Answers
Your hair stays wet after shower, molecules of water are actually sticking to your hair. This property is sticking of water with unlike substance is called Unlike substance attraction bonding, due to hydrogen bond.
The word may or may not contain ions that satisfy this condition, depending on the context. A collection of two or more atoms linked together by the alluring forces known as chemical bonds is referred to as a molecules. In the domains of quantum physics, organic chemistry, and biology, the distinction between polyatomic ions and ions is commonly lost. A molecule can be homonuclear, which is a molecule made up of atoms of one chemical element, such as the two atoms in the oxygen molecule (O2), or heteronuclear, which is a chemical compound made up of atoms of more than one chemical element, such as water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term "molecule" is usually used to refer to any gaseous particle, regardless of its composition. The requirement that a molecule consist of two or more atoms is waived because the noble gases are single atoms. Atoms and complexes connected by non-covalent interactions like hydrogen bonds or ionic bonds are frequently absent from single molecules.
To know more about molecules please refer: https://brainly.com/question/19556990
#SPJ4
Related Questions
Step 7: Put the Metal in the Water and Measure
Temperature Changes (Lead)
Measure the initial temperature of the water to the
nearest 0.1°C. Record in the data table.
Initial temperature of metal =
Initial temperature of water
Final temperature of both = [
27
DONE
26
25
24
23
Intro
Continue
AL
250 ml
=பா-©
200
150
100
1
27
O
°C
°C
answer 100,22.6,23.3,0.7, 76.6
Answers
The answer of temperature change is mentioned below
What is Temperature Change ?
The change in temperature is given by
ΔT=Final Temperature −Initial Temperature
Measurements of temperature
It is a measure of the degree of hotness or coldness of a body
To determine temperature changes,
the initial and final temperature of the iron and water is recorded.
For Iron
Iron at higher temperature is dipped in water
Water is at lower temperature so there will be change in temperature fo both
These data are recorded
Temperature change = Final temperature - Initial temperature.
a) Temperature change for metal = 200 - 100 = 100 0C
Temperature change for water = 22.5
Temperature change for both = 23.3
b) For water 0.7
For iron Metal 76.6
To know more about temperature change
https://brainly.com/question/19724978
#SPJ1
Answer: initial temp of metal: 100 C, initial temp of water 22.4, final temp of both 27.1
Explanation:
Classify the following compounds as binary ionic, ternary ionic, or molecular.
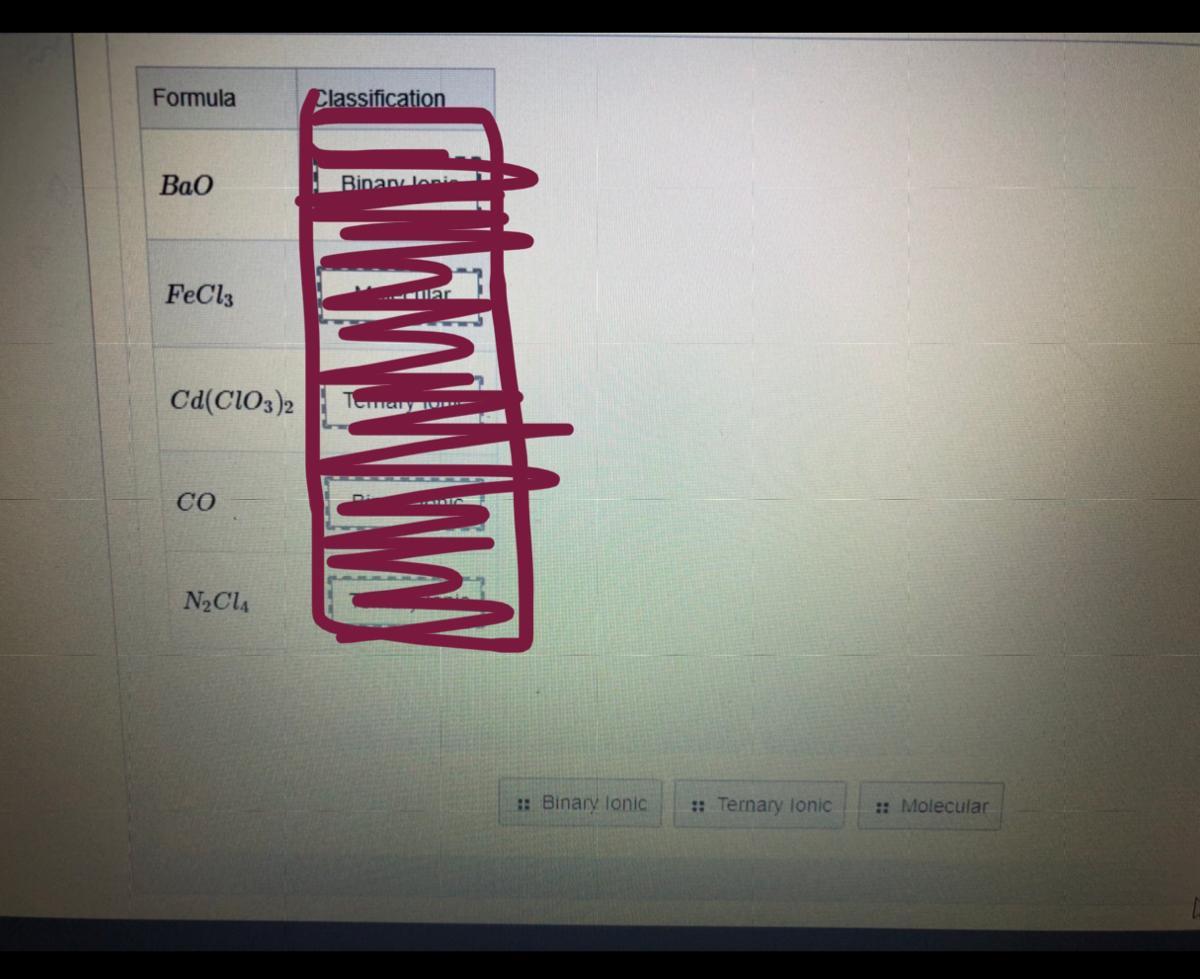
Answers
Answer:bro u already have an answer why are you asking?
Explanation:
Is this chemical equation balanced? yes or no
Mg + 2O ---> 2MgO
Answers
Answer:
yes
Explanation:
So the balanced equation is 2 Mg + O 2 2 MgO.. ... Example: 2Mg + O2 -> 2MgO Magnesium and oxygen combine to form the compound magnesium oxide.
Thus, to balance the O2 we put a 2 in front of the MgO. However, now there are 2 Mg's in the products and only one Mg in the reactants. To balance the Mg we then put a 2 in front of the Mg in the reactant. All atoms are now balanced in the reaction
Answer:
Yes.
Explanation:
Oxygen has 2 atoms, and so does Magnesium.
Hope this helped! Have a good night!
Directions: The picture below shows a model of Earth's layers. Use the picture to answer
any questions that follow.
Layer 5
Layer 4
Layer 3
Layer 2
Layer 1
1. Which layers compose the lithosphere?
A layers 6 and 4
B layers 4 and 3
a layers 3 and 2
D layers 2 and 1
Answers
Answer:
hfghfhjcbnggf
Explanation:
layer1
layer 2
how does the mass and size of an atom compare to the mass and size of the nucleus
Answers
Answer:
Explanation:
The nucleus of an atom is about 10-15 m in size; this means it is about 10-5 (or 1/100,000) of the size of the whole atom. ... (10-15 m is typical for the smaller nuclei; larger ones go up to about 10 times that.) Mass. Although it is very small, the nucleus is massive compared to the rest of the atom.
Answer:
The nucleus of an atom is about 10-15 m in size; this means it is about 10-5 (or 1/100,000) of the size of the whole atom. ... (10-15 m is typical for the smaller nuclei; larger ones go up to about 10 times that.) Mass. Although it is very small, the nucleus is massive compared to the rest of the atom.
Explanation:
What type of element would form an ionic bond with iodine?
1. metalloid
2. solid
3. metal
4. nonmetal
Answers
Guysss how to explain nuclear chemistry? And define nuclear chemistry ?
Answers
Answer:
How do amoeba respire.
Define Diffusion.
Name a substance/solution that could be used to distinguish alkane from an alkene.
Answers
Answer: Bromine water
Explanation:
Alkanes are saturated hydrocarbons with single bond and the alkenes as unsaturated hydrocarbons with double bonds.
Bromine water is a chemical reagent which is used to distinguish between alkane and alkene.
It is of orange color in solution form and turns to colorless in alkene.
The alkanes to show any reaction with bromine water.
Just wondering if this is correct I’m doing practice problems just want to make sure I got it right

Answers
We need to write our reaction:
Ti + 2 F2 ==> TiF4
We have 2 reactants with 2 quantities of each one. To calculate the moles of TiF4 we need to know first which one is the limiting reactant.
We proceed like this:
Moles of Ti = 12.28 moles
Moles of F2 = 3.87 moles
We work with stoichiometry,
1 mol Ti --------- 2 x 1 mole F2
12.28 moles Ti ---------- X = 24.56 moles
According to this result, for 12.28 moles of Ti, we need 24.56 moles of F2, but we only have 3.87. So, The limiting reactant is F2 (we use this to calculate the number of moles of TiF4)
Moles of TiF4)
2 x 1 mole F2 -------------- 1 mole TiF4
3.87 moles F2 -------------- X = 1.94 moles
Answer: 1.94 moles TiF4
7.0×107 ÷ 2.0×104
turn into a proper scientific notation. PLS HELP
Answers
The expression 7.0x\(10^7\) ÷ 2.0x\(10^4\) can be expressed in proper scientific notation as 3.5x10^3.
To express the division 7.0x\(10^7\) ÷ 2.0x\(10^4\) in proper scientific notation, we need to perform the division and adjust the result to the appropriate format.
Dividing the numbers, we get:
7.0x\(10^7\) ÷ 2.0x\(10^4\)= 3.5x\(10^{(7-4)\)= 3.5x\(10^3\)
The result of the division is 3.5, and we adjust the exponent by subtracting the exponent of the divisor from the exponent of the dividend (7 - 4 = 3).
Therefore, the proper scientific notation representation of the division 7.0x\(10^7\) ÷ 2.0x\(10^4\) is 3.5x\(10^3\).
Scientific notation is a way to express numbers using a coefficient (in this case, 3.5) multiplied by a power of 10 (in this case, 10^3). It allows for more concise representation of very large or very small numbers.
In this case, the division resulted in a number that is smaller than the dividend and has a positive exponent, indicating a smaller magnitude compared to the original numbers. The coefficient represents the significant digits of the result, while the power of 10 represents the scale or magnitude of the number.
For more such questions on scientific notation visit:
https://brainly.com/question/28468914
#SPJ8
What is pH defined as?
Answers
Answer:
pH is a measure of how acidic/basic water is. The range goes from 0 to 14, with 7 being neutral. pHs of less than 7 indicate acidity, whereas a pH of greater than 7 indicates a base. pH is really a measure of the relative amount of free hydrogen and hydroxyl ions in the water.
Explanation:
hope this helps if not let me know have a great day
Calculate the number of oxygen atoms in a 120.0 g sample of diphosphorus pentoxide (P2O5)- Be sure your answer has a unit symbol if necessary, and round it to 4 significant digits
Answers
The number of oxygen atoms in a 120.0 g sample of diphosphorus pentoxide is 2.553 x 10^24. This answer has 4 significant digits and the unit symbol is "oxygen atoms".
To calculate the number of oxygen atoms in a 120.0 g sample of diphosphorus pentoxide (P2O5), we need to use the formula for the number of moles, which is:
n = m/M
Where n is the number of moles, m is the mass of the sample, and M is the molar mass of the compound.
The molar mass of P2O5 can be calculated by adding the molar masses of 2 phosphorus atoms and 5 oxygen atoms:
M = (2 x 30.97) + (5 x 16.00) = 141.94 g/mol
Now we can plug in the values into the formula:
n = 120.0 g / 141.94 g/mol = 0.8453 mol
To calculate the number of oxygen atoms, we need to multiply the number of moles by the Avogadro's number (6.022 x 10^23) and by the number of oxygen atoms in one molecule of P2O5 (5):
N = 0.8453 mol x 6.022 x 10^23 x 5 = 2.553 x 10^24 oxygen atoms
Therefore, the number of oxygen atoms in a 120.0 g sample of diphosphorus pentoxide is 2.553 x 10^24. This answer has 4 significant digits and the unit symbol is "oxygen atoms".
To know more about diphosphorus pentoxide, refer here:
https://brainly.com/question/28024394#
#SPJ11
The two boron atoms listed in the table are isotopes of the boron. The two carbon atoms and the two oxygen atoms are also called isotopes. Based on the patterns in the particle compositions of these atoms, write a definition for “isotopes”
Answers
Answer:
A type of an atom which has a different number of neutrons but the same atomic number, therefore making it the same element. This atom would still have the same properties as well. (Ex: Vanadium-51 is an isotope of Vanadium that has 51 neutrons but still has 23 protons, as its atomic number is 23.)
Isotopes are variants of an element that have the same number of protons in their atomic nucleus, identifying them as the same chemical element, but differ in the number of neutrons.
The variations in neutron numbers among isotopes lead to differences in their atomic masses, resulting in isotopes having slightly different physical properties while retaining similar chemical behavior.
For example, in the case of boron, the two isotopes listed may have the same number of protons (5), but one has 6 neutrons, and the other has 7 neutrons, leading to slightly different atomic masses.
Similarly, for carbon and oxygen, the isotopes exhibit variations in neutron numbers while maintaining the same number of protons, defining them as isotopes of the respective elements. Isotopes play a crucial role in various scientific fields, including radiometric dating, nuclear energy, and medical imaging.
To know more about Isotopes here
https://brainly.com/question/27475737
#SPJ3
What is the concentration of chloride ions when 2.5 g FeCl is dissolved in 150 mL water?
Answers
The concentration of chloride ions when 2.5 g of FeCl is dissolved in 150 mL of water is approximately 0.54 M.
To determine the concentration of chloride ions when 2.5 g of FeCl is dissolved in 150 mL of water, we need to consider the molar mass of FeCl and perform some calculations.
The molar mass of FeCl is 55.85 g/mol (for iron) + 35.45 g/mol (for chlorine), which gives a total molar mass of 91.3 g/mol for FeCl.
First, we need to calculate the number of moles of FeCl present in 2.5 g of the compound. This can be done using the formula:
moles = mass / molar mass
moles of FeCl = 2.5 g / 91.3 g/mol = 0.027 moles
Next, we convert the volume of water from milliliters (mL) to liters (L):
volume of water = 150 mL = 150/1000 L = 0.15 L
Now, we can calculate the concentration of chloride ions (Cl-) using the formula:
concentration (Cl-) = moles of Cl- / volume of water
Since FeCl dissociates into one Fe3+ ion and three Cl- ions, the number of moles of Cl- is three times the moles of FeCl:
moles of Cl- = 3 * moles of FeCl = 3 * 0.027 moles = 0.081 moles
concentration (Cl-) = 0.081 moles / 0.15 L = 0.54 M.
For more such questions on concentration visit:
https://brainly.com/question/28564792
#SPJ8
A sample of SrCO3(s) is added to pure
water and allowed to come to equilibrium
at 25°C. The concentration of Sr²+ is 4.0 x
10-5 M at equilibrium. What is the value of
Ksp for SrCO₂?
Answers
The Ksp of SrCO3 is obtained as 1.6 * 10^-9.
What is the Ksp?The kind of salt used and the temperature of the solution both affect Ksp's value. It offers details on the maximum quantity of salt that can dissolve in the solvent under a specific set of circumstances.
A solution is referred to as supersaturated and the excess ions may precipitate out of solution if the concentration of the ions in it exceeds the value of Ksp.
We know that the Ksp for the SrCO3(s) can be obtained from;
Ksp = [Sr^2+] [CO3^2-]
Ksp = x^2
But [Sr^2+] = [CO3^2-] = 4 * 10^-5 M = x
Thus;
Ksp = (4 * 10^-5)^2
= 1.6 * 10^-9
Learn more about Ksp:https://brainly.com/question/27132799
#SPJ1
What is rate of reaction?
Answers
Answer:
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place. Reaction rate is defined as the speed at which reactants are converted into products. Reaction rates can vary dramatically.
Explanation:
PLS MAKE ME AS BRAINLIST
bond energy is the greatest for?. a: Ch4 b: O2 c: N2 d: Cl2
Answers
It's answer is c. N2
The maximum bond energy is of N2 because N2 molecule is formed by 3 covalent bonds and O2 molecule is formed by 2 covalent bonds.
Hope it helps :)
THIS QUESTION IS HARD BUT I NEED HELP AND ITS DUE IN 20 MINS. YOU HAVE TO ANSWER A AND B FOR EVERYTHING TO BE CORRECT. I KNOW YALL ARE SMART PLSS HELP. THANKS!!

Answers
What mass of aluminum has a total nuclear charge of 1.6 CC? Aluminum has atomic number 13. Suppose the aluminum is all of the isotope with 14 neutrons. slader
Answers
Answer:
aluminium is all of isotope with 14 neutrons .
Sort the following transitions from an excited state to a ground state according to the series into which they fall. Drag the appropriate transitions to their respective bins of lyman series, balmer series, or paschen series. The following transition are n=5 to n=2, n=7 to n=2, n=5 to n=1, n=7 to n=1, n=3 to n=2, n=4 to n=3
Answers
The following transition are n=5 to n=2 is balmer series, n=7 to n=2 is balmer series, n=5 to n=1 is lyman series, n=7 to n=1 is lyman series, n=3 to n=2 is balmer series, n=4 to n=3 is paschen series.
Energy level diagrams show us the various series of lines found in the hydrogen atom's spectrum. The diagram's horizontal lines represent various energy levels. An electron moves from a higher energy level to a lower energy level as shown by the vertical lines.
It is crucial that each transition correspond to a specific characteristic wavelength, as shown in the diagram. As a result, various transitions result in various line series. Among the different series acquired are the Lyman, Balmer, Paschen, Brackett, Pfund, and Henry series, to name a few.
to know more about wavelength visit
https://brainly.com/question/4112024
#SPJ4

In the reaction below what is the molar enthalpy if 1.73 mol A reacts with unlimited B and releases 4567 kJ of heat.
2 A+ 3 B - 2C

Answers
The standard enthalpy change for the reaction 2A+B⇌2C+2D is 664 kJ/mol and The heat that is absorbed when 3.70 mol of A reacts is 2456.8 J
The heat changes that take place as reactants combine to generate a product are measured by the enthalpy of a reaction.
The following formula can be used to determine the enthalpy change of a reaction:
Hess's law states that
Enthalpy of reaction = product's enthalpy - the reactant's enthalpy.
Considering the given reaction: 2A + B ⇌ 2C + 2D
Enthalpy of reaction = product's enthalpy - the reactant's enthalpy.
Enthalpy of reaction (ΔH°f) = (2 C + 2 D) - (2 * A + B)
Enthalpy of reaction (ΔH°f) = {[2(223) + 2(-523)] - [2(-245) + 2(-387)]}
Enthalpy of reaction (ΔH°f) = 664 kJ/mol
ΔH = q ÷ n
ΔH = molar enthalpy (heat) of solution
q = amount of energy (heat) released or absorbed
n = moles of solute
so. q = ΔH xn
q = ΔH xn
q = 664 kJ/mol x 3.70 mol
Q= 2456.8 J
Learn more about enthalpy of reaction at:
brainly.com/question/14291557
#SPJ1
Could use some help, thanks. Complete each row of the table below by filling in the missing prefix or missing exponent.

Answers
Answer:
if eel u gngg ferr
Explanation:
asdads
The angle between the two bonds is called the bond angle. The name of the molecular shape is shown on the molecule geometry in the mailbox. The first row of the following table it’s filled with the information for this model. To fill out the rest of the table, in the bonding box, click the white atom with a single bond to add a third atom to the molecule. Use this molecular model to complete the middle row of the table. Finally add a fourth white atom with single bond, and use The model to complete the last row of the table The angle between the two bonds is called the bond angle. The name of the molecular shape is shown on the molecule geometry in the mailbox. The first row of the following table it’s filled with the information for this model.

Answers
If number of bonds is three (3) then bond angle is 120° and structure is trigonal and if number of bonds is four( 4) then bond angle will be 109° 28' and structure is tetrahedral.
What does "bond angle" mean?The angle between two bonds in a complex molecule or between two orbitals that include bonding electron pairs encircling the core atom is the bond angle. It is calculated and represented in degrees using a spectroscopic method.
How is the bond angle changed?When a single electron pair there at central atom begins to resist the bound pair of electrons, the bond angle decreases and the bonds are slightly shifted inward. When there is a increase in back bonding, the bond angle increases.
To know more about bond angle visit :
https://brainly.com/question/6179102
#SPJ1
Strong acids and bases ____________ dissociate into the respective ions, while weak acids and bases only partially ________ into the H+ and OH- ions.
(60 points)
Answers
Answer:
Strong acids and bases completely dissociate into the respective ions, while weak acids and bases only partially dissociate into the H+ and OH- ions.
Answer: completely and dissociate
Explanation: Strong acids and bases completely dissociate into the respective ions, while weak acids and bases only partially dissociate into the H+ and OH- ions.
Write the formula for the following compounds.

Answers
The cation, anion, and formula for the given compounds are as follows:
sodium iodide: Na⁺; I⁻; Nalzinc sulfide: Zn²⁺; S²⁻; ZnSmagnesium nitride: Mg²⁺; N³⁻; Mg₃N₂barium phosphide: Ba²⁺; P³⁻; Ba₃P₂calcium chloride: Ca²⁺; Cl⁻; CaCl₂aluminum fluoride: Al³⁺; F⁻; AlF₃lithium oxide: Li⁺; O²⁻; Li₂OWhat is the formula of a compound?The formula of a compound is the formula that represents the compounds using the symbols of the elements and showing the ratio in which the elements combine to form the compound.
The ratio in which they combine is written as subscripts in the formula.
Learn more about the formula of compounds at: https://brainly.com/question/8007130
#SPJ1
Write a short essay about life in the Han Dynasty, comparing it to life today. Make sure to include key features:
-Family
-Government
-Social Structure
-Religion
-Trade
Answers
Answer:
Life in the Han Dynasty (206 BCE - 220 CE) differed significantly from today in family, government, social structure, religion, and trade. For example, the Han Dynasty emphasized a patriarchal family structure, where the eldest male held authority, and filial piety was highly valued. In contrast, contemporary societies embrace more egalitarian family dynamics with shared decision-making.
The government system of the Han Dynasty relied on a centralized bureaucracy and emphasized meritocracy, while modern societies often adopted democratic systems. Socially, the Han Dynasty followed a hierarchical model influenced by Confucian principles, whereas contemporary societies strive for greater equality and social mobility.
Religion in the Han Dynasty combined Confucianism, Taoism, and Buddhism, whereas modern societies exhibit diverse religious beliefs. Lastly, trade in the Han Dynasty thrived along the Silk Road, while modern trade was globally interconnected and facilitated by technological advancements. These differences highlight the evolution of society over time.
Explanation:
35 POINTS Y'ALL
What is the difference in petroleum and natural gas?
thanks,
-throckmorton
Answers
Answer:
:)
Explanation:
Petroleum gas is mainly C3 and C4 based (propane and butane), whilst natural gas is predominantly C1 and C2 (methane and ethane). Petroleum gas is generally produced via the cracking of naphtha, which is one of the components separated during crude oil refining.
Petroleum is C3 or C4 (propane and butane) while Natural Gas is C1 and C2. (methane and ethane)
Petroleum is also produced differently then Natural Gas.
"Petroleum gas is generally produced via the cracking of naphtha, which is one of the components separated during crude oil refining." - socratic.org
CxHy +O2 --> H2O + CO2
Question 1 options:
Decomposition
Combustion
Synthesis
Single Displacement
Look down below at the picture thank you

Answers
Single displacement because the oxygen is not balance
In using the Haber process in the formation of ammonia, what mass of hydrogen is needed to produce 51.0 grams of ammonia? 3 H₂(g) + N2 (g) → 2 NH3(g).
Answers
The mass of hydrogen needed to produce 51.0 grams of ammonia is ≈ 9.07 grams.
To determine the mass of hydrogen required to produce 51.0 grams of ammonia (NH3) using the Haber process, we need to calculate the stoichiometric ratio between hydrogen and ammonia.
From the balanced chemical equation:
3 H₂(g) + N₂(g) → 2 NH₃(g)
We can see that for every 3 moles of hydrogen (H₂), we obtain 2 moles of ammonia (NH₃).
First, we need to convert the given mass of ammonia (51.0 grams) to moles. The molar mass of NH₃ is 17.03 g/mol.
Number of moles of NH₃ = Mass / Molar mass
= 51.0 g / 17.03 g/mol
≈ 2.995 moles
Next, using the stoichiometric ratio, we can calculate the moles of hydrogen required.
Moles of H₂ = (Moles of NH₃ × Coefficient of H₂) / Coefficient of NH₃
= (2.995 moles × 3) / 2
≈ 4.493 moles
Finally, we can convert the moles of hydrogen to mass using the molar mass of hydrogen (2.02 g/mol).
Mass of H₂ = Moles × Molar mass
= 4.493 moles × 2.02 g/mol
≈ 9.07 grams
Therefore, approximately 9.07 grams of hydrogen is needed to produce 51.0 grams of ammonia in the Haber process.
Know more about the mass of hydrogen here:
https://brainly.com/question/14083730
#SPJ8
The sun provides _____ to a plant, when a person eats the plant, ____ is released and then transforms into___ when the person goes for a run
Answers
Answer: no answer
Explanation: