Answers
Answer:
32
Explanation:
because the number of heliem is your aswer
Answer:
so the balloon can fill up with air. If the balloon pops, then the air goes away.
Related Questions
Electronic configuration PLZZZ GUYSS(sub shell distribution) for L shell will be .........................
Answers
Answer:
Shells
The maximum number of electrons that can be accommodated in a shell is based on the principal quantum number (n). It is represented by the formula 2n2, where ‘n’ is the shell number. The shells, values of n, and the total number of electrons that can be accommodated are tabulated below.
Shell and ‘n’ value Max. Electrons in the Electron Configuration
K shell, n=1 2*12 = 2
L shell, n=2 2*22 = 8
M shell, n=3 2*32 = 18
N shell, n=4 2*42 = 32
L shell: n =2
hence,
2n²
= 2(2)²
i.e. 8 electrons can fit in the l shell
hence max no. of electrons is 8
so configuration in L shell is,
2, 8...
In a particular redox reaction, NO is oxidized to NO−3
and Cu2+ is reduced to Cu+.
Complete and balance the equation for this reaction in acidic solution. Phases are optional.
balanced redox reaction:
NO + Cu^{2+} -> NO_{3}^{-} + Cu^{+}
Answers
The balanced redox equation for the given reaction in acidic solution is:
\(3NO + 3Cu^2+ + 2e^- - > 3NO_3^- + 3Cu^+.\)
To balance the redox reaction:
\(NO + Cu^2+ - > NO_3^- + Cu^+\)
First, let's assign oxidation states to each element/ion in the equation:
Oxidation state of N in NO: +2
Oxidation state of N in \(NO_3^-: +5\)
Oxidation state of Cu in \(Cu^2+: +2\)
Oxidation state of Cu in \(Cu^+: +1\)
From the given oxidation states, we can see that N is being oxidized from +2 to +5, and Cu is being reduced from +2 to +1. We need to balance both the charge and the number of atoms on each side of the equation.
Balancing the nitrogen:
We need three NO molecules on the reactant side to balance the nitrogen atoms on the product side. Thus, the equation becomes:
\(3NO + Cu^2+ - > NO_3^- + Cu^+\)
Balancing the charge:
The charge on the reactant side is 0 (since NO is neutral), while on the product side, we have 1- charge from NO_3^- and 1+ charge from Cu^+. To balance the charges, we need two electrons on the reactant side.
Final balanced equation:
\(3NO + 3Cu^2+ + 2e^- - > 3NO_3^- + 3Cu^+\)
In acidic solution, we need to balance the hydrogen ions \((H^+)\). In this case, there are no hydrogen ions on either side of the equation, so no additional steps are needed.
For more such information on: redox equation
https://brainly.com/question/27907895
#SPJ8
Method A: Dilute 10.00 mL up to 100 mL with a transfer pipet and volumetric flask. Then take 10.00 mL of the dilute solution and dilute it again to 100 mL.
Answers
Based on dilution methods, the dilution given is a serial dilution with dilution factor of 1/10.
What is serial dilution?A serial dilution is the stepwise dilution of a substance in solution usually using a constant dilution factor at each step of dilution.
The above dilution step is a serial dilution.
Dilution factor = original volume/final volume.Therefire, the dilution factor of the dilution above is:
initial volume = 10 mL
final volume = 100 mL
Dilution factor = 10/100
Dilution factor = 1/10
Therefore, the dilution given is a serial dilution with dilution factor of 1/10.
Learn more about serial dilution at: https://brainly.com/question/2167827
Deduce the identity of the following compound from the spectral data given. C3H4BrN: H NMR, δ 2.98 (2H, triplet), 3.53 (2H, triplet) 13C NMR, δ 21.05 (triplet), 23.87 (triplet), 118.08 (singlet) (ppm):JR, 2963, 2254 cm" Express your answer as a chemical formula.
Answers
Answer: Br-CH₂-CH₂-C≡N (3-bromopropanenitrile)
Explanation:
The question tells us to " Deduce the identity of the following compound from the spectral data given. C3H4BrN: H NMR, δ 2.98 (2H, triplet), 3.53 (2H, triplet) 13C NMR, δ 21.05 (triplet), 23.87 (triplet), 118.08 (singlet) (ppm):JR, 2963, 2254 cm" Express your answer as a chemical formula".
Answer: Another Alternative to this is seen below
The Double Bond Equivalent of C₃H₄BrN = 3+1-4/2-1/2+1/2 = 2
2254 cm-1 Vc ≡N [IR analysis]
2963.4 = sp³Vc-H streek [IR analysis]
N≡C-CH₂-CH₂-Br (C₃H₄NBr)where the first CH₂ = b = 2.98 (2H,triplet) and
the second CH₂ = a = 3.53 (2H,triplet)
Note: where both are 1HNMR.
N≡C-CH₂-CH₂-Br
where C = c = 118.08 (singlet)
CH₂ = b = 21.05 (triplet)
CH₂ = a = 23.87 ( triplet)
Note: where all are 13CNMR
Important:
I have attached a copy of the solution to enhance better understanding in case the typed solution isn't clear enough.

Describe 4 steps involved in balancing a chemical equation.
Answers
Answer:
1. Identify the most complex substance.
2. Beginning with that substance, choose an element(s) that appears in only one reactant and one product, if possible. Adjust the coefficients to obtain the same number of atoms of this element(s) on both sides.
3. Balance polyatomic ions (if present on both sides of the chemical equation) as a unit.
4. Balance the remaining atoms, usually ending with the least complex substance and using fractional coefficients if necessary. If a fractional coefficient has been used, multiply both sides of the equation by the denominator to obtain whole numbers for the coefficients.
5. Count the numbers of atoms of each kind on both sides of the equation to be sure that the chemical equation is balanced.
Explanation:
I know that there 5 steps instead of 4 but this is what I have been taught
1. Calculate the heat (expressed in calories) required to heat 115 g of water from
15.4 °C to 91.4 °C.
Answers
115 grams in water need 8740 calories' worth of heat to be heated to 15.4 °C to 91. 4 °C.
How are calories created?A calorie is an unit of energy measurement. It is, in fact, the quantity of energy needed to increase the temperature among one mL (or one gram) of freshwater with one degrees C.
q = m * c * ΔT
where:
q = heat (in calories)
m = mass of water (in grams)
c = specific heat capacity of water (in calories per gram per degree Celsius)
ΔT = change in temperature (in degrees Celsius)
The specific heat capacity of water is 4.18 J/g°C, or 1 calorie/g°C.
ΔT = 91.4 - 15.4 = 76
Substituting the values into the formula:
q = 115 g * 1 calorie/g°C * 76°C = 8740 calories
So, the heat required to heat 115 grams of water from 15.4 °C to 91.4 °C is 8740 calories.
To know more about calorie visit:
https://brainly.com/question/28545564
#SPJ1
carboxylamine reaction
Answers
Answer:
Explanation:
The direct reaction of a carboxylic acid with an amine would be expected to be difficult because the basic amine would deprotonate the carboxylic acid to form a highly unreactive carboxylate.
calculate the pH of the solution obtained if 40cm^3 of 0.2M HCl was added to 30cm^3 of 0.1M NaOH
Answers
To calculate the pH of the solution obtained by mixing HCl and NaOH, we need to consider the neutralization reaction between the two compounds. The reaction between HCl (hydrochloric acid) and NaOH (sodium hydroxide) produces water (H₂O) and forms a salt (NaCl).
Given:
Volume of HCl solution (V₁) = 40 cm³
Concentration of HCl solution (C₁) = 0.2 M
Volume of NaOH solution (V₂) = 30 cm³
Concentration of NaOH solution (C₂) = 0.1 M
1. Determine the moles of HCl and NaOH used:
Moles of HCl = Concentration (C₁) × Volume (V₁)
Moles of HCl = 0.2 M × 0.04 L (converting cm³ to L)
Moles of HCl = 0.008 mol
Moles of NaOH = Concentration (C₂) × Volume (V₂)
Moles of NaOH = 0.1 M × 0.03 L (converting cm³ to L)
Moles of NaOH = 0.003 mol
2. Determine the limiting reagent:
The stoichiometry of the reaction between HCl and NaOH is 1:1, meaning that they react in a 1:1 ratio. Whichever reactant is present in a smaller amount will be the limiting reagent.
In this case, NaOH is present in a smaller amount (0.003 mol), which means it will be fully consumed during the reaction.
3. Determine the excess reagent and its remaining moles:
Since NaOH is the limiting reagent, we need to find the remaining moles of HCl.
Moles of HCl remaining = Moles of HCl initially - Moles of NaOH reacted
Moles of HCl remaining = 0.008 mol - 0.003 mol
Moles of HCl remaining = 0.005 mol
4. Calculate the concentration of HCl in the resulting solution:
Volume of resulting solution = Volume of HCl solution + Volume of NaOH solution
Volume of resulting solution = 0.04 L + 0.03 L
Volume of resulting solution = 0.07 L
Concentration of HCl in the resulting solution = Moles of HCl remaining / Volume of resulting solution
Concentration of HCl in the resulting solution = 0.005 mol / 0.07 L
Concentration of HCl in the resulting solution ≈ 0.071 M
5. Calculate the pH of the resulting solution:
pH = -log[H⁺]
pH = -log(0.071)
Using logarithm properties, we can determine the pH value:
pH ≈ -log(0.071)
pH ≈ -(-1.147)
pH ≈ 1.147
Therefore, the pH of the solution obtained by mixing 40 cm³ of 0.2 M HCl and 30 cm³ of 0.1 M NaOH is approximately 1.147.
A student measures the mass and volume of a small cube made of an unknown metal. The mass of the cube is 25.0 g, and the volume of the cube is 3.19 cm³. The student is told that the cube is a sample of one of the four materials listed in the table. based on the Data given, the unknown material is most likely A gold Density 19.39, B iron Density 7.85, C silver Density 10.50 D tin density 7.28
Answers
The unknown material with a mass of 25.0g, and the volume is 3.19 cm³ is iron with a density of 7.85g/cm³.
DENSITY:
The density of a substance can be calculated by dividing the mass of the substance by its volume. That is;Density (g/cm³) = mass (g) ÷ volume (cm³)According to this question, the mass of the cube is 25.0g, and the volume of the cube is 3.19cm³. The density can be calculated as follows:Density = 25.0g ÷ 3.19g/cm³Density = 7.84g/cm³Therefore, the unknown material with a mass of 25.0g, and the volume is 3.19 cm³ is iron with a density of 7.85g/cm³.Learn more at: https://brainly.com/question/15164682?referrer=searchResults
if two substance are at the same temperature, their enthalpy
Answers
Answer:
cannot be measure
Hope this helps :) !!!
You are trying to determine the
freezing point for a 0.195 m
aqueous solution of K₂S.
How many particles does K2S
dissociate into?
Answers
Answer:
K2S dissociates into three particles in aqueous solution: 2 K+ ions and 1 S2- ion.
Why are solids not included in the Ksp formula?
Answers
Explanation:
When calculating the equilibrium constant expression we don't include pure solids or liquids because their concentrations stay constant during the reaction. Since their concentrations don't change significantly throughout the reaction (if enough is present) they don't affect the equilibrium so we don't include them in the ksp formula.
Answer: because the concentration of a purid solid remains constant during the reaction.
Use the following information to answer the next 4 questions. Consider the following equilibrium: 2N02(g) + 2NO(g) + 02(g) Initially, 0.600 mol of NO, is placed in a 1.00 L flask. At equilibrium, the concentration of NO was found to be 0.460 mol/L. •The equilibrium law expression would be Select one:

Answers
Answer:
Second Option ([O2]*[NO]^2)/[NO2]^2
Explanation:
To find the Kc value of a reaction, you must divide the concentration of the products over the concentration of the reactants (divide right side of the reaction by the left side of the reaction)
you must also remember to turn the multiplier prefix such as the '2' in the 2NO into an exponent for that particular molecule in the Kc equation
as such, the second option is correct
Which are the two key factors that affect the rate of weathering of rocks?
Select 2.
A Climate
B Rock Type
C Bedrock
Answers
Answer:
A and B.
Explanation:
Climate:
High temperatures and greater rainfall increase the rate of chemical weathering. Lower temperatures can break rocks to by freezing water inside of a rock cracks. When it freezes it expands the water and forces the crack of the rock to expand.
Rock Type:
Certain types of rock are very resistant to weathering. Igneous rocks, especially intrusive igneous rocks such as granite, weather slowly because it is hard for water to penetrate them. Other types of rock, such as limestone, are easily weathered because they dissolve in weak acids.
ORDS ONLY. 71.A broad, painless, pink-gray, wart like infectious lesions may develop on the vulva, perineum, or anus in syphilis is called----- 72. In suspected syphilis infection the term RPR stands for? 73. The recommended dosage of Benzathine Penicillin in an adult in Zambia is je 74. The recommended drug for treatment of gonorrhea when using syndromic management is 75. The causative organism for chancroid is called- 76. The commonest type of HIV is 77.The assertive, problem solving approach to identification and treatment of the patient's problems is called --- 78. A tumour arising from the cells producing melanin is also known as 1 79. The type of sound described as drum like, loud, empty quality felt over gas-filled stomach, intestine or pneumothorax which is heard during percussion is called host wall into the pleural space to obtain
Answers
71. The broad, painless, pink-gray, wart-like infectious lesions that may develop on the vulva, perineum, or anus in syphilis are called "condyloma lata."
72. In suspected syphilis infection, the term RPR stands for "Rapid Plasma Reagin." It is a blood test used to screen for syphilis.
73. The recommended dosage of Benzathine Penicillin in an adult in Zambia may vary depending on the stage and severity of the syphilis infection. It is best to consult local guidelines or a healthcare professional for the specific recommended dosage in Zambia.
74. The recommended drug for the treatment of gonorrhea when using syndromic management may vary depending on local guidelines and antibiotic resistance patterns. Commonly used antibiotics include ceftriaxone in combination with azithromycin or doxycycline.
75. The causative organism for chancroid is called "Haemophilus ducreyi."
76. The commonest type of HIV is "HIV-1."
77. The assertive, problem-solving approach to the identification and treatment of the patient's problems is called "clinical decision-making."
78. A tumor arising from the cells producing melanin is also known as "melanoma."
79. The type of sound described as drum-like, loud, and empty quality felt over a gas-filled stomach, intestine, or pneumothorax during percussion is called "tympany."
To know more about melanoma:
https://brainly.com/question/14972277
#SPJ1
Oceanography does not include the study of which of the following topics?
A.the relationship between the Sun, Moon, and Earth
B.the relationship between the atmosphere, Earth, and the oceans
C.the Earth's oceans
D.the plants and animals that live in the oceans
Answers
I need help with this homework thank you

Answers
Plz help I WILL MARK BRAINLESS

Answers
Answer:
2 or more components
Explanation:
Compounds have to things in it and mixtures aswell
Which two terms represent types of chemical formulas?
1
fission and fusion
2.
oxidation and reduction
3
empirical and structural
4.
endothermic and exothermic
Answers
Answer: 3 empirical and structural
Explanation:
Just guessed on same question got it right
8.5 g of rubidium are reacted completely with water.
The reaction makes a solution of rubidium hydroxide.
The volume of this solution is 2.5 dm'.
Calculate the concentration of the rubidium hydroxide solution in g dm
(relative atomic mass: Rb = 85; relative formula mass: RbOH = 102)
please how do i work this out
Answers
Answer:
3.4g/dm³
Explanation:
Mass concentration = mass of solute/volume of solution
According to this question;
Mass of rubidium = 8.5g
Volume of rubidium hydroxide solution = 2.5dm³
Mass conc. = 8.5/2.5
Mass conc. 3.4g/dm³.
0.2g of sand in two-third in liter of ethanol . What is the concentration in g per dm cube
Answers
The mass concentration of sand in the ethanol solution is 0.299 g/dm³.
What is the concentration in grams per dm³?To find the concentration in grams per cubic decimeter (g/dm³), we first need to convert the volume from liters to cubic decimeters (dm³). Since 1 liter is equal to 1 cubic decimeter, we can directly convert the volume.
Given:
Mass of sand = 0.2 g
Volume of ethanol = two-thirds liter
Converting volume to dm³:
1 liter = 1 cubic decimeter
two-thirds liter = (2/3) cubic decimeter = 0.67 dm³ (rounded to two decimal places)
Now we can calculate the concentration in g/dm³ by dividing the mass of sand by the volume in dm³:
Concentration = Mass / Volume
Concentration = 0.2 g / 0.67 dm³
Concentration ≈ 0.299 g/dm³ (rounded to three decimal places)
Learn more about mass concentration at: https://brainly.com/question/23437000
#SPJ1
how many moles are in 22 grams of argon
Answers
Answer:
0.551 moles
Explanation:
To calculate the number of moles in 22 grams of argon, divide the mass by the molar mass:
Number of moles = Mass / Molar mass
Number of moles = 22 g / 39.95 g/mol
Number of moles ≈ 0.551 moles
Therefore, there are approximately 0.551 moles of argon in 22 grams of argon.
Select the correct structure that
corresponds to the name.
4-octyne
A. CH3CH₂CH₂C = CCH₂CH₂CH3
-CEC-
B.
C. both
Answers
Answer:
ugh this wont save anyway i think i'm just checking if ill save
Explanation:
if i does C both
hope this helps
The correct selection for 4-octyne structure is A. Hence option A is correct.
What is Molecule and atom ?Atom is smallest entity of a body. Body is made up of atoms. it is basic building block of a body. An atom consist of electrons, protons and neutrons as sub atomic particle. whole mass of the atom is concentrated at the center of the atom which we call it as nucleus, nucleus consist of proton and neutron. Electron revolve around the nucleus at determined(fixed) orbit. Total number of protons in the atom decides the atomic number and the elements in the periodic table.
Molecule is made up of atoms, it is formed when two or more atoms bound together. The electrons in the outermost orbit are responsible for the molecule formation. Electrons are shared by the atoms in the entire molecule.
4-Octyne, also known as dipropylethyne, is a type of alkyne with a triple bond at its fourth carbon. its chemical formula is C₈H₁₄.
C₈H₁₄ are bounded like . CH₃CH₂CH₂-C ΞC-CH₂CH₂CH₃
Hence option A is correct.
To know more about chalcogenides :
brainly.com/question/29215809
#SPJ7.
which compound is an electrolyte ? 1) butene 2) propane
Answers
Answer:
1.)
Explanation:
A sugar cube was placed into a beaker containing 100 mL of water at room temperature and completely dissolved into the water. This process is represented by the series of diagrams labeled A, B, and C below. Describe one way that the dissolved sugar at C could be separated from the water.
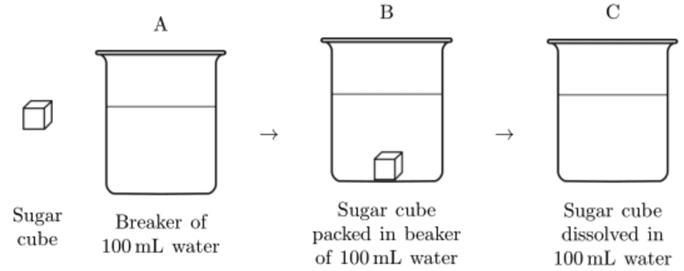
Answers
Answer:
Boil the water until it evaporates
Explanation:
If the water evaporates the sugar will no longer bond to it and then percipitate at the bottom of the beaker.
Bohr's Model of the atom
If an element has 7 electrons in its valence shell (outermost ring), which chemical family would you expect it to belong to?
Answers
Answer:
group 17 the halogen.as it has 7 electron in its outermost ring
What is the reduction half-reaction for the equation below?
5Fe2+(aq) + MnO4" (aq) + 8H+(aq) → 5Fe3+ (aq) + Mn2+(aq) +
4H2O(1)
Answers
Answer:
\(MnO_4^-+8H^++5e^-\rightarrow Mn^{2+}+4H_2O\)
Explanation:
Hello there!
In this case, since redox reactions are characterized by the presence of a reduction reaction, whereby the oxidation of the element decreases, and an oxidation reaction whereby the oxidation of the element increases.
In such a way, for the given chemical equation, we can see Fe is increasing its oxidation state from 2+ to 3+, which means it is oxidized. On the flip side, Mn is being reduced from 7+ (MnO₄⁻) to 2+ and this, the reduction half-reaction is:
\(MnO_4^-+8H^++5e^-\rightarrow Mn^{2+}+4H_2O\)
Whereas five electrons are carried.
Regards!
1. Which statement is true about the relationship between chromosomes, genes and
traits?
A. Genes are found within traits, and their codes are used to make chromosomes.
B. Chromosomes are found within genes, and their codes are used to make traits.
C. Genes are found within chromosomes, and their codes are used to make traits.
D. One gene is found on every chromosome and they are used to make traits.
Answers
Answer:
the answer is C.Explanation:
in any chemical reaction each type of atom is conserved
Answers
Answer:
Also, the number of atoms in a reaction remains the same. Mass cannot be created or destroyed in a chemical reaction. The law of conservation of mass states that the total mass of substances taking part in a chemical reaction is conserved during the reaction.
Images of the constellation Orion made using a telescope are what A. Myth. B. Science C. Pseudoscience
Answers
The Orion constellation which can be seen all around the world is positioned upon its celestial equator and it is among the noticeable and distinctive constellations in the sky. It is thought to be a Greek Mythology named after a hunter.
The Orion Nebula forms from dust, hydrogen as well as other ionized gases.
The tale and myth of Orion have various variations and explanations but one of the most popular ones is that Orion declared himself as the topmost and strongest hunter in the world.
Therefore, we can conclude that the constellation Orion made using a telescope are Myth.
Learn more about Orion Constellation here:
https://brainly.com/question/13387637?referrer=searchResults
