1. How is the activity series useful in predicting chemical behavior?
Answers
Answer:
It shows the most reactive to least reactive, so that we can predict whether certain reactions will occur. Any metal can replace any metal below it, but not above it.
Explanation:
1. The activity series is a type of ordering system for elements, which ranks how reactive a certain element is in relation to other elements.
2. The activity series determines the level of reactivity based on how well a certain element can displace hydrogen gas from acidic solutions and water.
3. The activity series of metals is an empirical tool used to predict products in displacement reactions and reactivity of metals with water and acids in replacement reactions and ore extraction.
4. It can be used to predict the products in similar reactions involving a different metal.
5. It helps in predicting whether a reaction will occur, and if so what the product will be.
Learn more:
brainly.com/question/19160471
Related Questions
Part 1. A chemist reacted 15.0 liters of F2 gas with NaCl in the laboratory to form Cl2 and NaF. Use the ideal gas law equation to determine the mass of NaCl that reacted with F2 at 280. K and 1.50 atm.
F2 + 2NaCl → Cl2 + 2NaF
Part 2. Explain how you would determine the mass of sodium chloride that can react with the same volume of fluorine gas at STP.
Answers
Taking into account the reaction stoichiometry and ideal gas law, the mass of NaCl that reacted with F₂ at 280 K, 15 L and 1.50 atm is 114.56 grams; and if you have the same volume of fluorine gas at STP, the mass of NaCl reacted is 78.323 grams.
Reaction stoichiometryIn first place, the balanced reaction is:
F₂ + 2 NaCl → Cl₂ + 2 NaF
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
F₂: 1 moleNaCl: 2 molesCl₂: 1 moleNaF: 2 molesThe molar mass of the compounds is:
F₂: 38 g/moleNaCl: 58.45 g/moleCl₂: 70.9 g/moleNaF: 42 g/moleThen, by reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
F₂: 1 mole×38 g/mole= 38 gramsNaCl: 2 moles×58.45 g/mole= 116.9 gramsCl₂: 1 mole×70.9 g/mole= 70.9 gramsNaF: 2 moles×42 g/mole= 84 gramsIdeal gas lawAn ideal gas is characterized by three state variables: absolute pressure (P), volume (V), and absolute temperature (T). The relationship between them constitutes the ideal gas law, an equation that relates the three variables if the amount of substance, number of moles n, remains constant and where R is the molar constant of gases:
P×V = n×R×T
STP conditionsThe STP conditions refer to the standard temperature and pressure. Pressure values at 1 atmosphere and temperature at 0 ° C are used and are reference values for gases. And in these conditions 1 mole of any gas occupies an approximate volume of 22.4 liters.
PART 1You know for F₂:
P= 1.50 atmV= 15 Ln= ?R= 0.082 (atm×L)÷(mol×K)T= 280 KReplacing in the definition of ideal gas law:
1.50 atm× 15 L = n× 0.082 (atm×L)÷(mol×K)× 280 K
Solving:
(1.50 atm× 15 L)÷ (0.082 (atm×L)÷(mol×K)× 280 K)= n
0.979965 moles= n
Then the following rule of three can be applied: if by stoichiometry of the reaction 1 mole of F₂ reacts with 116.9 grams of NaCl, 0.979965 moles of F₂ reacts with how much mass of NaCl?
mass of NaCl= (0.979965 moles of F₂× 116.9 grams of NaCl)÷ 1 mole of F₂
mass of NaCl= 114.56 grams
Finally, the mass of NaCl reacted is 114.56 grams.
PART 2In this case, you have the same volume of fluorine gas at STP:
P= 1 atmV= 15 Ln= ?R= 0.082 (atm×L)÷(mol×K)T= 273 KReplacing in the definition of ideal gas law:
1 atm× 15 L = n× 0.082 (atm×L)÷(mol×K)× 273 K
Solving:
(1 atm× 15 L)÷ (0.082 (atm×L)÷(mol×K)× 273 K)= n
0.67 moles= n
Then the following rule of three can be applied: if by stoichiometry of the reaction 1 mole of F₂ reacts with 116.9 grams of NaCl, 0.67 moles of F₂ reacts with how much mass of NaCl?
mass of NaCl= (0.67 moles of F₂× 116.9 grams of NaCl)÷ 1 mole of F₂
mass of NaCl= 78.323 grams
Finally, the mass of NaCl reacted is 78.323 grams.
Learn more about reaction stoichiometry, ideal gas law and STP conditions:
brainly.com/question/27809250
brainly.com/question/28096494
brainly.com/question/27815860
#SPJ1
What element is on period 5 group/family 11
Answers
Answer:
47
Ag
Silver
107.87
Explanation:
Scenario
HURRY I NEED HELP ASAP
A student masses 40 g copper (11) oxide Cuo, a black solid, and heats the substance using a Bunsen burner until the sample turns a reddish color. The chemical equation for the reaction issiven below. According to the reaction, the copper (1) oxide decomposes to produce copper(1) oxide and oxygen
4CuO → 2Cu2O + O2
Black solid Red solid Colorless gas
40 g. ? g
? g
Based on the evidence, write a scientific explanation describing the exact mass of the copper() oxide produced Provide mathematical evidence and give your reasoning in a way that demonstrates your understanding of the scientific principles involved.
Answers
72 g of Copper(i) oxide will be produced by 40 g of CuO base on the law of conservation of mass.
What mass of copper (i) oxide is produced?The law of conservation of mass states that mass remains constant at the end of every reaction.
The equation of the reaction is given below:
\(4CuO → 2Cu_2O + O_2\)
Based on the equation of reaction, 4 moles of copper (ii) oxide produces 2 moles of copper (i) oxide.
Mass of 4 moles of CuO = 4 × 80 = 160 g
Mass of 2 moles of copper (i) oxide = 2 × 144 g = 288 g
Mass of Copper(i) oxide produced by 40 g of CuO will be: 40 × 288/160 = 72 g
Therefore, 72 g of Copper(i) oxide will be produced by 40 g of CuO.
Learn more about law of conservation of mass at: https://brainly.com/question/20081187
In an experiment, how many variables should you change at a time? And why?
Answers
Answer:
it should test one variable at a time.
Explanation: because you will not be able to tell which variable is responsible for the observed results.
Why the graph isn't just a single sloped straight line.
Answers
Answer:
the equation could have mulitplication and square roots which may change the function of the line
a buffer solution contains 0.363 m c6h5nh3br and 0.289 m c6h5nh2 (aniline). determine the ph change when 0.069 mol koh is added to 1.00 l of the buffer.
Answers
7.765 is the ph change when 0.069 mol KOH is added to 1.00 l of the buffer solution.
What is pH ?The pH scale, which previously stood for "potential of hydrogen," is used to describe how acidic or basic an aqueous solution is. The pH values of acidic solutions are typically lower than those of basic or alkaline solutions.
What is Buffer Solution?A buffer solution is an aqueous mixture of a weak acid and either its conjugate base or base itself. When a modest amount of a strong acid or base is applied to it, the pH hardly changes at all.
As per given data;
We have acid as c6h5nh3br with NH3
and base as c6h5nh2 with NH2
now to find the ph change we need to apply the following
pH=pKa+ log[A−]/[HA]
here
ph=pKa(NH3)+log [A−]/[HA] here Ka value of NH3 is 5.56× 10−10 mol L−1 hence pKa value for NH3 =9.25
So
ph=pKa(9.25 )+log [0.289]/[0.363]
ph=9.25+(-1.485)
ph=7.765
Hence,7.765 is the ph change when 0.069 mol KOH is added to 1.00 l of the buffer solution.
To know more about the Buffer Solution visit
https://brainly.com/question/24262133
#SPJ4
What is the molecular formula of a compound with an empirical formula CHOCl and a molecular weight of 129 g
Answers
The molecular formula of the compound is C2H2O2Cl2.
To determine the molecular formula of a compound with the empirical formula CHOCl and a molecular weight of 129 g, we need to find the actual number of atoms of each element in the compound.The empirical formula CHOCl suggests that the compound contains one carbon (C), one hydrogen (H), one oxygen (O), and one chlorine (Cl) atom.To calculate the molecular formula, we need to compare the empirical formula's empirical mass to the compound's actual molecular weight. The empirical mass of CHOCl can be calculated by adding the atomic masses of the constituent elements: C (12.01 g/mol) + H (1.01 g/mol) + O (16.00 g/mol) + Cl (35.45 g/mol) = 64.47 g/mol.By dividing the molecular weight of 129 g by the empirical mass of 64.47 g/mol, we find that the compound's molecular formula is approximately C2H2O2Cl2.The molecular formula C2H2O2Cl2 indicates that the compound contains two carbon atoms, two hydrogen atoms, two oxygen atoms, and two chlorine atoms. This formula has a molecular weight of approximately 129 g, which matches the given molecular weight.
for such more questions on molecular
https://brainly.com/question/24191825
#SPJ8
Two large, nonconducting plates are suspended 5.61 cm apart. Plate 1 has an area charge density of +92.3μC/m2, and plate 2 has an area charge density of +19.1μC/m2. Treat each plate as an infinite sheet. How much electrostatic energy UE is stored in 1.54 cm3 of the space in region A ? UE= What volume V of the space in region B stores an equal amount of energy?
Answers
Two large, non conducting plates are suspended 5.61 cm apart. Plate 1 has an area charge density of +92.3 μC/m², and plate 2 has an area charge density of +19.1 μC/m².
The electrostatic energy UE is stored in 1.54 cm³ of the space in region A is 1.588 x 10⁻⁹ J.
The volume V of the space in region B stores an equal amount of energy is 1.541 x 10⁻⁶ m³.
To calculate the electrostatic energy stored in a given volume of space, we need to determine the electric field and then use it to calculate the energy density. The energy stored in a volume is given by the product of the energy density and the volume.
Distance between the plates (d): 5.61 cm
Charge density of plate 1 (σ₁): +92.3 μC/m²
Charge density of plate 2 (σ₂): +19.1 μC/m²
Volume in region A: 1.54 cm³
First, let's calculate the electric field between the plates:
The electric field between two infinite parallel plates with uniform charge densities is given by:
E = (σ₁ - σ₂) / (2ε₀)
where ε₀ is the permittivity of free space (ε₀ = 8.85 x 10⁻¹² C²/(N m²)).
Substituting the given values, we have:
E = (92.3 x 10⁻⁶ C/m² - 19.1 x 10⁻⁶ C/m²) / (2 x 8.85 x 10⁻¹² C²/(N m²))
E = 5.509 x 10⁶ N/C
Next, let's calculate the electrostatic energy stored in 1.54 cm³ of space in region A:
The energy density (u) is given by:
u = (1/2) ε₀ E²
Substituting the value of E we calculated earlier, we have:
u = (1/2) x 8.85 x 10⁻¹² C²/(N m²) x (5.509 x 10⁶ N/C)²
u = 1.031 x 10⁻³ J/m³
To calculate the energy (UE) stored in the given volume in region A, we multiply the energy density by the volume:
UE = u x V
UE = 1.031 x 10⁻³ J/m³ x 1.54 x 10⁻⁶ m³
UE = 1.588 x 10⁻⁹ J
Therefore, the electrostatic energy stored in 1.54 cm³ of space in region A is 1.588 x 10⁻⁹ J.
To determine the volume (V) in region B that stores an equal amount of energy, we rearrange the equation:
V = UE / u
V = 1.588 x 10⁻⁹ J / 1.031 x 10⁻³ J/m³
V = 1.541 x 10⁻⁶ m³
Therefore, a volume of 1.541 x 10⁻⁶ m³ in region B stores an equal amount of electrostatic energy.
To know more about non conducting plates here
https://brainly.com/question/33381740
#SPJ4
MAKE A POSTER OR SLIDES OF THE FOLLOWING INFORMATION
Important Details:
Due Date: October 8, 2021
Maximum group size: 2
Information to Include:
Origin –
ð Date of discovery
ð Discoverer
Background – Use these aspects of your element to describe your superhero (if you can’t think of how to show one of these, you could always use it as decoration on their costume):
ð: Name of the element
ð Element symbol
ð Atomic number
ð Atomic mass
ð Number of protons
ð Number of electrons (in a neutral atom)
ð Number of neutrons
ð Drawn picture of atom (YOU MUST DRAW IT)
ð Boiling point
ð Melting point
ð State of matter (at room temperature)
ð Uses for element
ð Type of element (metal, nonmetal, metalloid)
ð Group name (halides, lanthanides, noble gases, etc.)
ð Other elements in group
MUST BE COLORFUL AND DETAILED
Project Format (choose ONE):
Poster
Slides
flyer
Pamphlet
Criteria
4-5
3-3.99
2-2.99
0-1.99
Origin
All of the components for the origin criteria are present, explained fully, and are related to the context in an appropriate manner.
One component is missing; or, some components are not clearly explained.
Two components are missing; or, several components are not clearly explained.
More than two components are missing, or all components are not clearly explained.
Background
All of the components for the background criteria are present, explained fully, and are related to the context in an appropriate manner.
One component is missing; or, some components are not clearly explained.
Two components are missing; or, several components are not clearly explained.
More than two components are missing, or all components are not clearly explained.
Powers
All of the components for the criteria of the power are present, explained fully, and are related to the context in an appropriate manner.
One component is missing; or, some components are not clearly explained.
Two components are missing; or, several components are not clearly explained.
More than two components are missing, or all components are not clearly explained.
Other Heroes/Villains
All of the components for the other heroes/villains criteria are present, explained fully, and are related to the context in an appropriate manner.
One component is missing; or, some components are not clearly explained.
Two components are missing; or, several components are not clearly explained.
More than two components are missing, or all components are not clearly explained.
Work Quality
The work is of high quality, clean, neat, and easy to follow.
One component is missing; or, some components are not clearly explained.
Two components are missing; or, several components are not clearly explained.
More than two components are missing, or all components are not clearly explained.
Individual Contribution
The individual participated equally with their partner in the project with little to no drama and minimal prompting.
The individual participated in the project but was slightly less than an equal partner. May have required prompting to participate.
The individual participated, but was less than an equal partner, or required multiple prompts to participate.
Little to no participation in the project, requiring many prompts.
Score:
/30
Answers
Answer:
This is something you have to do yourself. The teacher is even letting you have a partner. Nobody is going to do this project for you because it´s your responsibility to finish it on your own.
Explanation:
Elements that are in the same _____ have the same number of electrons in their outer ______. These outer electrons are so important in determining the chemical ______ of an element that a special way to represent them has been developed. A(n) _____ uses the symbol of the element and dots to represent the electrons in the outer _______
Answers
Elements that are in the same group have the same number of electrons in their outer shell. These outer electrons are so important in determining the chemical properties of an element that a special way to represent them has been developed. A(n) electron dot diagram uses the symbol of the element and dots to represent the electrons in the outer shell.
The elements in the same group have the same number of electrons in their outer shell because they have the same number of valence electrons. These valence electrons are responsible for the chemical properties of an element, which is why elements in the same group have similar chemical properties. The symbol of the element is used in the electron dot diagram to represent the element, and the dots are used to represent the valence electrons in the outer shell. This diagram is a useful tool for understanding the chemical behavior of an element and for predicting how it will react with other elements.
You can learn more properties of elements at: brainly.com/question/30388913
#SPJ11
what is the second most abundant gas in the atmosphere
Answers
four different containers are labeled C+O2, CO, CO2, and Co. Based on the labels, classify each as an element, a compound, a homogeneous mixture, or a heterogeneous mixture. Explain your reasoning.
Answers
Answer:
Element - Co. This is because an element is an atom that is made up of purely its own element. In this case, we have Co, also known as Cobalt on the periodic table of elements
Compound - CO. A compound is a substance that can be broken down into simple stable substances. Each compound is made from the atoms of two or more elements that are chemically bonded. As you can see, C (carbon) and O (oxygen) are chemically bonded together
Homogeneous mixture - C+O2
Heterogeneous mixture - CO2
At what temperature do the combined effects of contraction and expansion produce the smallest volume of water?.
Answers
4°c, the temperature of maximum density for water.
Below that point, the crystalline lattice starts to form and the water begins to expand. The density decreases above that temperature as a result of the elevated molecular vibrational activity.
Temperature is a numerical expression of how hot a substance or radiation is. There are three different kinds of temperature scales: those that depend only on macroscopic properties and thermodynamic principles, like Kelvin's original definition; those that depend only on practical empirical properties of particulate matter rather than theoretical principles; and those that depend on the average translational kinetic energy per freely moving microscopic particle, such as an atom, molecule, or electron, in a body, like the SI scale.
A thermometer is used to determine temperature. It is calibrated using a variety of temperature scales, each of which has a distinct historical definition determined by a specific set of reference points and thermometric materials. The Celsius scale is the most widely used scale.
To learn more about Temperature visit here:
brainly.com/question/11464844
#SPJ4
The equation below represents a chemical reaction that occurs in living cells.
C6H12O6 + 6O2 ------> 6CO2 + 6H2O + energy
The reactants contain a total of _____?
atoms.
Answers
Answer:
36 atoms
Explanation:
The reactants in the equation contain 6 carbon atoms, 12 hydrogen atoms, and 18 oxygen atoms. So the total number of atoms in the reactants is:
6 (carbon atoms) + 12 (hydrogen atoms) + 18 (oxygen atoms) = 36 atoms
Why does Buckminsterfullerene, with a formula of C60, have a simple covalent structure instead of a giant covalent structure?
Answers
Answer:
HOPE THIS HELPS
Explanation:
They are made up of large molecules so are not classed as giant covalent networks . Weak intermolecular forces exist between buckyballs. These need little energy to overcome, so substances consisting of buckyballs are slippery and have lower melting points than graphite or diamond .
lee, c. e. a. atomically thin p–n junctions with van der waals heterointerfaces. nature nanotechnology 9, 6 (2014)
Answers
The citation you provided is from a scientific paper titled "Atomically Thin p-n Junctions with van der Waals Heterointerfaces" by C.E.A. Lee, published in the journal Nature Nanotechnology in 2014.
The term "atomically thin" refers to the extremely thin nature of the junction, which is only a few atomic layers thick. This is made possible by the use of van der Waals heterointerfaces, which are interfaces between different layered materials held together by weak van der Waals forces. Examples of layered materials include graphene and transition metal dichalcogenides.
The paper explores the fabrication and characterization of these atomically thin p-n junctions, highlighting their potential for future electronic devices with high performance and low power consumption.
To know more about p-n Junctions visit:-
https://brainly.com/question/24303357
#SPJ11
Hi can you help me please 
Answers
4.564000 x 10^-4 express how many significant figures
Answers
Answer: 7
Explanation:
Before a number but after a decimal. The zeros at the end would usually mean that it doesn't count but since the numbers are before the zeros and after a decimal it's 7 sig figs
EXPLAIN Describe the patterns you see among the chemical formulas. How
does the placement of the elements on the periodic table appear to relate to the
numbers in the chemical formula?
PLEASE HELP ME
Answers
The patterns among chemical formulas relate to the placement of elements on the periodic table through their valence electrons and bonding capacity.
Chemical formulas exhibit patterns based on the periodic table's organization. Elements in the same group share similar properties and bonding capacities due to their valence electrons.
For example, elements in Group 1 have one valence electron and typically form +1 ions, while Group 17 elements have seven valence electrons and usually form -1 ions. When combining elements, the numbers in the chemical formula reflect the ratio of atoms required to achieve a stable electron configuration.
For instance, sodium (Na, Group 1) and chlorine (Cl, Group 17) form NaCl, where one sodium atom donates an electron to one chlorine atom, resulting in a stable compound. By understanding the periodic table's arrangement, we can predict chemical formulas and the properties of compounds.
To know more about periodic table click on below link:
https://brainly.com/question/11155928#
#SPJ11
What two of the following organisms are secondary consumers in this food web?
Answers
Secondary consumers are organisms that primarily feed on herbivores or other primary consumers.
They occupy the next trophic level above the primary consumers in a food web. They obtain energy by consuming the primary consumers and play an important role in regulating the population of herbivores.
Examples of commonly observed secondary consumers include:
Carnivorous mammals: Animals such as wolves, lions, and tigers that feed on herbivores like deer, zebras, or gazelles.
Birds of prey: Species like eagles, hawks, and owls that consume small mammals, reptiles, or other birds.
Carnivorous fish: Fish like pike, barracuda, or bass that prey on smaller fish or aquatic invertebrates.
Predatory insects: Insects such as spiders, mantises, or dragonflies that feed on other insects, including herbivorous insects.
In a specific food web, the identification of secondary consumers would depend on the specific organisms present and their feeding interactions. It would be necessary to analyze the trophic relationships among the organisms in the food web to determine the secondary consumers accurately.
For more such questions on Secondary consumers visit:
https://brainly.com/question/28631974
#SPJ8
Could any one help on this fill in questions

Answers
Suppose that 5.2 L of methane at a pressure
of 782 Torr is transferred to a vessel of volume
2.2 L. What is the final pressure of methane
if the change occurs at constant temperature?
Answer in units of Torr.
Answers
Answer:
Final pressure = 1848.36 Torr
Explanation:
Given that,
Initial volume, V₁ = 5.2 L
Initial pressure, P₁ = 782 Torr
Initial volume, V₂ = 2.2 L
We need to find the final pressure. We know that the relationship between pressure and volume is given by :
\(P\propto \dfrac{1}{V}\\\\\dfrac{P_1}{P_2}=\dfrac{V_2}{V_1}\\\\P_2=\dfrac{P_1V_1}{V_2}\\\\P_2=\dfrac{782\times 5.2}{2.2}\\\\P_2=1848.36\ torr\)
So, the final pressure is equal to 1848.36 Torr.
I am an element in period 4 with 7 valence electrons.
Answers
Answer:
The elements in this family are fluorine, chlorine, bromine, iodine, and astatine. Halogens have 7 valence electrons, which explains why they are the most active non-metals.
Explanation:
Answer:
Selenium
Explanation:
When V increases, I _______ . It is a direct relationship
A. increases
B. decreases
C. remains constant
Answers
Answer:
B
Explanation:
A sample of air at room temperature in a 4.00 L container holds approximately 0.562 moles of O2and 2.11 moles of N2 along with other trace elements to total 2.67 moles. What is the mole fraction of N2 in the mixture
Answers
Taking into account the definition of mole or molar fraction, the mole fraction of N₂ in the mixture is 0.79.
Molar fractionThe molar fraction is used to express the concentration of a solute in a solution and expresses the proportion in which a substance is found with respect to the total moles of the solution, which are calculated by adding the moles of solute(s) and solvent.
The mole fraction "x" of the elements in a compound is defined as a ratio between the number of moles of each of the different elements present in the compound and the total number of moles of them:
x=number of moles of the solute÷ total number of moles
Mole fraction of N₂ in the mixtureIn this case, you know that:
number of moles of N₂: 2.11 molestotal number of moles: 2.67 molesReplacing in the definition of mole fraction:
x=2.11 moles÷ 2.67 moles
Solving:
x= 0.79
Finally, the mole fraction of N₂ in the mixture is 0.79.
Learn more about mole fraction
brainly.com/question/14434096
brainly.com/question/10095502
#SPJ1
What will happen to the density of the water if i put salt in it.
Answers
Answer:When salt is dissolved in fresh water, the density of the water increases because the mass of the water increases.
Explanation:
A pipe 10 m long and of radius r = 7 cm is to be coated by insulation material to a thickness of dr = 2 mm. Approximate the volume V of insulation material required in m³. Please use pi for л (rather than a decimal approximation) in your answer. Insulation volume (m³): You have not attempted this yet
Answers
The volume of insulation material required is approximately 0.003606 cubic meters (m³).
To calculate the volume of insulation material, we can subtract the volume of the inner pipe (original pipe) from the volume of the outer pipe (original pipe + insulation).
Given:
Length of the pipe, L = 10 m
Radius of the pipe, r = 7 cm = 0.07 m
Thickness of the insulation, dr = 2 mm = 0.002 m
The outer radius of the larger pipe is R = r + dr.
Using the formula for the volume of a cylinder, V = π(R² - r²)L, we can substitute the values and calculate:
V = π((0.07 + 0.002)² - 0.07²) × 10
V ≈ 3.606 × 10⁻³ m³
Therefore, the volume of insulation material required is approximately 0.003606 m³ (cubic meters).
Learn more about cylinder volumes from the given link
https://brainly.com/question/28058531
#SPJ11.
Help me pleaseeeeeee!!!!
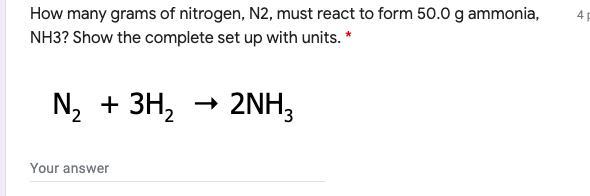
Answers
HELP DUE TODAY this is for science.
What is the advantage of viewing a
specimen at 40x as opposed to at 400x
magnification?

Answers
The advantage of viewing a specimen at 40x as opposed to at 400x magnification is increased field of view and provide more details about the specimen.
What are specimen?Specimen is defined as a single plant or animal that is investigated by experts as an example of a specific species or type.
It can also be defined as a component or person used to represent a whole mass or number; a characteristic plant, mineral, component, animal, etc. a portion of a chemical or material for testing or research: a tissue sample; a urine sample. a specific or odd type of person.
Thus, the advantage of viewing a specimen at 40x as opposed to at 400x magnification is increased field of view and provide more details about the specimen.
To learn more about specimen, refer to the link below:
https://brainly.com/question/14686485
#SPJ1
Match the provided labels to the appropriate point on the titration curve Buffering region 14 12 10 Excess titrant point 6 4 2 Volume of strong base (mb Part 2 (1 point) O See Hint Use the titration curve for the weak acid to calculate the pH of a 0.15 Msolution of that weak acid. Enter your answer with two significant figures. Round the pKa to the nearest whole number for the calculation, and use two significant figures for your final answer.
Answers
To match the provided labels to the appropriate point on the titration curve:
- Buffering region: 10
- Excess titrant point: 2
- Volume of strong base (mb): 6
As for the second question, to calculate the pH of a 0.15 M solution of a weak acid using the titration curve, we first need to determine the pKa of the acid. We can do this by finding the halfway point of the buffering region on the curve, which is at pH 4. This corresponds to a 50/50 mix of the weak acid and its conjugate base, which means that the pKa is equal to the pH.
So, the pKa of the weak acid is 4. We can then use the Henderson-Hasselbalch equation to find the pH of the 0.15 M solution:
pH = pKa + log([A-]/[HA])
We know that [HA] = 0.15 M, and [A-] = 0.85 M (since the solution is 85% conjugate base after the acid has been completely ionized). Plugging these values into the equation, we get:
pH = 4 + log(0.85/0.15) = 3.2 (rounded to two significant figures)
Therefore, the pH of the 0.15 M solution of the weak acid is 3.2.
to know more about labels intake pls visit:
https://brainly.com/question/29566278
#SPJ11