents a hydrocarbon among the given options:
A) C₂H6: This formula represents ethane, which is a hydrocarbon. Ethane consists of two carbon atoms bonded together with single bonds and six hydrogen atoms.
B) C₂H5OH: This formula represents ethanol, which is not a hydrocarbon. Ethanol contains a hydroxyl group (-OH), indicating the presence of oxygen in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
C) C₂H5Cl: This formula represents ethyl chloride, which is not a hydrocarbon. Ethyl chloride contains a chlorine atom (Cl) in addition to carbon and hydrogen atoms. It is a haloalkane, not a hydrocarbon.
D) C₂H6O: This formula represents ethanol, which, as mentioned before, is not a hydrocarbon. Ethanol contains an oxygen atom (O) in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). It consists only of carbon and hydrogen atoms, making it a suitable representation of a hydrocarbon.
In summary, the formula C₂H6 (option A) represents a hydrocarbon, while the other options contain additional elements (oxygen or chlorine) that make them non-hydrocarbon compounds. Option A
For more such questions on hydrocarbon visit:
https://brainly.com/question/21281906
#SPJ8
In the diatomic molecule HCl, the H and the Cl share a pair of
electrons. By doing so, the hydrogen atom attains the electron
configuration of while chlorine attains the electron
configuration of
Answers
Answer:
helium and argon
Explanation:
The helium dimer is a van der Waals molecule containing two helium atoms with the formula He2. This chemical is the biggest diatomic molecule, which is a molecule made up of two atoms that are bound together. This dimer's connection is so weak that it will break if the molecule spins or vibrates too much.The noble gases (helium, neon, argon, krypton, xenon, and radon) are also monatomic gases at STP. To distinguish them from other gases that are chemical compounds, homonuclear diatomic gases and noble gases are referred to as "elemental gases" or "molecular gases."Which of the following has the highest electromagnivity
Answers
Answer:
Where are the answer choices
?
In the Haber Process, Ammonia is created. The balanced reaction is as follows: 3H2 + N2 --> 2NH3 What type of reaction is this?
Decomposition
Single Replacement
Synthesis
Double replacement
Answers
In the Haber Process, Ammonia is created. The balanced reaction is as follows: 3H2 + N2 ⇒ 2NH3. The reaction is decomposition reaction. Therefore, option A is correct.
What is decomposition reaction ?A decomposition reaction occurs when a compound is broken down into two or more simpler substances. A decomposition reaction has the general form: ABA+B. The majority of decomposition reactions require an energy input in the form of heat, light, or electricity.
Carbonates are broken down into carbon dioxide and an oxide. Chlorate is broken down into oxygen gas and chloride. Water and an oxide are formed when hydroxides decompose. Water and a molecular oxide are formed when oxygen-containing acids decompose.
Thus, option A is correct.
To learn more about the decomposition reaction, follow the link;
https://brainly.com/question/16987748
#SPJ1
Which statement correctly compares what occurs when molecules absorb photons in the microwave region with what occurs when molecules absorb photons in the infrared region?
Microwave photons cause the molecules to increase their rotational energy states, whereas infrared photons cause the molecules to increase their vibrational energy states.
Microwave photons cause the molecules to increase their rotational energy states, whereas infrared photons cause the molecules to increase their vibrational energy states.
A
Microwave photons cause electrons in the molecules to increase their electronic energy states, whereas infrared photons cause the molecules to increase their rotational energy states.
Microwave photons cause electrons in the molecules to increase their electronic energy states, whereas infrared photons cause the molecules to increase their rotational energy states.
B
Microwave photons cause the molecules to increase their vibrational energy states, whereas infrared photons cause electrons in the molecules to increase their electronic energy states.
Microwave photons cause the molecules to increase their vibrational energy states, whereas infrared photons cause electrons in the molecules to increase their electronic energy states.
C
Microwave photons cause the molecules to increase their rotational energy states, whereas infrared photons cause electrons in the molecules to increase their electronic energy states.
Answers
d.) Microwave photons cause the molecules to increase their rotational energy states, whereas infrared photons cause electrons in the molecules to increase their electronic energy states.
Explanation:
Microwave: transitions in the molecular rotational levels
Infrared: transitions in molecular vibrational levels
UV/Visible: transitions in electronic energy levels.
what is a major consequence of the partial double bond character?
Answers
The amide group in the peptide link is planar due to the partial double bond, which causes them to be in either a cis or a trans conformation.
In an organic molecule, resonance results in partial double bonds. The partial double bond feature results from the pi electrons moving from one p-orbital to another. The peptide bonds that exist between the amino acids in proteins are a crucial illustration of this phenomenon. The resonance of amides is what gives the peptide bond its stability.
Resonance enables the nitrogen to shift electrons from the carbonyl double bond towards the oxygen, generating the oxygen anion, and transfer its single pair of electrons to the carbonyl carbon. The and bonds acquire a partial double bond nature as a result.
To know more about the partial double bond character
https://brainly.com/question/14331051
#SPJ4

a change in the composition of matter _____________ occurs during a chemical reaction.
Answers
Answer: During a chemical change, the composition of matter always changes
Explanation:
Can someone help me here please i need this :(
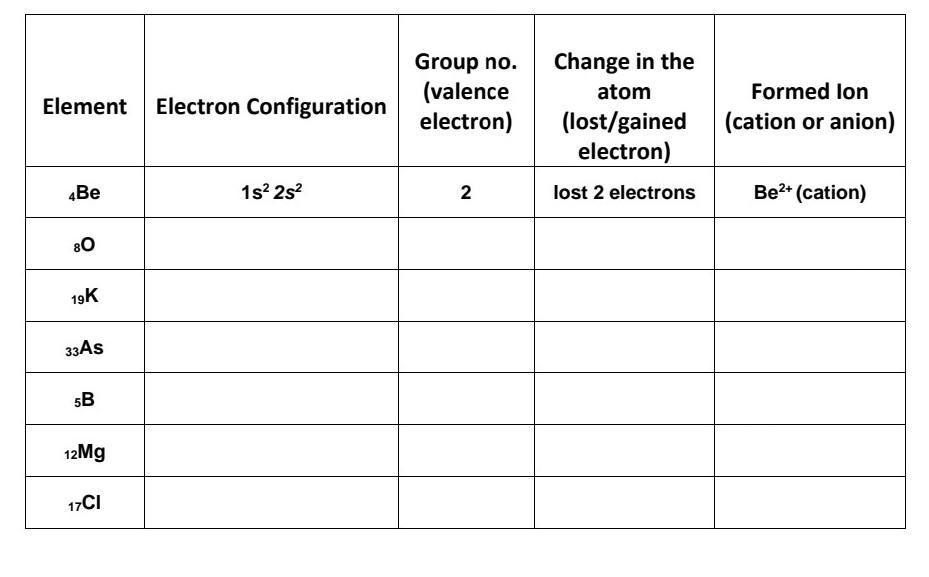
Answers
2Al + 6HCl → 2AlCl3 + 3H2
If the chemical reaction produces 129 grams of AlCl3, how many grams of H2 are also produced?
Answers
Answer: The reaction produces 2.93 g H₂.
M_r: 133.34 2.016
2Al + 6HCl → 2AlCl₃ + 3H₂
Moles of AlCl₃ = 129 g AlCl₃ × (1 mol AlCl₃/133.34 g AlCl₃) = 0.9675 mol AlCl₃
Moles of H₂ = 0.9675 mol AlCl₃ × (3 mol H₂/2 mol AlCl₃) = 1.451 mol H₂
Mass of H₂ = 1.451 mol H₂ × (2.016 g H₂/1 mol H₂) = 2.93 g H₂
Explanation:
Give an example of how knowledge of physical properties of matter can be used in everyday life
Answers
Understanding physical properties of matter is essential in everyday life for a variety of purposes, from cooking to choosing materials.
Knowledge of physical properties of matter is extremely important in everyday life as it helps us understand the nature of substances we come into contact with. One example is the use of boiling points in cooking. Different substances have different boiling points which determine the temperature at which they boil. This information is crucial in determining cooking times and ensuring that food is cooked properly.
For instance, water boils at 100 degrees Celsius, while sugar syrup boils at a much higher temperature. If the wrong temperature is used, food may be undercooked or overcooked, leading to undesired outcomes. Knowledge of physical properties also helps in choosing the right materials for different purposes, such as choosing heat-resistant materials for cooking.
In conclusion, understanding physical properties of matter is essential in everyday life for a variety of purposes, from cooking to choosing materials.
To know more about matter visit:
brainly.com/question/28487167
#SPJ11
how does sustainable development support in a long term vision
Answers
Explanation:
meet .google .com/sxr-wgwg-vnc
What best explains the reason Jeff sees a double image of the trees on the water
Answers
Answer:
Nerve or muscle damage in the eye might cause double vision. Each eye creates its own image of the environment. The brain combines the representations from each eye and perceives them as one clear picture. Damage to the muscles that move the eyes or the nerves that control eye movement can create a double image.
Explanation:
A formula that shows how many atoms of each element a substance contains is called a(n) ____.
A. ionic formula
B. molecular formula
C. structural formula
D. empirical formula
Answers
Answer:
c
Explanation:
A formula that shows how many atoms of each element a substance contains
Which has the greatest number of hydrogen atoms? group of answer choices 1020 hydrogen atoms 100 g of a substance that is 2% h by mass 100 g of water 20 g of hydrogen gas
Answers
20gm of hydrogen will have highest number of hydrogen atoms.
We know that 1 mole of substance contains atoms equal to Avogadro number i.e. \(6.022*10^{23}\)
As molar mass of hydrogen is 1gm there 1 mole of hydrogen contains 1gm of hydrogen and \(6.022*10^{23}\) hydrogen atoms
If we take 1020 hydrogen atoms then it is very less than \(6.022*10^{23}\) hydrogen atoms.
In 100gm of substance if hydrogen is 2% by mass i.e. we have 2gm of hydrogen atom which means 2 mole of hydrogen atoms
Mass of 1 mole of water is 18gm
Thus 100 gm of water have 5.55 moles of water
In 1 mole of water we have 2 grams of hydrogen
Therefore 5.55 moles of water contains 11.11gm of water i.e. 11.11 mole of water
11.11gm of water = \(11.11* 6.022 * 10^{23}\) atoms
In 20gm of hydrogen we have 20 mole of hydrogen
i.e. \(20* 6.022 * 10^{23}\)
To know more about Mole concept
https://brainly.com/question/2350371
#SPJ4
What are the 4 different types of bonds and how are they formed?
Answers
There are four types of chemical bonds essential for life to exist: Ionic Bonds, Covalent Bonds, Hydrogen Bonds, and van der Waals interactions. We need all of these different kinds of bonds to play various roles in biochemical interactions. These bonds vary in their strengths.
To play a variety of roles in biochemical interactions, we require all of these diverse sorts of linkages. The tensile strength of these linkages varies. In chemistry, we consider the range of strengths between ionic and covalent bonds to be overlapping. This indicates that in water, ionic bonds usually dissociate. As a result, we shall consider these bonds from strongest to weakest in the following order:
Covalent is followed by ionic, hydrogen, and van der Waals.
To know more about 4 different types of bonds, visit;
brainly.com/question/17401243
#SPJ4
What type of energy transformation occurs during photosynthesis?
Answers
Answer: Photosynthesis is the process by which energy is converted to chemical energy in plant cells. In cellular respiration plants use the chemical energy stored during photosynthesis in basic life processes. During both photosynthesis and cellular respiration, energy is converted.
Explanation:
HELP MEEEEEE PLEASEEEEE WILL OFFER BRAINLIEST

Answers
Answer:
1.This answer would be option A.
2. I think that would be 1350
Explanation:
The model shows the first three periods of the Periodic Table. Which statement BEST describes the trend in the number of protons and valence electrons across a period?
A. Across a period, elements gain a proton and lose a valence electron.
B. Across a period, elements gain a proton and gain a valence electron.
C. Across a period, elements lose a proton and gain a valence electron.
D. Across a period, elements lose a proton and lose a valence electron.

Answers
Moving across the period the elements gain a proton and gain a valence electron.
What is an element and why elements gain proton across the period?An element is the building block of inorganic chemistry , the basic fundamental unit of compounds.There are total of 118 elements discovered yet , out of which the 118th element is orgasson.Moving across the period that is from left to right the size of element decreases because of the addition of protons.Moving across the period the nucleus have increased capacity of attraction.With increase in the positive charge the nucleus gains a proton and a valence electron.To know more about periodic table visit:
https://brainly.com/question/11155928
#SPJ13
which of the following statements are true of both acids and bases? this question will not give partial credit. question 1 options:when dissolved in water, they can conduct electricity they produce ions in solution they are electrolytes they dissolve in waterthey react with metalsthey contain more hydrogen ions than hydroxide ions
Answers
The reactions between bases and acids don't happen. Electrolytes are substances that dissolve in water (or another polar solvent) to form an electrically conducting solution.
When dissolved in water, can ions conduct electricity?Because they can move, ions can ionise in solutions. Because conductivity in water is caused by electricity moving back and forth between ions, it increases as salt (Na+) or chlorine (Cl-) combine to form table salt in saltwater.
What is simultaneously an acid and a base?A substance can function as both a Brnsted base and an acid. The table above has entries for H2O, OH, HSO4, and NH3 in both columns, for instance. Water serves as the ideal illustration of this behaviour because, when it creates the H3O+ or OH- ions, it functions both as an acid as well as a base.
To know more about Electrolytes visit:
https://brainly.com/question/29771118
#SPJ1
4. Explain the rock in a bucket of water analogy. What type of weather does this cause?
Answers
Answer:
The rock in a bucket of water analogy is used to describe how air masses interact. When a warm air mass is placed over a cold air mass, it is like a rock being placed in a bucket of water. The warm air mass will initially rise, creating an area of low pressure. The cold air mass will then rush in to fill the area of low pressure, creating a storm. This type of weather causes thunderstorms, heavy rains, and high winds.
Please help ASAP
Calculate the acceleration of this object. Remember, acceleration = net force / mass
Question 4 options:
1.7 m/s2
17,000 m/s2
2.3 m/s2
2 m/s2
Answers
Answer:
Put the actual question in the comments and I’ll solve it.
Explanation: