Answers
Answer:
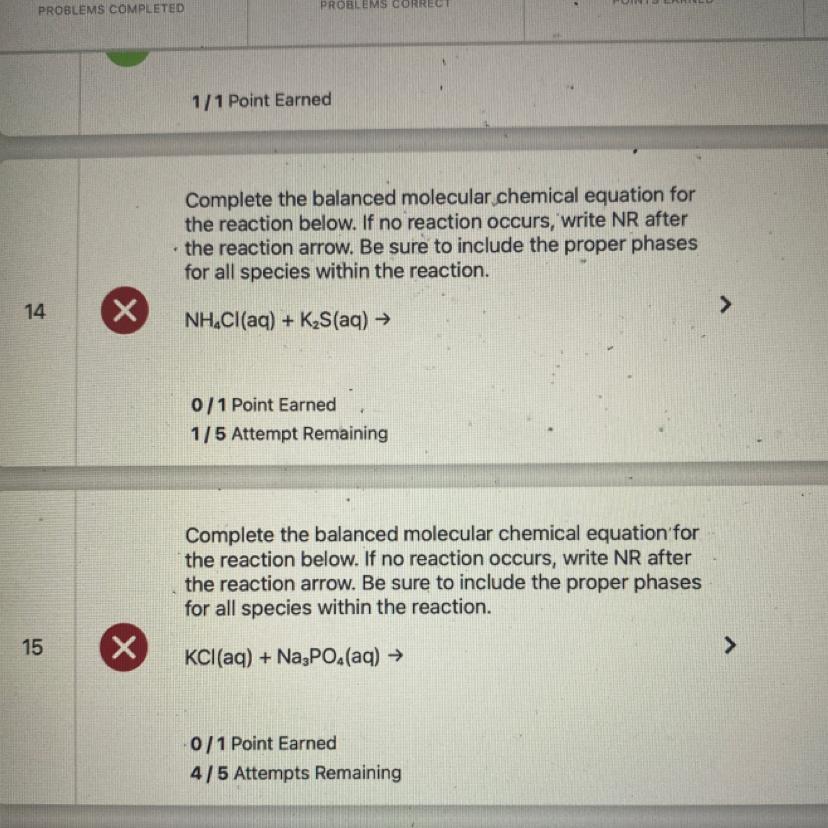
14. 2NH4Cl(aq) + K2S(aq) => 2KCl(aq) + (NH4)2S(aq)
15. 3KCl(aq) + Na3PO4(aq) = 3NaCl(aq) + K3PO4(aq)
Explanation:
14. 2NH4Cl(aq) + K2S(aq) => 2KCl(aq) + (NH4)2S(aq)
NH4 = 2
Cl = 2
K = 2
S = 1
15. 3KCl(aq) + Na3PO4(aq) = 3NaCl(aq) + K3PO4(aq)
K = 3
Cl = 3
Na = 3
PO4 = 1
Related Questions
please help ASAP!!
Determine the volume of 1.5 mol of butane gas if a 2.5 mol
sample of butane has a volume of 38.5 L. Assume
temperature and pressure are kept constant.
Answers
Answer: 23.1 L
Explanation: To solve this problem, you can use the ideal gas law, which states that the volume of a gas is directly proportional to the number of moles of gas, as long as the temperature and pressure are kept constant. This means that if you double the number of moles of gas, the volume will also double.
You can set up the following proportion:
(volume of 1.5 mol of butane) / (volume of 2.5 mol of butane) = (1.5 mol) / (2.5 mol)
Solving for the volume of 1.5 mol of butane, we find that it is equal to (1.5 mol) / (2.5 mol) * (38.5 L) = 23.1 L.
Therefore, the volume of 1.5 mol of butane gas at constant temperature and pressure is approximately 23.1 L.
Which of these gases will have the greatest density at the same specified temperature and pressure?
A. H2
B. CO 2
C. CCIF 3
D. C2H6
E. CF 4
Answers
Answer:
Neon
The highest density among the inert gases is of Neon (Ne). This a factual data. Thus the highest density among given options is of Ne, as all the options are of inert gases.
Explanation:
hope it helps u
FOLLOW MY ACCOUNT PLS PLS
CClF3 will have the greatest density at the same specified temperature and pressure.
• It is known that one mole of a gas under the standard temperature and pressure occupied 22.4 L of volume.
Now, the density of each of the gas under the similar condition will be,
• H2:
The molar mass is 2 and the volume is 22.4 L. The density is,
\(Density = \frac{mass}{volume}\)
\(=\frac{2}{22.4} \\= 0.089 g/L\)
• CO2:
The molar mass is 44 and the volume is 22.4 L. Now the density is,
\(= \frac{44}{22.4} \\= 1.96 g/L\)
• CClF3:
The molar mass is 104.5 and the volume is 22.4 L. The density is,
\(=\frac{104.5}{22.4} \\= 4.665 g/L\)
• C2H6:
The molar mass is 30 and the volume is 22.4 L. The density is,
\(= \frac{30}{22.4} \\= 1.339 g/L\)
• CF4:
The molar mass is 88 and the volume is 22.4 L. The density is,
\(= \frac{88}{22.4} \\= 3.928 g/L\)
Thus, CClF3 will have the greatest density at the same specified temperature and pressure.
To know more about:
https://brainly.com/question/14036556
How many of the 7 traits of living things have
Answers
Answer:
What do you mean by this?
Explanation:
when adenosine diphosphate (adp) binds an additional phosphate group to create adenosine triphosphate (atp), this type of reaction is considered .
Answers
When adenosine diphosphate (ADP) binds an additional phosphate group to create adenosine triphosphate (ATP), this type of reaction is considered as Phosphorylation.
The terminal oxygen atom of the disphosphate group of ADP attacks the P atom of an inorganic phosphate in a process called phosphorylation. In the biological cell, ATPs are synthesized from ADP through (1) substrate level phosphorylation and (2) oxidative phosphorylation.
Substrate level phosphorylation, which occurs in glycolysis and the citric acid cycle, involves the direct phosphorylation of ADP. This process uses the free energy released from a coupled reaction. On the other hand, oxidative phosphorylation harvests the energy from the proton gradient of the mitochondria.
Learn more about Phosphorylation from the link given below.
https://brainly.com/question/11835776
#SPJ4
Zn+HNO3 --> Zn(NO3)2+H2
PLS ANSWER IT FAST I REALLY NEED IT!!!!
Answers
The given equation represents the reaction between zinc (Zn) and nitric acid (HNO3). The balanced chemical equation for this reaction is : Zn + HNO3 → Zn(NO3)2 + H2Zinc is a metal, and nitric acid is an acid.
This reaction is a redox reaction as the oxidation state of Zinc is changed from 0 to +2, and the oxidation state of Nitrogen in Nitric acid is changed from +5 to +4.The reactants in the equation are zinc and nitric acid. Zinc is a solid metal, while nitric acid is a colorless, corrosive liquid. In this reaction, zinc reacts with nitric acid to form zinc nitrate and hydrogen gas. Zinc nitrate is a white crystalline substance that dissolves in water easily. Hydrogen gas is a colorless, odorless gas.The balanced chemical equation for this reaction is derived by ensuring that the total number of atoms of each element in the reactants is equal to the total number of atoms of the same element in the products. The coefficients in front of each substance show the number of atoms or molecules of each substance needed for the reaction to occur.In this case, one atom of zinc reacts with one molecule of nitric acid to form one molecule of zinc nitrate and one molecule of hydrogen gas.
The reaction between zinc and nitric acid is an exothermic reaction as heat is released during the reaction.The reaction between zinc and nitric acid is an important reaction as it is used in the production of zinc nitrate, which is used in the manufacture of other zinc compounds.
for such more questions on equation
https://brainly.com/question/28818351
#SPJ8
which among the materials have a varying amount of components are they compounds or mixtures
Answers
Answer:
Matter can be broken down into two Zalo categories: pure substances and mixtures. Pure substances are further broken down into elements and compounds. Mixtures are physically combined structures that can be separated into their original components.
What volume of CO2(g), measured at STP is produced if 15.2 grams of CaCO(s) is heated?
Answers
Answer:
Volume = 3.4 L
Explanation:
In order to calculate the volume of CO₂ produced when 15.2 g of CaCO₃ is heated, we need to first write out the balanced equation of the thermal decomposition of CaCO₃:
CaCO₃ (s) + [Heat] ⇒ CaO (s) + CO₂ (g)
Now, let's calculate the number of moles in 15.2 g CaCO₃:
mole no. = \(\mathrm{\frac{mass}{molar \ mass}}\)
= \(\frac{15.2}{40.1 + 12 + (16 \times 3)}\)
= 0.1518 moles
From the balanced equation above, we can see that the stoichiometric molar ratios of CaCO₃ and CO₂ are equal. Therefore, the number of moles of CO₂ produced is also 0.1518 moles.
Hence, from the formula for the number of moles of a gas, we can calculate the volume of CO₂:
mole no. = \(\mathrm{\frac{Volume \ in \ L}{22.4}}\)
⇒ \(0.1518 = \mathrm{\frac{Volume}{22.4}}\)
⇒ Volume = 0.1518 × 22.4
= 3.4 L
Therefore, if 15.2 g of CaCO₃ is heated, 3.4 L of CO₂ is produced at STP.
Determine E° for a galvanic (voltaic) cell if ∆G° = -6.3 kJ/mol and n = 3. (F = 96,500 J/(V・mol))
Answers
The E° for a galvanic cell is 0.000217 volts if ∆G° = -6.3 kJ/mol and n = 3. (F = 96,500 J/(V・mol).
A galvanic cell or voltaic cell, named after the scientists Luigi Galvani and Alessandro Volta, respectively, is an electrochemical cell in which an electric current is generated from spontaneous Oxidation-Reduction reactions. A common apparatus generally consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge or separated by a porous membrane.
E°=ΔG°/-nF= -6.3/-3×96500=0.000217 V.
Learn more about galvanic cell,here:
https://brainly.com/question/30268944
#SPJ1
The chemical formula for magnesium oxide is MgO. A chemist determined by measurements that 0.030 moles of magnesium oxide participate in a chemical reaction. Calculate the mass of magnesium oxide that participates.
Answers
Answer:
1.209g of MgO participates
Explanation:
In this problem, we have 0.030 moles of MgO that participates in a particular reaction.
And we are asked to solve for the mass of MgO that participates, that means, we need to convert moles to grams.
To convert moles to grams we need to use molar mass of the compound:
1 atom of Mg has a molar mass of 24.3g/mol
1 atom of O has a molar mass of 16g/mol
That means molar mass of MgO is 24.3g/mol + 16g/mol = 40.3g/mol
And mass of 0.030 moles of MgO is:
0.030 moles MgO * (40.3g/mol) =
1.209g of MgO participatesHow much of the original energy of the producers is available to an organism in the third trophic level?
Answers
Answer:
Energy is passed up a food chain or web from lower to higher trophic levels.
Explanation:
However, generally only about 10 percent of the energy at one level is available to the next level. This is represented by the ecological pyramid in Figure below.
Answer:
generally only about 10 percent of the energy at one level is available to the next level
Explanation:
thank you
Predict the missing component in the nuclear equation.

Answers
Since what we have is an alpha decay, the missing component is 234/90 Th
What is Alpha decay?Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle, which is a helium nucleus consisting of two protons and two neutrons. During alpha decay, the atomic number of the parent nucleus decreases by two, and the mass number decreases by four.
Apha particles can be stopped by a thin layer of material such as paper or skin, and they do not penetrate very far into matter.
Learn more about Alpha decay:https://brainly.com/question/27870937
#SPJ1
use the ideal gas law to calculate the concentrations of nitrogen and oxygen present in the air at a pressure of 1.0 atm
Answers
The concertation of oxygen is 0.0086 M while the concentration of nitrogen is 0.032 M
What is the concentration?From the ideal gas law, we have
P V = n RT
Let us take it that the volume that the gas occupies is 1 L
Mole fraction of N₂ = 0.78
Mole fraction of O₂ = 0.21
Mole fraction of noble gases = 0.01
We have to keep in mid that air is mere a mixture of the gases mentioned above
1 atm . 1L = n * 0.082L.atm/mol.K * 298K
n = 1 L.atm / 0.082L.atm/mol.K * 298K → 0.0409 moles
We know that;
N₂ moles / Total moles =0.78 * 0.0409 mol = 0.032 moles N₂
O₂ moles / Total moles = 0.21 * 0.0409 mol = 0.0086 moles O₂
Concentration of N₂ = 0.032 moles/ 1 L = 0.032 M
Concentration of O₂ = 0.0086 moles/ 1 L = 0.0086 M
Learn more about ideal gas equation:https://brainly.com/question/15379358
#SPJ1
Missing parts;
Use the ideal gas law to calculate the concentrations of nitrogen and oxygen present in air at a pressure of 1.0 atm and a temperature of 298 K. Assume that nitrogen comprises 78% of air by volume and that oxygen comprises 21%. Express your answers using two significant figures separated by a comma.
Which of the following is a future consequence of using windmills for wind energy?
It can harm birds and species nearby.
Weather affects the quality of wind.
It produces less noise than other energy.
Wind cells are used in isolated locations.
Answers
It can harm birds and species nearby.
What is the consequence of wind mills?Wind mills, also known as wind turbines, are devices that generate electricity from wind energy. While wind energy is a renewable and clean source of energy, the installation and operation of wind turbines can have both positive and negative consequences on the environment and society.
The consequences of wind mills depend on many factors, such as their location, size, design, and the management practices used to mitigate their impacts. Careful planning and management can help to maximize the positive consequences of wind mills while minimizing their negative impacts.
Learn more about wind mills:https://brainly.com/question/17279920
#SPJ1
Ionic compounds do not conduct electricity in the solid state, but do conduct electricity in what 2 states
Answers
Ionic compounds conduct electricity in an aqueous solution and molten state but do not conduct electricity in a solid state.
What are ionic compounds?Ionic compounds can be defined as crystalline solids generated by closely packed ions with opposite charges. An Ionic compound can be described as generally formed when one metal reacts with a non-metal.
In ionic compounds, the ions in the compound are usually held together by ionic bonds. The ions are produced by gaining or losing electrons in order to attain the nearest noble gas configuration.
In a reaction, the metals commonly lose electrons to achieve their complete octet while non-metals gain electrons to get a full octet.
In the solid state, Ionic compounds do not conduct electricity because ions are at fixed positions and do not move.
Learn more about ionic compounds, here:
brainly.com/question/17217225
#SPJ1
Using the periodic table and your knowledge of patterns and trends on the table, which of the following elements is the most reactive?
A. Titanium (Ti, #22)
B. Silicon (Si, #14)
C. Oxygen (0, #8)
D. Argon (Ar, #18)
Answers
Based on the periodic trends in the periodic table, the most reactive element is oxygen; option C
What are periodic trends in the periodic table?Periodic trends in the periodic table refers to the periodic variation in the properties of the elements which is observed in the periodic table as one moves across the periodic table from left to right across a period or down a group in the periodic table.
This regular variation is also known as periodicity in the properties of elements.
Some of the periodic trends observed in the periodic table include:
the reactivity of metals increase down a group and from right to left across a periodthe reactivity of non-metals increase in a group from down to top and from left to right across a period across a period.Considering the most reactive element based on the periodic trends:
Titanium, Ti is a transition metal whose reactivity is intermediate as it is found in between group 2 and 3 of the periodic table
Silicon is metalloid which is not very reactive
Oxygen, O is a very reactive non-metal found in group 6A
Argon, is a noble gas which is almost inert.
Therefore, the most reactive element is oxygen.
Learn more about periodic trends at: brainly.com/question/28161428
#SPJ1
When fossil fuels are burned, they emit carbon dioxide into the atmosphere. After centuries of large amounts of carbon dioxide accumulating in the atmosphere, the earth's temperature increases by 1°C.
What is the connection between increasing carbon dioxide and increasing temperature?

Answers
The connection between increasing carbon dioxide and increasing temperature is: carbon dioxide absorbs heat from the sun and traps it in earth's atmosphere. Since the heat cannot escape, it causes the earth's temperature to increase which is the first option.
When carbon dioxide (CO₂) and other greenhouse gases are present in the atmosphere, they act as a natural blanket, allowing sunlight (solar radiation) to pass through and reach the Earth's surface. Some of this solar radiation is absorbed by the Earth's surface, while the rest is reflected back towards space as heat (infrared radiation). However, greenhouse gases like carbon dioxide have the property of absorbing and re-emitting infrared radiation.
Learn more about fossil fuel here
https://brainly.com/question/2029072
#SPJ1
Which best describes a codon?
Answers
Answer:
Which best describes a codon? 1. a cell structure that gives the master instructions for an organism 2. a segment of DNA that is the basis of heredity in organisms 3. the sequence of three bases that codes for a specific amino acid 4. the basic unit of structure and function of all living things
Answer:
The Correct answer is C
Explanation:
The sequence of three bases which codes for a specified amino acid.
Look back at parts A and B to compare the properties of the unknown elements with the properties of the known
elements. Based on these properties, match each unknown element to its group in the periodic table.
Drag each tile to the correct box.
Tiles
element 1 element 2
Pairs
group 1
group 2
group 11
group 14
group 17
group 18
element 3
element 4
element 5
element 6

Answers
Based on the properties of elements, elements can be arranged into groups in the periodic table as follows:
Group 1 to 3 - metals
Group 14 - non-metals, metalloids, and metals
Group 15 to 18 - non-metals
What are groups and periods in the periodic table?Groups are the names given to the periodic table's columns. In the table, individuals who belong to the same group make bonds of the same kind and have an equal number of electrons in their atoms' outermost shells.
Periods are the horizontal rows found in the periodic table.
Learn more about the periodic table at: https://brainly.com/question/25916838
#SPJ!
Why does increasing the temperature of a chemical reaction speed up the reaction
Answers
Answer:
An increase in temperature typically increases the rate of reaction. An increase in temperature will raise the average kinetic energy of the reactant molecules. Therefore, a greater proportion of molecules will have the minimum energy necessary for an effective collision.
Identify the substance that has formula mass of 133.5amu.
(a) MgCI
b)SCI
c)BCI
D) AICI
Answers
The calculated formula masses to 133.5 amu, we find that the substance with a formula mass closest to 133.5 amu is (d) AlCl3. Therefore, the answer is option D.
To identify the substance with a formula mass of 133.5 amu, we need to calculate the formula mass of each given substance and compare it to 133.5 amu.
(a) MgCl2:
The formula mass of MgCl2 can be calculated by adding the atomic masses of magnesium (Mg) and chlorine (Cl).
Mg: atomic mass = 24.31 amu
Cl: atomic mass = 35.45 amu
Formula mass of MgCl2 = (24.31 amu) + 2(35.45 amu) = 95.21 amu
(b) SCl:
The formula mass of SCl can be calculated by adding the atomic masses of sulfur (S) and chlorine (Cl).
S: atomic mass = 32.07 amu
Cl: atomic mass = 35.45 amu
Formula mass of SCl = 32.07 amu + 35.45 amu = 67.52 amu
(c) BCl:
The formula mass of BCl can be calculated by adding the atomic mass of boron (B) and chlorine (Cl).
B: atomic mass = 10.81 amu
Cl: atomic mass = 35.45 amu
Formula mass of BCl = 10.81 amu + 35.45 amu = 46.26 amu
(d) AlCl3:
The formula mass of AlCl3 can be calculated by adding the atomic mass of aluminum (Al) and 3 times the atomic mass of chlorine (Cl).
Al: atomic mass = 26.98 amu
Cl: atomic mass = 35.45 amu
Formula mass of AlCl3 = 26.98 amu + 3(35.45 amu) = 133.78 amu. Option D
For more such questions on masses visit:
https://brainly.com/question/24191825
#SPJ8
Based on the ideal gas law, there is a simple equivalency that exists between the amount of gas and the volume it occupies. At standard temperature and pressure (STP; 273.15 K and 1 atm , respectively), one mole of gas occupies 22.4 L of volume. What mass of methanol ( CH3OH ) could you form if you reacted 7.82 L of a gas mixture (at STP) that contains an equal number of carbon monoxide ( CO ) and hydrogen gas ( H2 ) molecules?
Answers
You could form approximately 5.8 grams of methanol (CH3OH) from the given gas mixture at STP.
What is the mass?
Using the Ideal gas equation;
n = PV / RT
n = (1 atm * 7.82 L) / (0.0821 L·atm/(mol·K) * 273.15 K)
From the question;
nCO = n / 2
nH2 = n / 2
Then;
n = (1 atm * 7.82 L) / (0.0821 L·atm/(mol·K) * 273.15 K)
n = 0.362 mol
nCO = n / 2 = 0.362 mol / 2 = 0.181 mol
nH2 = n / 2 = 0.362 mol / 2 = 0.181 mol
By stoichiometry;
methanol= nCO = 0.181 mol
Mass of methanol = 0.181 mol * 32.04 g/mol
= 5.8 g
Learn more about Ideal gas equation:https://brainly.com/question/30935329
#SPJ1
Given: S03(g) + H20(1) -> H2SO4(1); AH° = -130. kJ
determine AH° for the following thermochemical equation.
5H2S04(1) -> 5S03(8) + 5H20(1)
Answers
Considering the definition of enthalpy of reaction, the enthalpy change for the reaction is 650 kJ.
Enthalpy of reactionThe enthalpy of a chemical reaction as the heat absorbed or released in a chemical reaction when it occurs at constant pressure. That is, the heat of reaction is the energy that is released or absorbed when chemicals are transformed into a chemical reaction.
Enthalpy in this caseIn this case you want to calculate the enthalpy change of:
5 H₂SO₄ → 5 SO₃ + 5 H₂O
You know the following reaction, with his corresponding enthalpie:
SO₃ + H₂O → H₂SO₄ ΔH = –130 kJ
To obtain the enthalpy of the desired chemical reaction you need 5 moles of H₂SO₄ on reactant side. The given equation has 1 mole of H₂SO₄ on the product side, soit is necessary to locate it on the reactant side (invert it) and multiply it by 5.
When an equation is inverted, the sign of delta H also changes. And since enthalpy is an extensive property, that is, it depends on the amount of matter present, since the equation is multiply by 2, the variation of enthalpy also.
In summary, you know that the enthalpy change is 650 kJ.
Learn more about enthalpy of a chemical reaction:
brainly.com/question/5976752
brainly.com/question/13707449
#SPJ1
In which stage do cells spend most of their time?
Interphase
Answers
Answer:
Interphase
Explanation:
I think you answered your own question chief? lol
Answer:
Explanation:
Heres your answer! (or what I think is) A cell cycle is a series of events that takes place in a cell as it grows and divides. A cell spends most of its time in what is called interphase, and during this time it grows, replicates its chromosomes, and prepares for cell division. The cell then leaves interphase, undergoes mitosis, and completes its division.
what does the roman numeral stand for in copper(1) oxide should it not be copper(II) oxide
Answers
Answer:
The roman numeral in copper(I) oxide indicates that the oxidation number of copper in the compound is 1.
Explanation:
Roman numeral is used to indicate the oxidation number of an element in a compound.
The roman numeral in copper(I) oxide indicates that the oxidation number of copper in the compound is 1.
This can be seen from the following illustration:
copper(I) oxide => Cu₂O
Oxidation number of O = –2
Oxidation number of Cu₂O = 0
Oxidation number of Cu =?
Cu₂O = 0
2Cu + O = 0
2Cu – 2 = 0
Collect like terms
2Cu = 0 + 2
2Cu = 2
Divide both side by 2
Cu = 2/2
Cu = 1
Thus, we can see that the oxidation number of Cu in Cu₂O is 1. Hence the name of Cu₂O is copper(I) oxide indicating that the oxidation number of of copper (Cu) in the compound is 1.
For copper(II) oxide, we shall determine the oxidation number of Cu. This can be obtained as follow:
copper(II) oxide, CuO => CuO
Oxidation number of O = –2
Oxidation number of CuO = 0
Oxidation number of Cu =?
CuO = 0
Cu + O = 0
Cu – 2 = 0
Collect like terms
Cu = 0 + 2
Cu = 2
Thus, the oxidation number of Cu in CuO is 2. Hence the name of CuO is copper(II) oxide indicating that the oxidation number of of copper (Cu) in the compound is 2.
From the above illustrations,
We can see that the roman numeral in both copper(I) oxide, Cu₂O and copper(II) oxide, CuO are different because the oxidation number of Cu in both cases are different.
The map shows the average high temperatures in July for two cities in Texas.
Average July High Temperatures
Texas
Del Rio
36°C
Galveston
32°C 12
Answers
Answer:
Highest temperature which was recorded in Texas was 40 degrees.
Explanation:
Texas has variating weather. In summer Texas city turns to be very hot and during winters the city of Texas experience fall in temperature. There is very few rainfall in Texas due the climatic conditions.
Your little sister asks you a scientific question: "Does chocolate milk come from brown cows?" In order to answer the question, you decide to form a hypothesis.
Explain whether or not the following statements are effective hypotheses.
i. Brown cows produce chocolate milk.
ii. Brown cows never produce chocolate milk.
iii. Brown cows produce white milk.
Answers
A hypothesis is a proposed explanation or prediction based on limited evidence or observations, which can be tested through further investigation or experimentation. It should be specific, testable, and based on existing knowledge.
Now, let's evaluate each statement as a hypothesis:Brown cows produce chocolate milk.This statement can be considered an effective hypothesis as it proposes a relationship between the color of cows and the color of milk they produce. It is specific and testable, as one could observe and analyze the milk produced by brown cows to see if it is indeed chocolate milk. However, based on existing knowledge, we can confidently say that this hypothesis is not accurate, as the color of a cow does not determine the color of the milk it produces.Brown cows never produce chocolate milk.This statement can also be considered an effective hypothesis because it makes a specific claim that can be tested. However, based on existing knowledge, we can say that this hypothesis is not accurate. While the color of a cow does not determine the color of the milk, it is possible for chocolate milk to be produced by adding chocolate syrup or cocoa powder to regular white milk.Brown cows produce white milk.This statement is not an effective hypothesis as it is a general statement that aligns with existing knowledge. It does not propose any specific relationship or prediction to be tested. In the context of this question, the statement is not accurate as milk produced by cows is typically white, regardless of their coat color.For such more question on hypothesis
https://brainly.com/question/606806
#SPJ8
A 54.2 g sample of polystyrene, which has a specific heat capacity of 1.880 J-gc, is put into a calorimeter (see sketch at
right) that contains 100.0 g of water. The temperature of the water starts off at 21.0 °C. When the temperature of the water stops
changing it's 34.3 °C. The pressure remains constant at 1 atm.
Calculate the initial temperature of the polystyrene sample. Be sure your answer is rounded to the correct number of significant
digits.
thermometer.
insulated
container
water
sample.
a calorimeter
Answers
Tthe initial temperature of the polystyrene sample is 39.4°C.
Given: Mass of polystyrene sample = 54.2 gSpecific heat of polystyrene = 1.880 J-g°CWater mass = 100.0 g Initial water temperature = 21.0°CWater final temperature = 34.3°CPressure remains constant at 1 atmFormula used:Heat gained by water = heat lost by polystyreneHence,Heat lost by polystyrene = Heat gained by water=> mcΔT = mcΔTwhere,m = mass of polystyrene or waterc = specific heat capacityΔT = change in temperatureThe temperature change is ΔT = 34.3°C - 21.0°C = 13.3°CNow we can use this temperature change to calculate the initial temperature of the polystyrene.Taking the water's specific heat capacity, c = 4.184 J/g°CHeat gained by water = (100.0 g)(4.184 J/g°C)(13.3°C) = 5574 JHeat lost by polystyrene = 5574 JTaking the polystyrene's specific heat capacity, c = 1.880 J/g° ) = 13.3°C Now let's calculate the mass of polystyrene using the specific heat capacity formula.5574 J = (54.2 g)(1.880 J/g°C)(13.3°C - Ti)Ti = 39.4°C
for more questions on polystyrene
https://brainly.com/question/15913091
#SPJ8
Initial temperature of metal =
°℃
Initial temperature of water =
°℃
Final temperature of both =
√°C
Subtract to find the temperature changes
for the water and the metal.
AT (water) =
AT (metal)=-C
Answers
The temperature changes for the water and the metal can be calculated by subtracting their initial temperatures from the final temperature.
AT (water) = √°C - °℃
AT (metal) = √°C - °℃
The above equations give the temperature changes for the water and the metal, respectively. The specific values of the temperatures and the final temperature are not provided, so the actual temperature changes cannot be determined without knowing these values.
In general, the temperature change of a substance is given by the difference between the final and initial temperatures. When a warmer object comes into contact with a cooler one, heat energy is transferred from the warmer object to the cooler one until they reach thermal equilibrium, where their temperatures become equal.
The magnitude of the temperature change depends on factors such as the specific heat capacity of the substances involved and the amount of heat exchanged between them.
To accurately calculate the temperature changes, the specific heat capacities of water and the metal would be needed. Additionally, the masses or quantities of the substances would be necessary to determine the amount of heat exchanged. Without these specific values, it is not possible to provide a precise numerical answer.
for such more questions on temperature
https://brainly.com/question/4735135
#SPJ8
7. Which of the following compound is the most soluble in CC14? C. NH3 A. HF B. NaCl D. C10H22
Answers
C10H22 is a compound which is the most soluble in CC14 because both are non-polar in nature.
Why polar solute soluble in polar solvent?We know that like dissolve like which means that polar solutes only dissolve in polar solvent while on the other hand, non-polar solutes only dissolve in non-polar solvent.
So we can conclude that C10H22 is a compound which is the most soluble in CC14 because both are non-polar in nature.
Learn more about soluble here: https://brainly.com/question/23946616
#SPJ1
when two water molecules are near each other, a hydrogen bond will form between the more positive and the more negative atoms of neighboring water molecules. t or f
Answers
It is false that when two water molecules are near each other, a hydrogen bond will form between the more positive and the more negative atoms.
What is the composition of a water particle?A water particle is composed of two hydrogen atoms and one oxygen atom.
How do water particles bond?When there are two water particles close a bond is formed between the hydrogen atom of one particle and the oxygen atom of a neighbor water particle, which means there is a hydrogen-oxygen bond rather than a hydrogen-hydrogen bond.
Learn more about water in https://brainly.com/question/28465561
#SPJ1
It is True that when two water molecules are near each other, a hydrogen bond will form between the more positive hydrogen atom of one molecule and the more negative oxygen atom of the neighboring molecule.
How is water molecules formed?A water molecule is made up of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. This is because the oxygen atom, forms a bond with hydrogen atoms. It also carries two pairs of unshared electrons. All of the electron pairs (shared and unshared) repel each other.
Hydrogen bond will form between the more positive and the more negative atoms of neighboring water molecules when two water molecules are near each other. This is due to the polar nature of water molecules, which have a slightly positive charge on the hydrogen atoms and a slightly negative charge on the oxygen atoms. These hydrogen bonds give water many of its unique properties, such as its high surface tension and high heat of vaporization.
Learn more about water molecules on
https://brainly.com/question/29413538
#SPJ1