you run a reaction which will make ammonia (nh3) as a product. you predict that you should make 8.41 x 1024 molecules of ammonia in your reaction. how many grams of ammonia will you make if your prediction is correct?
Answers
The molecules of ammonia in your reaction. the grams of ammonia will you make is 2.39 g.
given that :
the no. of molecules of the ammonia : 8.41 × 10²⁴
molar mass of ammonia : 17 g /mol
no. of molecules = (mass / molar mass ) × Avogadro's number
8.41 × 10²⁴ = (mass / 17 ) × 6.022 × 10²³
mass = 17 / 7.1
mass = 2.39 g
Thus, a reaction which will make ammonia as a product. you predict that you should make 8.41 × 10²⁴ molecules of ammonia in your reaction. grams of ammonia will you make is 2.39 g.
To learn more about molecules here
https://brainly.com/question/2486276
#SPJ4
Related Questions
2. When two oppositely charged particles are brought near each other, they produce a torceted the stone
shows the force around two changed particles
Which force do the arrows represent?
A an unbalanced force
B. a frictional force
C.an electrical force
D.a gravitational force
Answers
Answer:
A
Explanation:
An unbalanced force is your answer.
calculate the amount of water of crystalization in 10 g of Na2co3 .10 H2o
Answers
Answer:
the mass percentage of water of crystallization in washing soda is 62.9 %.
Explanation:
A laser used in eye surgery to fuse detached retinas produces radiation with a wavelength of 6.4 x 10^-7 m. What is the frequency of the laser?
Speed of light = 3.00 x 10^8 m/s
(Hint: Remember to round your answer to the lowest number of significant figures.)
(Speed of light) / (Wavelength) = Frequency
show steps please.

Answers
Answer:
ν ≈ 4.7 × 10¹⁴ Hz
General Formulas and Concepts:
Chem
Speed of Light = Wavelength times Frequency
c = λν c = 3.0 × 10⁸ m/sExplanation:
Step 1: Define
λ = 6.4 × 10⁻⁷ m
Step 2: Solve for ν
3.0 × 10⁸ = 6.4 × 10⁻⁷ · ν
ν = 4.6875 × 10¹⁴ Hz
Step 3: Check
We are given 2 sig figs. Follow sig fig rules.
4.6875 × 10¹⁴ Hz ≈ 4.7 × 10¹⁴ Hz
What else is produced when sodium carbonate decomposes?
Na2CO3 → Na2O + ?
Answers
Answer:
Na2CO3 → Na2O + CO2
Explanation:
CO2 is your answer
Answer:
CO2
Explanation:
I Just Took The Quiz
Have A Good Day :)
please help, this is for an important grade i really need this..
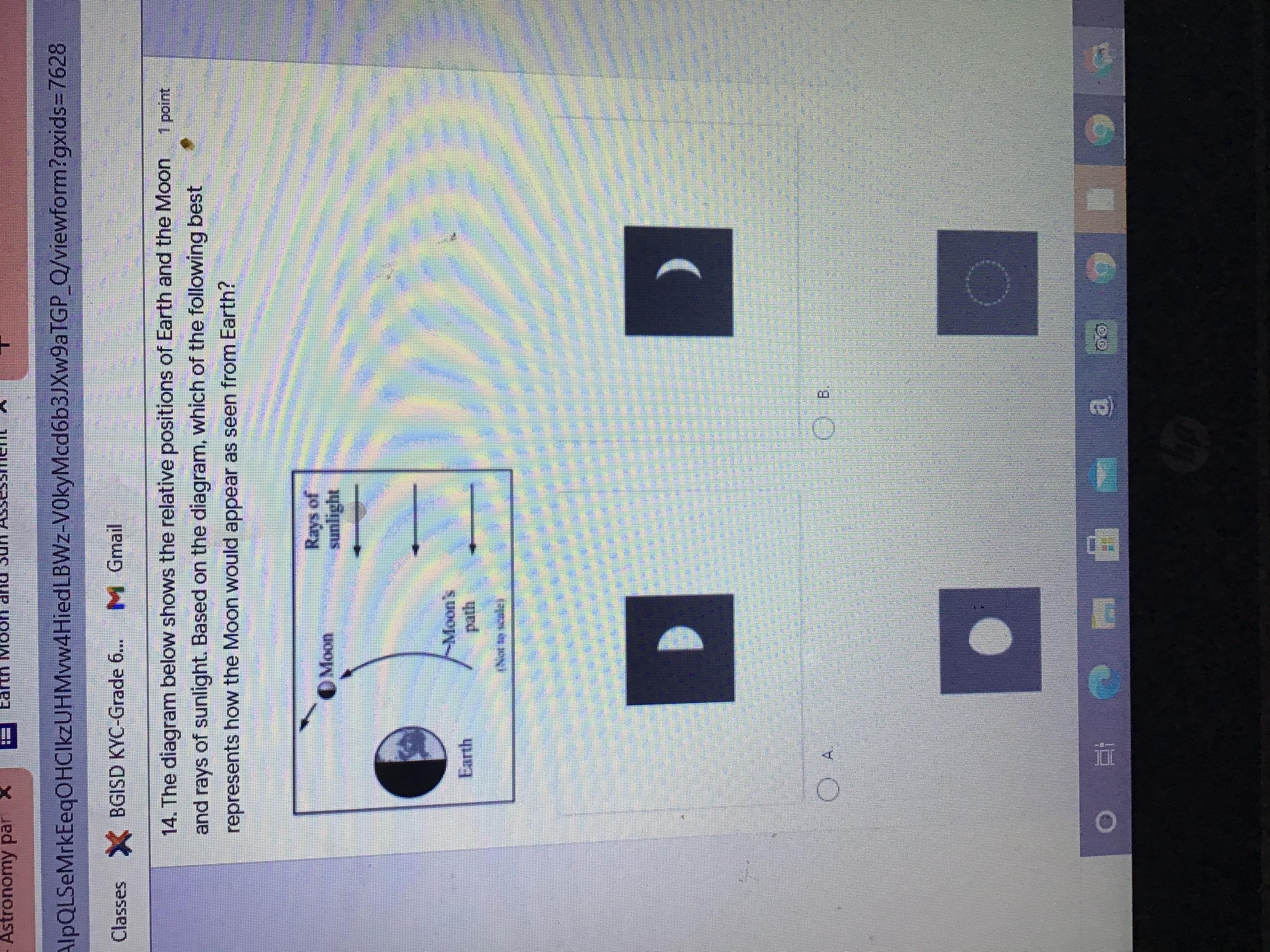
Answers
Answer:
B.
You won't be able to see it cause the dark part of the moon is facing the Earth and isn't receiving any sunlight from the sun
c) Calculate the frequency of light required to remove the valence electron(s).

Answers
The frequency of light required to remove the valence electron(s) from an atom is known as the ionization energy. The ionization energy is dependent on the atomic structure and is specific to each element.
What is calculation of frequency of light?This energy can be calculated using the equation: E = hf = hc/λ, where E is the ionization energy, h is Planck's constant, f is the frequency of the light, c is the speed of light, and λ is the wavelength of the light.E = hf = hc/λwhere E is the ionization energy, h is Planck's constant, f is the frequency of the light, c is the speed of light, and λ is the wavelength of the light.To calculate the frequency of light required to remove the valence electron(s) from an atom, the ionization energy of the atom must be known, and then the equation can be rearranged to solve for frequency:f = E/h = hc/λEIt is important to note that the ionization energy for each element is unique and can be found in periodic table or in the reference book.To learn more about frequency of electron refer:
brainly.in/question/37881991
#SPJ1
Help me with this plz

Answers
Answer:
3.6 moles
Explanation: i got that quistion right .
What is the charge of electrons?
Answers
Answer:
-1
Explanation:
When 1.50 g of Ba(s) is added to 100.00 g of water in a container open to the atmosphere, the reaction shown below occurs and the temperature of the resulting solution rises from 22.00°C to 33.10°C. If the specific heat of the solution is 4.18 J/(g ∙ °C), calculate for the reaction, as written. Ba(s) + 2 H2O(l) → Ba(OH)2(aq) + H2(g)
Answers
Answer:
The amount of heat is 431.12 kJ/mol.
Explanation:
Given that,
Mass of Ba = 1.50 g
Mass of water = 100.0 g
Initial temperature = 22.00°C
Final temperature = 33.10°C
The reaction is,
\(Ba+2H_{2}O\Rightarrow Ba(OH)_{2}+H_{2}\)
We need to calculate the heat
Using formula of heat
\(Q=ms\Delta T\)
Where, m = mass
s = specific heat
\(\Delta T\) temperature
Put the value into the formula
\(Q=(1.50+100)\times4.18\times(33.10-22.00)\)
\(Q=4709.397\ J\)
We need to calculate the amount of heat per mole
Using formula of energy per mole
\(per\ mole\ Q=\dfrac{4709.397}{moles\ of\ Ba}\)
Put the value into the formula
\(per\ mole\ Q=\dfrac{4709.397}{\dfrac{1.5}{137.32}}\)
\(per\ mole\ Q=431129.59\ J/mol\)
\(per\ mole\ Q=431.12\ kJ/mol\)
Hence, The amount of heat is 431.12 kJ/mol.
why can the charge of the nucleus never be +8
Answers
Answer:
it could destroy the nucleus
Explanation:
due too much power going into it
in the ostwald process, 4 molecules of ammonia and 5 molecules of oxygen react to produce 4 molecules of nitric oxide and 6 molecules of water. which
Answers
In the Ostwald process, 4 molecules of ammonia and 5 molecules of oxygen react to produce 4 molecules of nitric oxide and 6 molecules of water.
What is the role of the platinum-rhodium gauze catalyst in the Ostwald process?The platinum-rhodium gauze catalyst is used in the first step of the Ostwald process to catalyze the oxidation of ammonia with oxygen to produce nitric oxide and water. The catalyst helps to lower the activation energy of the reaction and increase the rate of reaction.
The Ostwald process is a chemical process used to produce nitric acid (HNO3) from ammonia (NH3) and oxygen (O2). The process involves two main steps. In the first step, ammonia is oxidized with oxygen to produce nitric oxide (NO) and water (H2O) according to the following reaction:
4NH3 + 5O2 → 4NO + 6H2O
This reaction is exothermic and is typically carried out in a catalytic reactor at temperatures between 800°C and 900°C using a platinum-rhodium gauze catalyst.
In the second step, the nitric oxide is further oxidized with air to produce nitrogen dioxide (NO2) which is then absorbed in water to produce nitric acid:
2NO + O2 → 2NO2
3NO2 + H2O → 2HNO3 + NO
The nitrogen dioxide (NO2) is typically produced by passing the nitric oxide (NO) through a bed of vanadium oxide catalysts and then mixing it with air.
The Ostwald process is an important industrial process for the production of nitric acid, which is used in the production of fertilizers, dyes, and other chemicals.
Learn more about Ostwald process here:
https://brainly.com/question/28295415
#SPJ1
rank the species (carbonate chloride iodate and sulfate) from most to least soluble
Answers
The order of solubility from most to least can be given as carbonate>sulfate>iodate>chloride. Carbonates are the most soluble.
What is solubility?The maximum amount of a material that may dissolve in another is known as its solubility. A saturated solution is created when a solvent can dissolve its maximum quantity of solute before reaching equilibrium. A supersaturated solution results when extra solutes are dissolved past its equilibrium solubility point under specific circumstances.
Dissolution is the action of disintegrating. In contrast to the speed of solution, which specifies how rapidly a molecule dissolves in a solvent, solubility is not a feature of matter. The order of solubility from most to least can be given as carbonate>sulfate>iodate>chloride.
Therefore, the order of solubility from most to least can be given as carbonate>sulfate>iodate>chloride.
To know more about solubility, here:
https://brainly.com/question/14366471
#SPJ1
2. Do you think you would have to exercise longer to use the energy that can be released
from three grams of carbohydrate, fat or protein? Why?
Answers
Which of the following is the correct name for CCl4? O A. Carbon chlorine O B. Carbon tetrachloride O C. Carbon chloride D. Carbon tetrachlorine
Answers
how can we calculate the energy efficiency of the fuel cell is it through 1) the electrical energy divided by the chemical energy , or 2) the energy output divided by energy input, and what does the (energy input) mean if the redox reaction occurs spontaneous?
Answers
The energy efficiency of a fuel cell can be calculated by dividing the electrical energy output by the energy input. In a fuel cell, the energy input refers to the chemical energy stored in the fuel that is converted to electrical energy through a redox reaction.
This redox reaction is spontaneous, meaning that it releases energy when it occurs. Therefore, energy input is calculated by measuring the heat released by the reaction, which is proportional to the chemical energy of fuel. The energy output is determined by measuring the electrical energy produced by fuel cell. The energy efficiency is expressed as percentage, where the higher the percentage, more efficient the fuel cell is in converting chemical energy to electrical energy.
To know more about redox reaction, here
brainly.com/question/13293425
#SPJ1
I need help with answers c and d please help

Answers
The limiting component or reactant in a chemical process is the one that controls how much product is created. Because metals and compounds respond in a balanced chemical equation according to their mole ratios, a limiting reactant is necessary.
What is the limiting reagent?When a chemical reaction is complete, the limiting reagent—also known as the limiting reactant or limiting agent—is the component that has been completely consumed. Since the process cannot proceed without this reagent, the quantity of product that can be produced is constrained.
Excess reagents or surplus reactants are any chemicals that are present in amounts greater than those necessary to cause a reaction with the limiting reagent (sometimes abbreviated as "xs").
Learn more about limiting reagent
https://brainly.com/question/11848702
#SPJ1
When a strontium compound is heated in a flame, red light is produced. When a barium
compound is heated in a flame, yellow-green
light is produced. Explain why these colors are
emitted.
Answers
Predict the ground‑state electron configuration of each ion. Use the abbreviated noble gas notation.
Cr2+ : Cu2+ : Co3+ :
Answers
The electron configuration of an atom or ion is the arrangement of electrons in the orbitals of the atom or ion. It is represented by a list of occupied atomic orbitals in order of increasing energy, with the number of electrons in each orbital given in superscript. The electron configuration of an atom or ion can be used to predict its chemical behavior and reactivity. It is determined by the number of protons in the nucleus, which determines the number of electrons in the atom or ion, and the arrangement of these electrons in the atomic orbitals. The electron configuration of an atom or ion is written using the Periodic Table and the principles of quantum mechanics.
Learn more about electron configuration, here https://brainly.com/question/29757010
#SPJ4
What is the balanced equation for the reaction that takes place when a solution of sodium sulfate is mixed with strontium chloride?
Answers
The balanced chemical equation for the reaction that takes place when a solution of sodium sulfate is mixed with strontium chloride is
Na2SO4 + SrCl2 → 2Nacl + SrSO4
Balanced chemical equation explained.Balanced chemical equation is a chemical reaction in which the number of atoms and moles of the reactant side is the same as the product. This means the reactant side has equal number of atoms and moles with the product.
The balanced equation for the reaction that takes place when a solution of sodium sulfate is mixed with strontium chloride is
Na2SO4 + SrCl2 → 2Nacl + SrSO4
In this reaction, sodium sulfate with strontium chloride to form sodium chloride and strontium sulfate. The coefficients in the balanced equation indicate the stoichiometric ratios of the reactants and the products, ensuring that the same number of atoms of each element is present on both sides of the equation.
Learn more about balanced chemical equation below.
https://brainly.com/question/11904811
#SPJ1
to burn 1 molecule of c3h8 to form co2 and h2o (complete combustion), how many molecules of o2 are required?
Answers
1 molecule of propane combines with 5 molecules of oxygen to produce 3 molecules of carbon dioxide (CO2) and 4 molecules of water (H2O).
To burn 1 molecule of C3H8 completely, 5 molecules of O2 are required. This reaction can be written as follows:
C3H8 + 5O2 → 3CO2 + 4H2O
The balanced equation shows that for every molecule of C3H8 burned, 5 molecules of O2 are needed to completely react with the carbon and hydrogen in the fuel. This information can be useful for calculating the amount of oxygen required for a given amount of fuel, as well as for understanding the environmental impact of burning hydrocarbons.
To burn 1 molecule of propane (C3H8) in a complete combustion reaction, you need 5 molecules of oxygen (O2). The balanced chemical equation for this reaction is: C3H8 + 5O2 -> 3CO2 + 4H2O. In this reaction, 1 molecule of propane combines with 5 molecules of oxygen to produce 3 molecules of carbon dioxide (CO2) and 4 molecules of water (H2O).
To know more about combustion visit:
https://brainly.com/question/31123826
#SPJ11
Write the equilibrium constant expression for the reaction H₂SO₄(aq)+CaF₂(s)⇌2 HF(g)+CaSO₄(s).
Answers
The equilibrium constant expression for the given reaction is K = [HF]² / [H₂SO₄] [CaF₂].
The equilibrium constant expression is derived from the balanced chemical equation. In the given reaction, H₂SO₄(aq) and CaF₂(s) react to form 2 HF(g) and CaSO₄(s). The equilibrium constant expression is written by taking the concentration of the products (HF) raised to the power of their stoichiometric coefficient (²) and dividing it by the concentration of the reactants (H₂SO₄ and CaF₂).
Therefore, the equilibrium constant expression for the reaction is K = [HF]² / [H₂SO₄] [CaF₂].
You can learn more about equilibrium constant expression at
https://brainly.com/question/11353995
#SPJ11
From the molarity of IO3- in saturated solution #1 (8.84 x 10-6), determine the equilibrium concentration of Ca2+ in that solution, and determine the molar solubility of Ca(IO3)2 in saturated solution #1.
Answers
From the molarity of \(IO^{3-}\)in saturated solution #1 (\(8.84 . 10^{-6}\)), the equilibrium concentration of \(Ca^{2+}\) in that solution is: 4.42 x \(10^{-6}\) M and the molar solubility of \(Ca(IO^3)^2\) in saturated solution is: 4.42 x \(10^{-6}\) M.
To determine the equilibrium concentration of Ca2+ in saturated solution #1 with a molarity of \(IO^{3-}\) at 8.84 x 10-6, and determine the molar solubility of\(Ca(IO^3)^2\) in that solution, follow these steps:
1. Write the balanced chemical equation for the dissolution of Ca(IO3)2:\(Ca(IO^3)^2\) (s) ↔ \(Ca^{2+}\) (aq) + 2 \(IO^{3-}\) (aq)2. Use the stoichiometry of the reaction to relate the concentrations of the ions. Since there is a 1:2 ratio between \(Ca^{2+}\) and \(IO^{3-}\)in the balanced equation, the concentration of \(Ca^{2+}\) will be half of the concentration of \(IO^{3-}\).
[\(Ca^{2+}\) ] = 0.5 * [IO3-]3. Plug in the given molarity of \(IO^{3-}\)(8.84 x\(10^{-6}\) )to find the equilibrium concentration of \(Ca^{2+}\):
[ \(Ca^{2+}\)] = 0.5 * (8.84 x 10-6) = 4.42 x \(10^{-6}\)M4. The molar solubility of \(Ca(IO^3)^2\) is equal to the concentration of \(Ca^{2+}\) in the saturated solution:
Molar solubility of \(Ca(IO^3)^2\)= [ \(Ca^{2+}\)] = 4.42 x \(10^{-6}\)M
So, the equilibrium concentration of \(Ca^{2+}\) in saturated solution #1 is 4.42 x \(10^{-6}\) M, and the molar solubility of \(Ca(IO^3)^2\) in that solution is 4.42 x \(10^{-6}\) M.
To know more about "Molarity" refer here:
https://brainly.com/question/13601876#
#SPJ11
new government regulations require that foods containing trans fats be labeled appropriately. a trans fat is formed when food manufacturers turn liquid oils into solid fats by adding hydrogen to vegetable oils. how would this hydrogenation process produce a solid fat?
Answers
The hydrogenation process is a chemical reaction that involves adding hydrogen to vegetable oils, which are naturally liquid at room temperature. This process is typically carried out by food manufacturers to convert liquid oils into solid fats.
During hydrogenation, unsaturated fats present in vegetable oils are chemically modified. These unsaturated fats have double bonds in their molecular structure. The hydrogenation process aims to break some of these double bonds and add hydrogen atoms to the fatty acid molecules. The addition of hydrogen atoms to the unsaturated fatty acid chains changes their structure and causes them to straighten out. This straightening effect leads to the formation of a more solid or semi-solid fat. The degree of hydrogenation determines the final consistency of the fat. Fully hydrogenated fats are solid, while partially hydrogenated fats tend to be semi-solid or have a creamy texture.
Learn more about hydrogenation process here:
https://brainly.com/question/30064037
#SPJ11
what is the minimum mass of caco3 required to establish equilibrium at a certain temperature in a 6.50-l container if the equilibrium constant (kc) is 0.50 for the decomposition reaction of caco3 at that temperature
Answers
the reaction of the equilibrium for caco3 was found to be: CaCO 3(s)⇌CaO(s)+CO 2(g); K c=0.005 mole/litre A 3.25 g
what is calcium carbonate (caco3) ?
CaCO3 is the chemical formula for calcium carbonate. It is found in rocks as the minerals calcite and aragonite (most notably as limestone, a form of sedimentary rock composed primarily of calcite) and is the primary component of eggshells, gastropod shells, shellfish skeletons, and pearls. Calcareous refers to things that contain or resemble calcium carbonate. The active element in agricultural lime is calcium carbonate, which is formed when calcium ions in hard water combine with carbonate ions to form limescale. It is used in medicine as a calcium supplement or as an antacid, but excessive ingestion can be harmful, causing hypercalcemia and digestive problems.
the reaction of the equilibrium for caco3 was found to be: CaCO 3(s)⇌CaO(s)+CO 2(g); K c=0.005 mole/litre A 3.25 g
To learn more about calcium carbonate follow the given link: https://brainly.com/question/12024964
#SPJ4
Show your calculations/solutions to earn a full marks of 25 points.
A piece of silver of mass 362 g has a heat capacity of 85. 7 J/°C. What is the specific heat of silver?
Answers
To calculate the specific heat of silver, you will need to use the formula:
specific heat (c) = total heat absorbed (Q) / mass (m) * change in temperature (ΔT)So for this example, we can calculate the specific heat of silver as follows:
c = 85.7 J / (362 g * (65.3 - 42.1))c = 0.523 J/g°CTherefore, the specific heat of silver is 0.523 J/g°C.
The specific heat of silver is a measure of the amount of energy required to increase the temperature of one gram of silver by one degree Celsius. This value is important in understanding how different materials respond to temperature changes and can be used in many areas of science and engineering.
Learn more about the specific heat of silver:
https://brainly.com/question/9422819
#SPJ4
What is the name of NoCk4? Explain how you determined the bond type and the steps you used to determine the naming convention for the compound.
Answers
emission of a 1282 nm photon lowers an electron in hydrogen from n=5 to which value of n?
Answers
The emission of a 1282 nm photon lowers an electron in hydrogen from n=5 to n=3.
The energy of the photon corresponds to the energy difference between the two energy levels in the hydrogen atom. The energy of a photon is given by the equation E = hc/λ, where h is Planck's constant, c is the speed of light, and λ is the wavelength of the photon.
Plugging in the given values, we find that the energy of the 1282 nm photon is E = (6.626 x 10⁻³⁴ J s)(3 x 10⁸ m/s)/(1282 x 10⁻⁹ m) = 1.55 x 10⁻¹⁸ J.
This energy corresponds to the difference between the energy levels of n=5 and n=3 in the hydrogen atom, so the electron drops from n=5 to n=3 after emitting the photon.
Learn more about energy of photon here: https://brainly.com/question/27856390.
#SPJ11
identify the parts of the compound light microscope.
Answers
A compound light microscope consists of the following main parts:
Base: Supports the entire microscope and provides stability.
Body Tube: Connects the eyepiece to the objective lenses.
Arm: Supports the body tube and connects it to the base.
Nosepiece: Holds the objective lenses and allows them to rotate for easy magnification changes.
Objective Lenses: Provide magnification and are usually 4 lenses of different magnification powers (4x, 10x, 40x, 100x).
Condenser Lens: Focuses light onto the specimen and is adjustable for optimal illumination.
Diaphragm: Controls the amount of light that reaches the specimen.
Illumination Source: A bulb that provides light to the specimen.
Eyepiece: Magnifies the image and is usually 10x magnification.
Stage: Supports the slide and has clips to hold it in place.
Focus Knobs: Allow the user to adjust the focus by moving the stage and/or the body tube up and down.
Find out more about compound light microscope
brainly.com/question/30126710
#SPJ4
Answer number 5 please if you say for point I will report you:)

Answers
Answer:
A
Explanation:
Lighting a fireworks the potential energy is converted to all forms of energy like light, heat and sound energy
Name and describe the two types of observations. Please help!
Answers
Answer:
Quantitative and Qualitative :)
Explanation: