12. A malleable sheet of metal used to wrap food in the kitchen is made of only aluminum. What can be said about the metal sheet?
Answers
Answer:
is aluminium prepared in thin metal leaves with a thickness less than 0.2 mm (7.9 mils); thinner gauges down to 6 micrometres (0.24 mils) are also commonly used.[1] In the United States, foils are commonly measured in thousandths of an inch or mils. Standard household foil is typically 0.016 mm (0.63 mils) thick, and heavy duty household foil is typically 0.024 mm (0.94 mils). The foil is pliable, and can be readily bent or wrapped around objects. Thin foils are fragile and are sometimes laminated with other materials such as plastics or paper to make them stronger and more useful. Aluminium foil supplanted tin foil in the mid 20th century.
Related Questions
Help please I don’t know

Answers
Answer:
I don't EXACTLY know but hopefully the description below helps you find the answer!
Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. This exchange results in a more stable, noble gas electronic configuration for both atoms involved. An ionic bond is based on attractive electrostatic forces between two ions of opposite charge.
Explanation:
From each of the following ion concentrations in a solution, predict whether a precipitate will form in the solution. (Hint: Calculate Q and compare Q to Ksp)
a) [Ba2+] = 0.020 M, [F-] = 0.015 M Ksp= 1.0x10-6
BaF2(s) Ba2+(aq) + 2F-(aq)
b) [Pb2+] = 0.035 M, [Cl-] = 0.15M Ksp= 1.6x10-5
PbCl2(s) Pb2+(aq) + 2Cl-(aq)
Answers
If the ionic constant Q is greater than Ksp the solution forms precipitate if it is lower than the solubility constant Ksp then there will be no precipitate. Both of the given solutions forms precipitate.
What is ionic constant?The ionic constant Q of a solution is the product of molar concentrations of reactants. Solubility constant Ksp is the product of solubilities of the reactant ions when the reaction is in equilibrium.
For the first solution Q = 0.020 M × 0.015M = 0.003.
Ksp = 0.000001. Hence, Q is greater than Ksp which means the solution forms precipitate of BaF₂.
For the second solution Q = 0.035 M × 0.15 M =0.0025.
This is greater than Ksp = 1.6 × 10⁻⁵. Hence, this solution forms a precipitate of PbCl₂.
To find more about solubility, refer here:
https://brainly.com/question/28170449
#SPJ1
Given: (52.6 cm)(1.214 cm)
What is the product expressed to the correct number of significant figures?
1.
64 cm2
2.
63.9 cm2
3.
63.86 cm2
4.
63.8564 cm2
Answers
Answer:
2.
63.9 cm2
Explanation:
All non-zero digits are consider significant figures like 1, 2, 3, 4, 5, 6, 7, 8, 9.
Leading zeros are not consider as a significant figures. e.g. 0.06 in this number only one significant figure present which is 6.
Zero between the non zero digits are consider significant like 108 consist of three significant figures.
The zeros at the right side e.g 240000 are also significant. There are six significant figures are present.
When we add or subtract the values the number of significant figures after decimal in result must be equal to the given measurement having less number of decimal places. For example,
The difference between 7.69 and 4.0.
7.69 - 4.0 = 3.7
When we multiply or divide the values the number of significant figures must be equal to the less number of significant figures in given value.
For example in given q:
52.6 cm × 1.214 cm = 63.9 cm²
The term used to identify anything that occupies space is called:
A:
a) a gas b) matter (correct) c) a solid d) organic
Answers
The term used to identify anything that occupies space is matter. The correct option is b).
Matter refers to anything that has mass and takes up space. It includes all physical substances, such as solids, liquids, gases, and plasma. Matter is composed of atoms, which are the building blocks of all substances. Atoms consist of a nucleus of protons and neutrons, surrounded by a cloud of electrons.
The properties of matter can be described in terms of its physical and chemical characteristics, such as its mass, density, color, and reactivity. Understanding the properties of matter is essential for many fields of science, including physics, chemistry, and materials science. Therefore, the correct is b).
To know more about chemistry refer here:
https://brainly.com/question/13428382#
#SPJ11
At what temperature is breathing rate the highest? *
10 c
15 c
20 c
25 c
Answers
Answer:
Depends on what you're doing, you could be running and it goes higher, temperature isn't too much of a difference.
Explanation:
you need to make an aqueous solution of 0.180 m potassium sulfide for an experiment in lab, using a 300 ml volumetric flask. how much solid potassium sulfide should you add?
Answers
4.2228 g of solid potassium sulfide should be added to make an aqueous solution of 0.180 M potassium sulfide for an experiment in lab, using a 300 ml volumetric flask.
The given molarity of the aqueous solution of potassium sulfide is 0.180 M and the volume of the solution is 300 mL. We are required to find out the amount of solid potassium sulfide required to make the solution.
The formula to calculate the number of moles is: Number of moles = Molarity x Volume (in liters) 1. Convert the volume into liters.300 mL = 0.3 L2. Substitute the given values in the above formula.Number of moles = 0.180 M x 0.3 LNumber of moles = 0.054 mol3. The molecular formula of potassium sulfide is K2S. It means there are two moles of K for one mole of K2S. Hence, we can calculate the moles of K.Number of moles of K = 2 x 0.054
Number of moles of K = 0.108 mol4. The molar mass of K is 39.1 g/mol. Hence, we can calculate the mass of K required to make 0.108 mol.Number of grams of K = Number of moles x Molar massNumber of grams of K = 0.108 mol x 39.1 g/mol
Number of grams of K = 4.2228 g. Hence, 4.2228 g of solid potassium sulfide should be added to make an aqueous solution of 0.180 M potassium sulfide for an experiment in lab, using a 300 ml volumetric flask.
To learn more about aqueous visit;
https://brainly.com/question/30215562
#SPJ11
Pls help:)! Describe the steps you would take to write the chemical formula for the compound iodine trioxide
Answers
Answer:
IO3(.) (IO3)(.) Iodine trioxide is an iodine oxide and an inorganic radical.
Explanation:
In order to form water, two hydrogen atoms and one oxygen atom must be
A. dissolved.
B. bonded.
C. divided.
D. mixed.
Answers
Answer:
B bonded
Explanation:
Two molecules of hydrogen gas (two hydrogens bonded together) are combined with one molecule of oxygen gas (two oxygen atoms bonded together) in order to form two molecules of water (two hydrogen atoms bonded to one oxygen atom). What are covalent bonds? Covalent bonds occur when two or more atoms share electrons.
In order to form water, two hydrogen atoms and one oxygen atom must be bonded. The correct option is B.
What are covalent bonds?Covalent bonds between two or more atoms occur when they share electrons. with each other.
Two molecules of hydrogen gas are combined with one molecule of oxygen gas which forms two molecules of water. Hydrogen take one electron and oxygen can donate two electrons. One oxygen shares two electron with two hydrogen with one electron gaining capacity.
Thus, in order to form water, two hydrogen atoms and one oxygen atom must be bonded. The correct option is B.
Learn more about covalent bond.
https://brainly.com/question/10777799
#SPJ2
secretin, a hormone produced by cells lining the duodenum, stimulates the pancreas to release bicarbonate ions.
Answers
Secretin, a hormone produced by duodenal cells, triggers the release of bicarbonate ions from the pancreas. This response helps neutralize stomach acid in the duodenum, aiding in digestion and maintaining pH balance.
Secretin is a hormone produced by specialized cells lining the duodenum, the first part of the small intestine. When the duodenum detects the presence of acidic chyme (partially digested food) from the stomach, it releases secretin into the bloodstream. Secretin then travels to the pancreas, where it stimulates the release of bicarbonate ions (HCO3-) into the duodenum. Bicarbonate ions act as a buffer and help neutralize the acidic chyme, maintaining an optimal pH for digestion. This process is crucial in preventing damage to the duodenum and ensuring efficient digestion by balancing the acidity and alkalinity in the gastrointestinal tract.
Learn more about bicarbonate ions here:
https://brainly.com/question/13164182
#SPJ11
ANSWER QUICKLY calcium carbide reacts with water to form calcium hydroxide and acetylene gas according to the reaction below what mass of Cac2 is used when 2.00 moles of water react
Answers
Answer: Calcium carbide (CaC2) reacts with water to produce acetylene (C₂H₂):. CaC2 (s) + 2H2O (g) → Ca(OH)2 (s) + C₂H₂ (g).
we have not visited jupiter to collect samples, so how do we know what the atmosphere is composed of?
Answers
We have collected information from space organizations about the Jupiter, and we say that atmosphere of Jupiter is mainly composed of helium and hydrogen gas.
What does atmosphere means?Atmosphere is the space where we are living and in this space gases are present.
We have not visited the Jupiter to collect samples, but the study done from the space and research organization provides the in formation that the atmosphere of Jupiter is composed of mainly helium and hydrogen gas with little amount of ammonia and methane also. Due to the present of these gases the atmosphere of Jupiter is dense.
Hence, the atmosphere of Jupiter is mainly composed of helium and hydrogen gas.
To now more about atmosphere of Jupiter, visit the below link:
https://brainly.com/question/5973837
Give the o.N. Of each of the elements magnesium and oxygen in the reactants and in the products 2Mg + O2=2MgO
Answers
Answer:
\(2Mg^0 + O_2^0\rightarrow2Mg^{2+}O^{2-}\)
Explanation:
Hello there!
In this case, according to the rules for the oxidation states in chemical reactions, it is possible to realize that lone elements have 0 and since magnesium is in group 2A, it forms the cation Mg⁺² as it loses electrons and oxygen is in group 6A so it forms the anion O⁻²; therefore resulting oxidation numbers are:
\(2Mg^0 + O_2^0\rightarrow2Mg^{2+}O^{2-}\)
Best regards!
do all molecular structures have at least one imaginary frequency
Answers
No, not all molecular structures have at least one imaginary frequency. It depends on the specific geometry and symmetry of the molecule.
In general, molecular structures with three or more atoms will have one or more imaginary frequencies.This is because these structures have multiple vibrational modes, and not all of these modes correspond to physical vibrations of the molecule. Instead, some modes involve a distortion of the molecular geometry that is energetically unfavorable or impossible. These modes are represented by imaginary frequencies in the calculated vibrational spectrum of the molecule. However, some simple diatomic molecules, such as HCl or N₂, may not have any imaginary frequencies due to their limited vibrational modes.
Learn more about molecules here: brainly.com/question/19922822
#SPJ4
Calculate the amount of energy required to boil 25.00 g of mercury.

Answers
Answer:
C. 7.4 kJ
Explanation:
Let assume that Mercury is at room temperature (25 °C). The energy required to boil the sample of mercury is the sum of sensible and latent heats. Mercury has a fussion and boiling points of -38.83 °C and 356.7 °C, respectively, and a specific heat of \(138\,\frac{J}{kg\cdot ^{\circ}C}\). Then:
\(Q = (25\,g)\cdot \left[\left(0.138\,\frac{J}{g\cdot ^{\circ}C} \right)\cdot (356.7^{\circ}C - 25^{\circ}C) + 296\,\frac{J}{g} \right]\)
\(Q = 8544.365\,J\)
The option that offers the best approximation to the result is C.
During which event is more energy produced compared to other days to turn a turbine? el niño a tsunami during a spring tide during a low tide
Answers
A spring tide represents a natural process where more energy is produced when compared to other days in order to turn a turbine.
What do renewable energies mean?A renewable energy can be defined as any source of energy that is reversibly obtained from the nature.
A spring tide can be considered a powerful renewable source capable of generating electricity.In conclusion, a spring tide represents a natural process where more energy is produced compared to other days in order to turn a turbine.
Learn more about renewable energies here:
https://brainly.com/question/545618
#SPJ4
Answer:
C. during a spring tide
Explanation:
:D
The smallest particle of an element that retains the properties of that element is a(n) ____. a. atom c. proton b. electron d. neutron
Answers
Answer: proton
Explanation:
They are what give an atom it’s identity
I need help with the electron configurations shown in the picture

Answers
Answer:
1s2 2s2 2p6 3s1
Explanation:
How many moles of Ca are in 525g Ca?
Step 1 What is the molar mass (g/1 mol) of Ca?
Step 2 setup (525g x 1mol/grams Ca = mol)
Answers
There are approximately 13.11 moles of calcium in 525 g of Ca.
What is Molar Mass?
It is a physical property of a substance that is calculated by adding up the atomic masses (in atomic mass units, or amu) of all the atoms or molecules that make up the substance.
Molar mass is an important concept in chemistry because it allows us to convert between the mass of a substance and the number of moles of that substance, which is necessary for many chemical calculations.
Step 1: The molar mass of calcium (Ca) is 40.08 g/mol.
Step 2: To calculate the number of moles of calcium in 525 g of Ca:
mol = 525 g / 40.08 g/mol
mol = 13.11 mol (rounded to two decimal places)
Therefore, there are approximately 13.11 moles of calcium in 525 g of Ca.
Learn more about Molar Mass from given link
https://brainly.com/question/30459969
#SPJ1
Sodium azide (NaN3) is used in some automobile air bags. The impact of a collision triggers the decomposition of NaN3 as follows: 2NaN3(s) → 2Na(s) + 3N2(g). The nitrogen gas produced quickly inflates the bag between the driver and the windshield and dashboard. Calculate the volume of N2 generated at 80°C and 823 mmHg by the decomposition of 60.0 g of NaN3. (Answer: V = 36.9 L) I need the solution to this problem, the answer is already provided.
Answers
The volume of the N₂ generated at the 80 °C and the 823 mmHg and the decomposition of the 60.0 g of the NaN₃ is the 26.72 L.
The chemical reaction is as :
2NaN₃(s) ---> 2Na(s) + 3N₂(g)
The pressure of the gas = 823 mmHg = 1 atm
The temperature of the gas = 80 °C = 353 K
The mass of the NaN₃ = 60 g
The molar mass of the NaN₃ = 65 g/mol
The moles of NaN₃ = 60/65
The moles of NaN₃ = 0.92 mol
The ideal gas law is :
P V = n R T
V = n R T / P
V = ( 0.92 × 0.0823 × 353 ) / 1
V = 26.72 L.
The volume is 26.72 L with 1 atm pressure and the 353 K temperature.
To learn more about ideal gas here
https://brainly.com/question/14826347
#SPJ4
1. Which property makes metals good conductors of electricity?
A. The electrons in a metal can move freely.
B. The positively charged metal ions attract free electrons around them.
C. The electrons are shared equally by all of the atoms that make up the metal.
D. The negative charge of the electrons cancels the positive charge of the metal ions.
Answers
Answer:
I think it will be A
"Write the general form of beta decay."
Answers
Answer:
In beta minus decay, a neutron decays into a proton, an electron, and an antineutrino: n Æ p + e - +.
please help me solve this im lost

Answers
n H2 = m H2 / Mr H2
n H2 = 2 / 2
n H2 = 1 mol
n O2 = m O2 / Mr O2
n O2 = 4 / 32
n O2 = 0.125 mol
O2 is a limiting reactant
n H2O = (coef. H2O / coef. O2) • n O2
n H2O = (2 / 1) • 0.125
n H2O = 0.25 mol
m H2O = n H2O • Mr H2O
m H2O = 0.25 • 18
m H2O = 4.5 gr
I'll mark Brainliest please help me


Answers
Answer: Number 20 is A and number 14 is D
Explanation:
What is the p value for the following scenario: Out of 300 male inpatients, there are 195 that have a MCC and out of 450 female inpatients 205 have a MCC. Question 4 options: .A. 49
B.53
C.59
D.50
Answers
The p-value would depend on the calculated chi-square statistic and the degrees of freedom associated with the test.
To determine the p-value for the given scenario, we need to perform a statistical test, such as a chi-square test, to assess the association between gender and having a major co-existing condition (MCC).
The observed data can be summarized in a contingency table as follows:
Male 195 105
Female 205 245
To calculate the p-value, we would perform a chi-square test on this contingency table, comparing the observed frequencies to the expected frequencies under the assumption of independence between gender and MCC.
After conducting the chi-square test, the resulting p-value will indicate the probability of observing the given data or data more extreme if there is truly no association between gender and MCC.
However, without the expected frequencies or the results of the chi-square test, it is not possible to determine the exact p-value. Therefore, none of the provided options (A. 49, B. 53, C. 59, D. 50) can be considered as the correct answer. The p-value would depend on the calculated chi-square statistic and the degrees of freedom associated with the test.
Learn more about chi-square statistic from below link
https://brainly.com/question/4543358
#SPJ11
Question 8
What is the effective nuclear charge for a 2nd row electron in sulfur
Answers
Answer:
The effective nuclear charge for a 2nd row electron in Sulfur is +8
Explanation:
Zeff = Z (# of protons) - S (# of shielded electrons)
Since there are 8 electrons in the first and second rows combined, there are 8 shielding electrons.
The number of protons in Sulfur is 16.
Therefore,
Zeff = 16 - 8
Zeff = 8
(It's been awhile, so I am not 100% sure)
The effective nuclear charge for a 2nd row electron in sulfur is 14.
Sulfur has 16 atomic number means 16 number of protons and we know that in the first shell or orbit 2 electrons are present which produces a shielding effect due to its fast motion.
Its 2s electrons are shielded only by the two 1s electrons, therefore the second row electron experience an effective nuclear charge i.e. Z effective = 16 − 2 = 14 so we can conclude that the effective nuclear charge for a 2nd row electron in sulfur is 14.
Learn more: https://brainly.com/question/20556852
Select all the names that could be used to describe the figure
A parallelogram
B quadrilateral
c rhombus
D square
1017 Answered
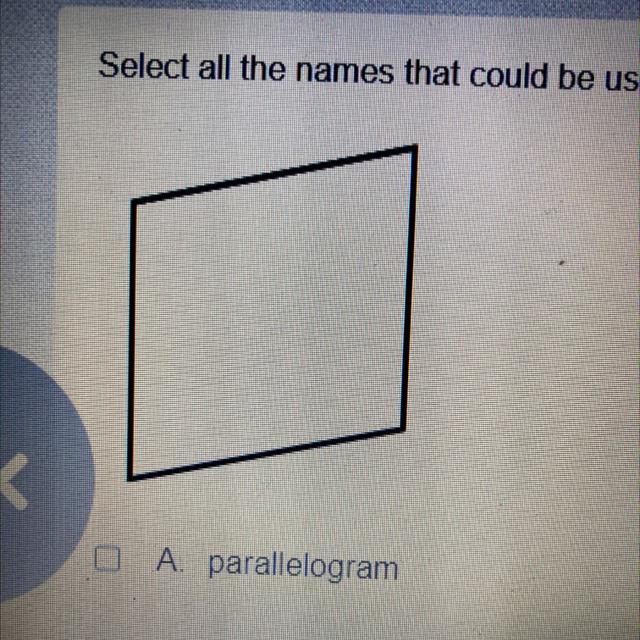
Answers
Answer:
Quadrilateral because there is no parallel and equal
what effect does polarity have on the melting point of a pure compound? select the single best answer
Answers
Polarity affects the melting point of a pure compound because it can affect the strength of intermolecular forces between molecules.
Polar molecules tend to have stronger intermolecular forces, which results in higher melting points for compounds that have a greater degree of polarity. This means that molecules with higher molar masses and that are polar will have the highest melting points.
Polarity of a pure compound refers to the degree to which the electrons in the compound are shared unequally between the atoms. This can be determined by looking at the electronegativity of the atoms involved in the compound. If the atoms have similar electronegativities, the electrons will be shared equally and the bond will be nonpolar.
Learn more about polarity of a pure compound:
https://brainly.com/question/29647466
#SPJ4
The Difference Between a Hot Cup of Water and a Cold One in Terms of Thermal Energy
Answers
Answer:
The difference between a hot cup of water and a cold one in terms of thermal energy can be described as the amount of heat energy that is present in each cup. The hot cup of water contains more thermal energy than the cold one due to its higher temperature. This means that the hot cup has more energy to transfer to its surroundings, and will cool down faster than the cold cup. The thermal energy in a cup of water is related to the temperature, with hotter water having a higher thermal energy than colder water.
Explanation:
if a molecule has bond angles of 109.5° between the atoms, what type of hybrid orbitals are on the central atom in the molecule? group of answer choicesa. spb. sp^2c. sp3
Answers
The type of hybrid orbitals on the central atom in a molecule with bond angles of 109.5° between the atoms is sp3 (option C)
Hybrid orbitals are a combination of two or more atomic orbitals that create a new hybrid orbital with different properties. The type of hybrid orbital depends on the number of atomic orbitals that are combined. In the case of sp3 hybrid orbitals, four atomic orbitals (one s orbital and three p orbitals) are combined to create four new hybrid orbitals. These sp3 hybrid orbitals have a tetrahedral shape with bond angles of 109.5° between the atoms. Therefore, if a molecule has bond angles of 109.5° between the atoms, it indicates that the central atom has sp3 hybrid orbitals. (option C)
Learn more about hybrid orbitals here: https://brainly.com/question/27956623
#SPJ11
a sample of solid x is carefully weighed and put inside a vented flask. the flask is heated until oxygen gas starts being produced. after no more oxygen gas is produced, the contents of the flask are removed and weighed, and from the decrease in weight the value of o may be calculated.
Answers
The value of O can be calculated by measuring the decrease in weight of the flask after the production of oxygen gas.
To determine the value of O, we need to calculate the mass of oxygen gas produced during the reaction. This can be done by measuring the decrease in weight of the flask before and after the reaction.
Let's assume the initial mass of the flask and solid X is M1, and the final mass of the flask and remaining solid (after the reaction) is M2. The decrease in weight, ΔM, can be calculated as:
ΔM = M1 - M2
The decrease in weight corresponds to the mass of oxygen gas produced during the reaction.
Once we have the mass of oxygen gas, we can calculate the value of O using the following formula:
O = (mass of oxygen gas produced) / (molar mass of oxygen)
By carefully weighing the solid X, placing it in a vented flask, and heating it to produce oxygen gas, we can determine the value of O by measuring the decrease in weight of the flask. The mass of oxygen gas produced can be calculated from the weight loss, and then the value of O can be determined by dividing the mass of oxygen gas by the molar mass of oxygen.
To know more about oxygen gas, visit;
https://brainly.com/question/30780592
#SPJ11