You have the compound CH4. The first element is a
(metal, nonmetal) and the second element is a
(metal, nonmetal). You would predict that this would form a(n).
(ionic, covalent) bond.
ASAP

Answers
Answer:
1. Nonmetal
2. Nonmetal
3. Covalent
Explanation:
CH4 is the chemical formula for the methane molecule in which four atoms of hydrogen is chemically bonded to one atom of carbon. Both carbon, which is the first element and hydrogen the second element are non-metals, and hence can only form a covalent bond.
A covalent bond is a type of chemical bond in which two atoms share their valence electrons. It occurs between two non metallic atoms. In this molecule, CH4, carbon and hydrogen share their valence electrons to form a covalent bond.
Related Questions
Which are steps of mitosis? Select 4 options.
prophase
metaphase
interphase
anaphase
telophase
Answers
Metaphase
Anaphase
Telophase
Answer:
Prophase, metaphase, anaphase, telophase
Explanation:
Pb(NO3)2(aq) + KCl(aq) ---- → KNO3(aq) + PbC12(s)
Answers
Pb(NO3)2(aq) + 2KCl(aq) -> 2KNO3(aq) + PbCl2(s)
Just remember: the equations at the end is Cl not C12
Note: the small number on the bottom (subscripts) apply to the one element if it’s inside the bracket and if the small number is on the outside of the bracket it applies to all the elements. For example the 3 in (NO3)2 applied only to the O (oxygen) and the 2 applies to both N and O but don’t forget it’s multiplied. So it would be 2 N’s and 6 O’s bc the 3 multiplies with the 2 only for the O.
A pan containing 20.0 grams of water was allowed to cool from a temperature of 95.0 °C. If the amount of heat released is 1,200 joules, what is the approximate final temperature of the water?
75 °C
78 °C
81 °C
87 °C
Answers
Answer:
81 °C
Explanation:
This is a calorimetry question so a few things you will need for this. The calorimetry equation q=mcΔT & the specific heat of water (4.2J/g•°C). Other definitions are:
q = heat added/released by a sample
m = mass of sample
c=specific heat of sample
ΔT = change in temperature
from here we can rearrange the equation to state:
q/(mc) = ΔT
1200J/((20.0g)(4.2J/g•°C)) = ΔT
14°C = ΔT
If the starting temperature was 95.0°C and we know that the temperature was cooled by 14°C then the final temperature of the water would be 81.
Answer:
C. 81 c
Explanation:
took the test :)
Gas is confined in a metal tank represented by the figure below. At 283.2K 283.2 K , the gas exerts a pressure of 7.571atm 7.571 a t m . After heating the tank, the pressure of the gas increases to 12.846atm 12.846 atm.
Answers
The gas in the tank has a final temperature of roughly 480.5 K.
Why is the gas under pressure?Gas molecules interacting with an object's surface generate force, which is what creates gas pressure. Although though there is very little force involved in each impact, any surface with a sizeable area is subject to many of them quickly, which can lead to a high pressure.
As per the ideal gas law,
PV = nRT
We can write: Provided that the volume and quantity of moles of gas remain constant.
P1/T1 = P2/T2
where the initial pressure and temperature are P1 and T1, and the ultimate pressure and temperature are P2 and T2.
With the values provided, we have:
P1 = 7.571 atm
T1 = 283.2 K
P2 = 12.846 atm
We can solve for T2:
T2 = (P2/P1) * T1
= (12.846 atm / 7.571 atm) * 283.2 K
= 480.5 K
To know more about gas pressure visit:-
https://brainly.com/question/9099789
#SPJ1
What is the definition of Chemical Symbol
Answers
A chemical symbol is a notation of one or two letters representing a chemical element.
help me plss. A DIRECTION. Determine the percentage composition of all the elements in the compounds. Write your answer on the space provided.

Answers
%element:
\(\tt \%=\dfrac{Ar\times subscript}{Mr} \)
Ag₂O:
\(\tt \%Ag=\dfrac{108\times 2}{232}=93.1\%\\\\\%O=\dfrac{16}{232}=6.9\% \)
KClO₃:
\(\tt \%K=\dfrac{39}{122.5}=31.8\%\\\\\%Cl=\dfrac{35.5}{122.5}=29\%\\\\\%O=\dfrac{16\times 3}{122.5}=39.2\%\\\\or\:100-(31.8+29)=39.2\% \)
SO₃:
\(\tt \%S=\dfrac{32}{80}=40\%\\\\\%O=\dfrac{16\times 3}{80}=60\% \)
CCl₄:
\(\tt \%C=\dfrac{12}{154}=7.8\%\\\\\%Cl=\dfrac{35.5\times 4}{154}=92.2\%\\\\or\:100-7.8=92.2\% \)
does earth's tilt change over the course of a year
Answers
The slanted axis of the Earth points in the same general direction as it revolves around the Sun. As a result, during the year, the Sun's direct rays reach various locations on Earth. Around June, the North Pole will occasionally tilt toward the Sun. Other times, the South Pole will do similarly.
What is earth ?The elements of Earth are land, air, water, and life. Mountains, valleys, and flat places can all be found on the ground. Different gases, namely nitrogen and oxygen, make up the air. Rain, snow, ice, rivers, lakes, oceans, and streams are all examples of water.
To its orbital plane, the earth's spin axis is inclined. The seasons are brought on by this. It is summer in that hemisphere when the axis of the earth faces the sun. Winter is to be anticipated when the axis of the planet is pointing away.
Thus, during the year, the Sun's direct rays reach various locations on Earth.
To learn more about the earth, follow the link;
https://brainly.com/question/12041467
#SPJ1
Need help please ! if someone can answer any of these that would be great !!

Answers
Answer:
NH4 ClBa Br2Al4 C3Na2 OK3 PO4Ca F2Li3 NBa CO3Al2 (SO4)3Mg (NO3)2Li2 SNa2 SO4Ca (HCO3)2Ag CH3 COOZn (OH)2Explanation:
Here are all of them- your picture had #1-13, but here's #1-15!
what do the formulas, arrow, and plus signs in a chemical equation tell you?
Answers
The formulas in a chemical equation represent the different compounds or molecules involved in the reaction. The arrow indicates the direction of the reaction, usually pointing from the reactants to the products.
In a chemical equation, the formulas, arrow, and plus signs convey important information about the chemical reaction taking place. The plus signs indicate that multiple reactants or products are present.
1. Formulas: These represent the chemical compounds involved in the reaction, with each formula showing the elements and their proportions in the compound. The formulas on the left side of the equation are the reactants, and those on the right side are the products.
2. Arrow: The arrow in the equation (→) represents the direction of the reaction, indicating that the reactants on the left side are converted into the products on the right side. It can be read as "yields" or "forms."
3. Plus signs: These denote that two or more reactants or products are involved in the reaction. A plus sign between reactants or products indicates they are separate entities participating in or resulting from the chemical reaction.
In summary, the formulas, arrow, and plus signs in a chemical equation describe the reactants, products, and the process of the chemical reaction taking place.
Learn more about chemical equation:
brainly.com/question/30087623
#SPJ11
what mass (in g) of potassium chlorate is required to supply the proper amount of oxygen needed to burn 134.9 g of methane? assume 100% yield for both reactions. enter to 0 decimal places. are the equations balanced?
Answers
Mass of potassium chlorate needed: 187 g. To find the mass of potassium chlorate needed.
we can use the balanced chemical equation for the reaction between potassium chlorate and heat to form potassium chloride and oxygen. The balanced equation is: 2KClO3 -> 2KCl + 3O2. Since 134.9 g of methane reacts with 8 g of oxygen to form carbon dioxide and water,
we can use this information to determine the amount of oxygen required for complete combustion. 134.9 g of methane requires 8 * 134.9 g = 1079.2 g of oxygen.
To produce this amount of oxygen from potassium chlorate, we would need 1079.2 g / 3 = 359.7 g of potassium chlorate.
Since the reaction only has a 100% yield, we would need 359.7 g of potassium chlorate. The equation is balanced.
Learn more about potassium chlorate here:
https://brainly.com/question/488887
#SPJ4
there are five basic taste sensations: sweet, sour, bitter, salty, and umami. b. fluid is needed to help dissolve foods for tasting. c. they react only with particles in solution.
Answers
Only particles in solution react during taste perception, which is also influenced by scent, appearance, and temperature.
How do umami, bitter, and sweet taste cells get activated?
G protein-coupled proteins (GPCRs) from the T1R and T2R groups are activated on tasting receptor cells to release the bitter, umami, and sweet sensations that mammals detect.
What causes the perception of sweetness?
The brain's neurological pathways for interpreting and responding to sweet stimuli are activated when sweet endings in the mouth—which are involved in the experience of sweet taste—are stimulated with either nutritional sweeteners or LCS.
To know more temperature about :
https://brainly.com/question/11464844
SPJ4
Is C6H1206 an element or compound
Answers
C6H1206 is a compound (of glucose).
2H,0
o,
2H,
rogen perode
oxygen
This chemical reaction shows that two molecules of hydrogen peroxide (H 0.) break
apart to form two molecules of water (H.0) and one molecule of oxygen (0,1
How does the arrangement of atoms and bonds
change?

Answers
Answer:
Due to removal of oxygen and number of hydrogen bonds.
Explanation:
The arrangement of atoms and bonds change due to removal of oxygen molecule from hydrogen peroxide forming water which has one oxygen less than hydrogen peroxide. In hydrogen peroxide, four identical hydrogen bonds are formed with Oxygen while on the other hand, in water, two atoms of hydrogen attached by covalent bonds to the same atom of oxygen.
When comparing ethanol, CH3CH2OH, and sodium ethoxide, NaOCH2CH3 ________________ is the better nucleophile because it ____________.
Answers
When comparing ethanol, CH3CH2OH, and sodium ethoxide, NaOCH2CH3, Sodium ethoxide, NaOCH2CH3, is the better nucleophile because it has a negatively charged oxygen atom which can easily donate its electron pair to another atom or molecule.
Sodium ethoxide, NaOCH2CH3, is the better nucleophile because it has a negatively charged oxygen atom which can easily donate its electron pair to another atom or molecule.
Ethanol, CH3CH2OH, on the other hand, has a polar covalent bond between the carbon and oxygen atoms, but does not have a negative charge on the oxygen atom. Therefore, it is not as strong of a nucleophile as sodium ethoxide.
In general, nucleophilicity is affected by several factors such as charge, electronegativity, steric hindrance, and solvation. In this case, the negative charge on the oxygen atom of sodium ethoxide makes it a strong nucleophile.
To learn more about Sodium ethoxide:
https://brainly.com/question/13130552
#SPJ4
As new information is discovered, scientific theories are changed. Sometimes, old theories are revised. Why is this a necessary practice for science? Group of answer choices Old theories are dependent on primitive technology that was flawed, so new technology must be used. The process allows for an increasingly accurate understanding of the world It ensures that the newest ideas are the ones used to develop medicines and important safety equipment. New scientists need to get credit for their ideas or no one will go into science professions
Answers
Answer:
Old theories are dependent on primitive technology that was flawed, so new technology must be used.
Explanation:
The process of science is an unending process of inquiry into the nature of the universe. This inquiry into the nature of the universe is aimed at understanding of how things work and how the natural course of the universe can be exploited for man's good.
As new scientific evidences emerge, old theories are revised because we understand the world better, better technology is used to investigate nature in order to get a clearer view of natural phenomena.
Most old theories depend on obsolete technology, hence modern day sophisticated equipment must be used to correct the flaws of old theories.
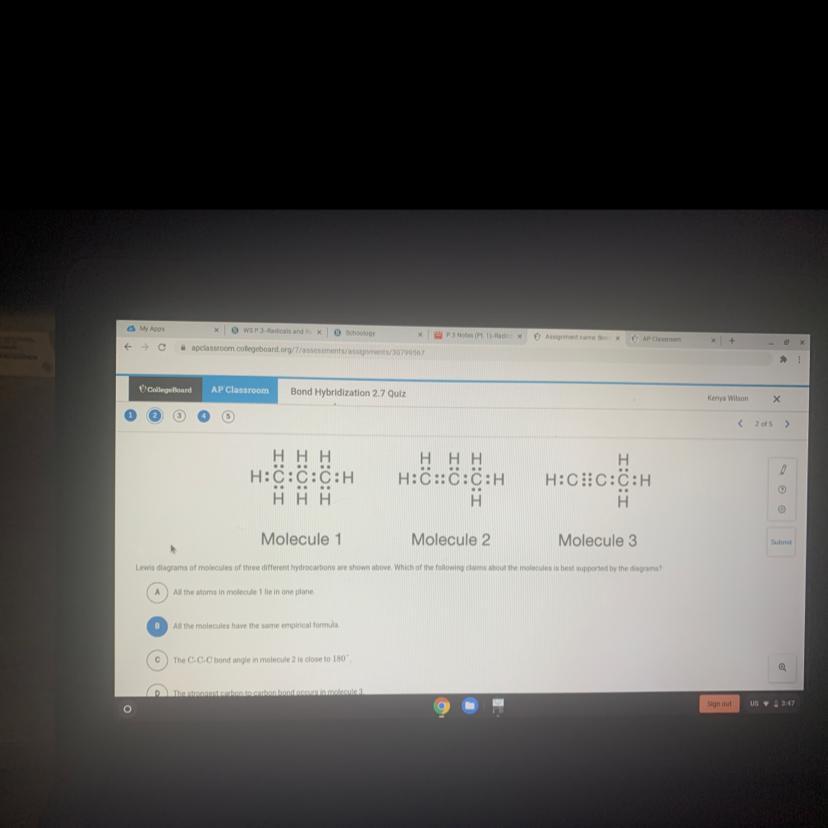
Lewis diagrams of molecules of three different hydrocarbons are shown above. Which of the following claims about the molecules is best supported by the diagrams?
Answers
Answer:
D
Explanation:
Seeing the Lewis formula for each of the molecules, we can attempt to write the condensed structural formula for each.
What we notice is that while there are purely single bonds between the carbon atoms in the first molecule, there is a double bond in the second molecule and a triple bond in the third
What we can deduce from this is that these are alkanes , alkenes and alkynes
The alkynes have the strongest of the three bonds and thus they have the strongest bonds in the mix
Since molecule 3 is an alkyne, then we can say that it has the strongest of the bonds
The correct claim about the molecules that best supports the diagrams is :
( D ) The strongest carbon to carbon bond occurs in molecule 3
The first molecule ( molecule 1 ) is an alkane because the molecule is made up of only single bonds connecting the carbon atoms.
while the second mole ( molecule 2 ) is an alkene because of the presence of a double bond connecting carbon atoms.
and the third molecule ( molecule 3 ) is an alkyne because of the presence of a triple bond connecting carbon atoms.
Therefore Alkynes have the strongest of of bonds between its carbon atoms because of the presence of a triple connecting carbon atoms.
Hence we can conclude that The strongest carbon to carbon bond occurs in molecule 3
Learn more about alkynes : https://brainly.com/question/22138155
In the tropical rainforests, birds have evolved to eat the brightly-colored fruits. The fruits have evolved their attractive color so the birds will eat them and spread their seed. What theory is this an example of? A artificial selection b coevolution c mutation d natural selection
Answers
Answer:
coevolution
Explanation:
Coevolution plays a key role in shaping the biodiversity on Earth. Coevolution is commonly defined as reciprocal evolutionary changes brought about by interactions between species, implying that interacting species impose selection on each other(Science direct).
We can see that the change that occurred in the plants of the tropical rainforests is closely related to their link to birds. The fruits evolved an attractive color so that birds, having evolved to eat the brightly-colored fruits, may eat them.
what is the predominant charge of the amino acid abbreviated e at ph 7?
Answers
At pH 7, the amino acid abbreviated as "E" (glutamic acid) is predominantly negatively charged (deprotonated). This is because at pH 7, the carboxyl group (-COOH) of glutamic acid tends to lose a proton (H+), resulting in the formation of a negatively charged carboxylate ion (-COO-).
The amino group (-NH2) of glutamic acid, on the other hand, remains protonated, carrying a positive charge. Therefore, the overall charge of the glutamic acid molecule at pH 7 is negative.
Glutamic acid (abbreviated as "E") is an amino acid that contains both a carboxyl group (-COOH) and an amino group (-NH2) in its structure. At pH 7, which is near neutral, the carboxyl group tends to lose a proton and become deprotonated. This results in the formation of a negatively charged carboxylate ion (-COO-). The amino group, however, remains protonated and carries a positive charge (+NH3+).
The deprotonation of the carboxyl group and the protonation of the amino group are influenced by the pH of the surrounding environment. At pH 7, which is close to the pKa value of the carboxyl group, the majority of glutamic acid molecules will have a negatively charged carboxylate group and a positively charged amino group, resulting in a net negative charge.
In summary, at pH 7, the amino acid abbreviated as "E" (glutamic acid) is predominantly negatively charged.
Learn more about amino acid here: brainly.com/question/31872499
#SPJ11
The predominant charge of the amino acid E at pH 7 is negative.
The predominant charge of an amino acid at a specific pH is determined by the ionization of its functional groups, specifically the amino group (\(NH_2\)) and the carboxyl group (COOH). The pH affects the ionization state of these groups.
Amino acids can be categorized into three groups based on their ionization behaviour:
1. Acidic amino acids: At pH 7, acidic amino acids like glutamic acid (abbreviated as E) have a carboxyl group (COOH) that is ionized and carries a negative charge (COO-). The amino group (\(NH_2\)) remains uncharged.
2. Basic amino acids: Basic amino acids, such as lysine or arginine, have an amino group (\(NH_2\)) that is ionized and carries a positive charge (\(NH_3^+\)). The carboxyl group (COOH) remains uncharged.
3. Neutral amino acids: Neutral amino acids, like glycine or alanine, have both the amino group (\(NH_2\)) and the carboxyl group (COOH) in their neutral, non-ionized forms at pH 7.
In the case of the amino acid abbreviated E (glutamic acid), at pH 7, the carboxyl group (COOH) is ionized and carries a negative charge (\(COO^-\)), while the amino group (\(NH_2\)) remains uncharged.
Therefore, the predominant charge of the amino acid E at pH 7 is negative.
To learn more about predominant charge from the given link
https://brainly.com/question/31035959
#SPJ4
When a chemical system is at equilibrium, the concentrations of the reactants are equal to:___________.
1. the concentrations of the products.
2. the concentrations of the reactants and products have reached constant values.
3. the forward and reverse reactions have stopped. the reaction quotient, , has reached a maximum.
4. the reaction quotient, , has reached a minimum.
Answers
Answer:
catalyst
Explanation:
A passenger elevator travels form a distance of 219 m in 32s. What is the elevator speed? Round up your answer to one decimal place.
Answers
The speed of the elevator rounded up to one decimal place is approximately 6.8 m/s.
What is the formula to calculate the speed of an object?The formula to calculate the speed of an object is given below:
Speed = distance covered / time taken.In the case of the elevator, the distance traveled by elevator is 219 m, and the time taken is 32 s.
Therefore, the speed of the elevator is:
Speed = 219 m / 32 s = 6.84 m/s (rounded to two decimal places)
Rounding up to one decimal place, the speed of the elevator is approximately 6.8 m/s.
Learn more about speed at: https://brainly.com/question/27888149
#SPJ1
Identify the acid associated with each conjugate base. I-SO4 2-Cl-OH -F-
Answers
The acid associated with each conjugate base is I-SO4 2= H2SO4 (sulfuric acid), ClO= HClO(hypochlorous acid), OH= H2O( water) and F- = HF (hydrofluoric acid).
For each conjugate base, the associated acid can be identified by adding a proton (H+) to the anion. The acid associated with each conjugate base is as follows:
I-SO4 2- : The conjugate base is derived from the acid H2SO4, which is sulfuric acid. The acid can donate two protons to form H+ ions and SO4 2- ions in aqueous solution.ClO- : The conjugate base is derived from the acid HClO, which is hypochlorous acid. The acid can donate a proton to form H+ and ClO- ions in aqueous solution.OH- : The conjugate base is derived from the acid H2O, which is water. The acid can donate a proton to form H+ and OH- ions in aqueous solution.F- : The conjugate base is derived from the acid HF, which is hydrofluoric acid. The acid can donate a proton to form H+ and F- ions in aqueous solution.Conjugate acid-base pairs are important in many chemical reactions, including acid-base reactions, redox reactions, and complex formation reactions. In acid-base reactions, for example, the acid donates a proton to the base to form its conjugate base and conjugate acid. The strength of the acid and base determines the position of the equilibrium in the reaction, with stronger acids and bases driving the reaction toward the formation of their weaker conjugate partners.
In summary, the concept of conjugate acids and bases is a fundamental aspect of acid-base chemistry and helps to explain the behavior of acids and bases in many different chemical reactions.
Learn more about conjugate base here:
https://brainly.com/question/30225100
#SPJ4
What happens to wavelength as wave frequency increases?
Answers
Answer:
As a wavelength increases in size, its frequency and energy (E) decrease. From these equations you may realize that as the frequency increases, the wavelength gets shorter. As the frequency decreases, the wavelength gets longer.
Explanation:
Which of the following polyatomic ions will form an ionic compound with two sodium ions? CO32− HCO31− NO21− NO31−
Answers
Answer:
CO32−
Explanation:
We have to consider the valencies of the polyatomic ions involved. Recall that it is only a polyatomic ion with a valency of -2 that can form a compound which requires two sodium ions.
When we look closely at the options, we will realize that among all the options, only CO32− has a valency of -2, hence it must be the required answer. In order to be double sure, we put down the ionic reaction equation as follows;
2Na^+(aq) + CO3^2-(aq) ---------> Na2CO3(aq)
Answer:
A). CO32−
Explanation:
For the reaction represented by the equation AgNO3 + NaCl ® NaNO3 + AgCl,
how many moles of silver chloride, AgCl, is produced from 7.0 mol of silver nitrate AgNO3?
Answers
Answer:
7
Explanation:
7 mol AgCL * \(\frac{1 mol AgNO3}{1 mol AgCl}\)
You can cancel out the "mol AgCl" to get 7 moles of AgNO3
Is melting cheese on a hamburger a form of radiation
Answers
yes it is
............
Required: a. Compute the acid-test ratio for each of the separate cases above. b. Which company is in the best position to meet short-term obligations? Complete this question by entering your answers
Answers
Among the three companies, Company A has the highest acid-test ratio (2.67), followed by Company B (2.00), and then Company C (1.75). The higher the acid-test ratio, the better the company's ability to meet short-term obligations without relying on inventory sales. Therefore, Company A is in the best position to meet short-term obligations.
To compute the acid-test ratio (also known as the quick ratio) for each company, we'll use the formula:
Acid-Test Ratio = (Current Assets - Inventory) / Current Liabilities
a. Compute the acid-test ratio for each company:
Company A:
Current Assets = $250,000
Inventory = $50,000
Current Liabilities = $75,000
Acid-Test Ratio for Company A = ($250,000 - $50,000) / $75,000
Acid-Test Ratio for Company A = $200,000 / $75,000
Acid-Test Ratio for Company A ≈ 2.67
Company B:
Current Assets = $150,000
Inventory = $30,000
Current Liabilities = $60,000
Acid-Test Ratio for Company B = ($150,000 - $30,000) / $60,000
Acid-Test Ratio for Company B = $120,000 / $60,000
Acid-Test Ratio for Company B = 2.00
Company C:
Current Assets = $300,000
Inventory = $90,000
Current Liabilities = $120,000
Acid-Test Ratio for Company C = ($300,000 - $90,000) / $120,000
Acid-Test Ratio for Company C = $210,000 / $120,000
Acid-Test Ratio for Company C = 1.75
To know more about Current Liabilities
https://brainly.com/question/29341666
#SPJ11
explain why is energy input required to add an electron to zinc
Answers
Answer: When you add an electron to zinc, it needs some extra energy. This is because zinc atoms naturally don't like having an extra electron. The extra electron and the electrons already present in zinc repel each other due to their negative charges. So, you have to give some energy to the zinc atom to overcome this repulsion and make it accept the additional electron. Basically, energy input is required to make zinc accept an extra electron because the electron doesn't fit easily and needs some force to be added.
Explanation: hope this helps
Summary of exothermic and endothermic
Please help with the chart 10 questions
1. Which type of energy is representing by the moving of objects?
2. What type of energy is stored?
3. Explain the lot of conversation of energy?
4. A metal spoon and a wooden spoon are placed in a pot of boiling water. What property of the metal spoon causes it to become hotter than the wooden spoon and why?
5. The thing we measure when we want to determine the average kinetic energy of random motion is the particles of a substance is ___________.
6. The __________ is the energy needed to raise the temperature of a substance by 1°C.
7. A(n) ________ reaction is one where the products have lower energy than the reactants.
8. __________ reaction requires energy in order to take place.
9. The __________ is used to describe how much energy is produced or used during a chemical change.

Answers
What is an Biomedical Engineering
Answers
as a plant roots grow they produce weak acids that slowly dissolve rock around the roots. lichens plant like organisms that grow on rocks also produce weak acids
Answers
Both plant roots and lichens have the ability to produce weak acids that slowly dissolve rock in their immediate surroundings.
Plant roots secrete weak acids, such as organic acids, as a part of their growth process. These acids aid in the breakdown of minerals in the soil, facilitating the uptake of essential nutrients by the plants. As roots grow and extend into the soil, the weak acids they release can gradually dissolve minerals present in the rocks surrounding them. Over time, this process can contribute to the weathering and erosion of the rock material.
Similarly, lichens, which are symbiotic organisms consisting of a fungus and an alga or a cyanobacterium, also produce weak acids. Lichens can grow on rocks and other substrates, utilizing their acid-producing capabilities to extract nutrients and minerals from the rocks. The weak acids they release can slowly break down the mineral content of the rocks, contributing to physical and chemical weathering.
Both plant roots and lichens play a role in the process of bioerosion, where living organisms contribute to the breakdown and alteration of rocks. Their production of weak acids enables them to interact with and modify their surrounding environment, albeit on a relatively slow timescale.
To learn more about Plant Roots
brainly.com/question/8647279
#SPJ11