You are asked to make a 1. 5 L solution of. 35 M HCl by diluting concentrated 16. 0 M HCI. What
volume of acid would be needed to make the dilution?
Answers
To make a 1.5 L solution of 0.35 M HCl using 16.0 M HCl, you will need 32.81 mL of concentrated acid.
1. Use the dilution formula: M1V1 = M2V2
2. M1 is the initial concentration (16.0 M), V1 is the volume of concentrated acid needed, M2 is the final concentration (0.35 M), and V2 is the final volume (1.5 L).
3. Plug in the values: (16.0 M)(V1) = (0.35 M)(1.5 L)
4. Solve for V1: V1 = (0.35 M)(1.5 L) / 16.0 M
5. V1 = 0.0328125 L, which is equal to 32.81 mL.
6. So, 32.81 mL of concentrated 16.0 M HCl is needed to make the 1.5 L solution of 0.35 M HCl.
To know more about concentrated acid click on below link:
https://brainly.com/question/30327123#
#SPJ11
Related Questions
6. The central selenium atom in selenium hexafluo-
ride forms an expanded octet. How many electron
pairs surround the central Se atom?
A. 4
C. 6
B. 5
D. 7
Answers
Given what we know, we can confirm that when forming an expanded octet, selenium hexafluo ride will hold 6 pairs of shared electrons around its center selenium atom.
What is an expanded octet?An expanded octet is when an atom can hold more than 8 valence electrons in its outer shell. This is possible for those elements in period four of the periodic table. Elements that are capable of this form what we call hypervalent compounds.Therefore, given that during an expanded octet formation, the central atom is capable of holding more than 8 valence electrons in its outer shell, the central atom of a selenium hexafluo ride compound will have 12 electrons being shared, which results in 6 pairs, making C the correct answer.
To learn more about valence electrons visit:
https://brainly.com/question/7223122?referrer=searchResults
1. You may be using medium for shoot regeneration from leaf explants of a plant in Expt-5. The plant media may contain the plant growth regulators (hoones) BA and NAA. The molecular weight of BK is 72 A : and NAA is 186. The media is pH to 5.8. (a) Before making the plant media, you found the pH to be 3.6. What would you add quiekly to get it to a pH of 5.8 (give a specific name of the solution)? Why? (1 pt) (b) How much BA will be weighed fot a 1M solution? (Y po) (c) Convert your answer from (b) to mg/ml. (Y/ pt) (d) Convert your answer from (c) to mg 1 . (1 pt) (e) How much BA will be weighed for a 5mM solution? (1/4pt) (f) Convert your answer from (c) to mg/ml. ( /4pt ) (g) Convert your answer from (f) to mg/L. (H/ pt) (h) Your stock solution of BA is 5mM and your working solution is 0.2mg/.. What volume of the stoc be added to 250ml of medium? [Hint: fook at the previous answers Keep to 4 decimal pts.) (3 pts Convert your answer from (h) to μI, and which pipettor will you use to aliquot the B. A? (1 pt)
Answers
(a) To get the pH of the media to 5.8, you would add NaOH solution. NaOH is used as a basic solution, and when it is added to a solution, it will increase the pH of the solution.
(b) The molecular weight of BA is 225.3. To prepare a 1M solution, you would have to weigh out 225.3 grams of BA.(c) To convert a 1M solution of BA to mg/mL, you can use the following equation: 1 mole = molecular weight in grams; 1000 millimoles = 1 mole. So, 1 M = 1000 mg/mL. Therefore, a 1M solution of BA is equivalent to 1000 mg/mL .(d) To convert a concentration of 1000 mg/mL .
Therefore, to calculate the weight required for a 5 mM solution, use the following formula :Mass of BA = molarity × volume × molecular weight= 5 × 0.001 × 225.3= 1.1265 grams(f) To convert a concentration of 5 mM to mg/mL, we use the following formula: Concentration (mg/mL) = (Concentration (mM) × Molecular weight) / 1000= (5 × 225.3) / 1000= 1.1265 mg/mL(g)
To convert a concentration of 1.1265 mg/mL to mg/L, we multiply by 1000, so 1.1265 mg/mL = 1126.5 mg/L.(h) Given that the stock solution of BA is 5 mM and the working solution is 0.2 mg/mL.
To know more about increase visit:
brainly.com/question/19383315
#SPJ11
Sample H is a mineral found in this sand! This mineral is black and will stick to you when you walk through the sand at the beach. Zoom in really close - sand is very cool to look at when it is magnified. We will look at more later in the class. The sand grains that you should focus on are black. Not all of the black grains are the same mineral however the mineral you're looking for is strongly magnetic. If you ran a magnet just over the top of this sample, some of black sand grains would stick. What mineral is it?
Answers
The mineral that's illustrated based on the information given will be magnetite.
What is a mineral?A mineral simply means any pure substance that has a unique composition and structure.
Since the mineral is black and will stick to you when you walk through the sand at the beach, it's magnetite.
Learn more about mineral on:
brainly.com/question/15844293
#SPJ12
An apple i reting on a table Clarie ay that there are no force acting on the apple becaue it i not moving i he right
Answers
Clarie is wrong by saying that no force is acting on the apple when it is rest on the table.
Forces always come in pairs. The gravitational force is acting upon the apple and the result is that the matter in the Earth is pulling the matter in the apple. When the apple is set rest on the table, the table exerts a force upward on the apple equal to the force exerted downward, and hence the apple stays without moving. This implies that resting object always have their own gravitational force.
Learn more about gravitational force from the link given below.
https://brainly.com/question/12528243
#SPJ4
Solve pls brainliest
The two green substances are not same thing because some of their properties are different and some of them are the same. If they were the same substance, all of their properties would have to be the same.
How could the explanation be improved?
Answers
Answer:
Even though the two substances possess many similarities, they have some unique properties. In turn, since they have the same properties, if they were the same substance, it would make matters worse, if the same chemical was in two different places, there would not be a difference between them since they are the same, just as it is with are two different chemicals would have differing properties since they are two properties would vary from one another since they are 2 totally different things!
What best describes the process of facilitated diffusion?
Answers
With the aid of a transport molecule, chemicals can be transported across a biological membrane from a region of higher concentration to an area of lower concentration.
What is facilitated diffusion ?The process of spontaneous passive transport of molecules or ions across a biological membrane by certain transmembrane integral proteins is known as facilitated diffusion. It is sometimes referred to as facilitated transport or passive-mediated transport.
The passage of certain chemicals through protein channels to traverse cell membranes. It is a sort of passive transport, thus it is not quite diffusion. proteins for transport. proteins that assist in moving materials across cell membranes and throughout the body.
Facilitated diffusion is demonstrated by the movement of amino acids and glucose into cells from the circulation. These molecules are ingested by active transport in the small intestine and subsequently released into the circulation.
Thus, the chemicals can be transported across a biological membrane from a region of higher concentration to an area of lower concentration.
Learn more about facilitated diffusion follow the link below;
https://brainly.com/question/18122054
#SPJ12
The equation below shows the decomposition of lead nitrate. How many grams of lead (II) oxide are also produced when 20.5 g NO2 is formed?
Answers
Answer:
ligma
Explanation:
Give the nuclear symbol for an atom with 9 protons and 10 neutrons
Answers
The nuclear symbol for an atom with 9 protons and 10 neutrons is 19F the nuclear symbol consists of the atomic number (number of protons) as the subscript and the mass number (sum of protons and neutrons) as the superscript.
In this case, the atomic number is 9, indicating the element is fluorine (F) since the atomic number determines the element's identity. The mass number is calculated by adding the number of protons (9) and neutrons (10), resulting in a mass number of 19. Therefore, the nuclear symbol for this atom is 19F, representing an atom of fluorine with 9 protons and 10 neutrons.
Learn more about atom here:
https://brainly.com/question/1566330
#SPJ11
PLEASEEEEEEEEEEEEEEE I NEED THEM DONE ASAPPPPPPPPP I CANT WAIT
THE QUESTIONS ARE GONNA DETERMINE 30% OF MY GRADE SO PLEASE EEEEEEEEEEEEEEEEEEEEEEEEEEEEEEE ._.





Answers
Answer:
lol good luck
Explanation:
u need it
The homogeneity of the chloride level in a water sample from a lake was tested by analyzing portions drawn from the top and from near the bottom of the lake, with the following results
Top (ppm Cl)
Bottom (ppm Cl)
26.30
26.22
26.43
26.32
26.28
26.20
26.19
26.11
26.49
26.42
Apply the t-test at the 95% confidence level to determine if the chloride level from the top of the lake is different from that at the bottom.
Now use the paired t-test and determine whether there is a significant difference between the top and bottom values at the 95% confidence level.
Why is a different conclusion drawn from using the paired t- test than from just pooling the data and using the normal t- test for differences in means?
Answers
The paired t-test yields a different conclusion than the normal t-test because it accounts for the paired nature of the data, comparing the measurements taken at the top and bottom of the lake separately.
In this scenario, the paired t-test is appropriate because it analyzes the data as pairs, considering the chloride levels measured at the top and bottom of the lake for each sample. By comparing the differences within each pair, the paired t-test determines whether there is a significant difference between the chloride levels at the top and bottom of the lake.
Using the paired t-test, the differences between the paired observations are calculated, and the null hypothesis assumes that the mean difference is zero (no significant difference between the top and bottom chloride levels). The test then determines whether the observed differences are statistically significant at a chosen confidence level, in this case, 95%.
The normal t-test for differences in means, on the other hand, would treat the top and bottom chloride levels as separate and unrelated groups, disregarding their paired nature. By pooling the data and conducting a standard t-test, the analysis assumes that the two sets of measurements are independent, which may not be appropriate in this case. This can lead to a different conclusion compared to the paired t-test.
The different conclusion drawn from using the paired t-test compared to pooling the data and using the normal t-test is due to the consideration of the paired nature of the measurements. The paired t-test takes into account the potential correlation or connection between the measurements taken at the same location (top and bottom of the lake) and assesses the differences within each pair.
Pooling the data and using the normal t-test treats the measurements as independent, disregarding the pairing. This can result in a loss of valuable information and may lead to an inaccurate conclusion. The paired t-test is more appropriate when dealing with dependent or related measurements, ensuring a more accurate assessment of the differences between the top and bottom chloride levels.
Learn more about paired t-test
brainly.com/question/32245864
#SPJ11
How many grams do 3.6 × 10^20 atoms of sodium weigh?
Answer in units of g.
Second time posting as someone else answered incorrectly.
Answers
Answer:
13.749 x 10 -3 g
Explanation:
take the number of atoms that you have divided by Avogadro's number and then multiply by the molar mass of the element. :)
If an element has a mass number of 16 and an atomic number of 8, how many neutrons does the element have? A. 16 B. 10 C. 8 D. 9
Answers
Answer: C
Explanation: because mass number = proton + neutron number and atomic number = proton number
so 16-8=8
Answer:
C. 8
Explanation:
number of neutrons = mass number - atomic number
16-8
8
How much heat is roguired to raise the temperature of 8.75 g of water from its melting point to its boiling pointsExpress your answer numerically in kilojoulos,
Answers
The heat required to raise the temperature of 8.75 g of water from its melting point to its boiling points is 3.662 kJ.
What exactly is specific heat?The amount of heat required to increase the temperature of one gram of a material by one degree Celsius (°C) is defined as specific heat.
What is the name of the specific heat formula?The equation q = mcΔt can be used to compute the amount of heat acquired or lost by a specific heat (q), where m is the mass of the sample, c is the specific heat, and Δt is the temperature change.
Given:
m = 8.75
c = 4.186 J/g°C
The melting point and boiling point of water is 0° and 100° respectively.
Δt = 100° - 0° = 100°
We know that,
q = mcΔt
= 8.75(4.186)100
= 3.662 kJ
Thus, the heat required to raise the temperature of 8.75 g of water from its melting point to its boiling points is 3.662 kJ.
Learn more about specific heat here:
https://brainly.com/question/21406849
#SPJ9
Which lists the structures, in correct order, through which light passes when it enters the eye?
cones, pupil, lens, sclera
sclera, iris, pupil, lens
vitreous humor, lens, pupil, cornea
cornea, pupil, lens, vitreous humor
hurry plz
Answers
Answer:
cornea, pupil, lens, vitreous humor
Answer:
cornea, pupil, lens, vitreous humor
Explanation:
PERIODIC TABLE PLS HELP 20 POINTS

Answers
\(\bold{\huge{\blue{\underline{ Answers}}}}\)
Ans 1.) Potassium ( 1 ) , palladium (2)
Metals are those which posses lustre, hardness, malleability, ductility etc.
Ans 2.) Oxygen ( 5 ) , Argon ( 4 )
Non metals are those elements which do not possess lustre, hardness, ductility, malleability etc... they are generally brittle in nature.
Ans 3.) Boron ( 3 )
Metalloids are those substances which shows both the properties of metals and nonmetals.
Ans 4.) Boron ( 3 )
Semiconductor are those substances whose conductivity is lie between insulators and conductors.
Ans 5.) Argon(4)
Least reactive gases are those gases which generally do not reactive with any element or having very least reactivity like argon which is noble gas.
Ans 6.) Oxygen (5)
Oxygen is dull, easily breakable and good insulator but only in solid form
Ans 7.) Palladium(2)
Malleability is the property of metals that is drawn metals into thin sheets .
A 0.5 kg arrow was shot at a target. The arrow accelerated at 200 m/s2. What was
the net force on the arrow to the nearest newton?
Record your answer and be sure to use the correct place value.

Answers
Answer: I think 100?
Explanation:
It’s asking for the net FORCE! And the formula to find force is to multiply the mass by the acceleration which would be in this case 0.5x200 which would be 100
Within the visible spectrum, red light has
Answers
Answer:
Within the visible spectrum, red light has the longest wavelength.
Hope that helps! :)
Explanation:
Nitrogen dioxide, NO2(g) (Delta. Hf = 33. 84 kJ/mol), is decomposed according to the following reaction: 2 upper N upper O subscript 2 (g) right arrow upper N subscript 2 (g) plus 2 upper O subscript 2 (g). What is the enthalpy change when 2. 50 mol of nitrogen dioxide decomposes? Use Delta H r x n equals the sum of delta H f of all the products minus the sum of delta H f of all the reactants. 13. 5 kJ of energy released 13. 5 kJ of energy absorbed 84. 6 kJ of energy released 84. 6 kJ of energy absorbed.
Answers
The heat of Reaction or Enthalpy is defined as the changes in the heat during a chemical reaction. The change in heat is calculated as the sum of all the heat change in products minus the sum of all the changes in the reactants.
How do you calculate the heat change in the reaction?The chemical reaction between nitrogen and oxygen is given as:
\(\rm 2 N O_2 \rightarrow N_2 + 2 O_2\\\\ \rm \Delta\; H_{f} &= 33.84 kJ/mol\)
Now, we know:
1 mol Nitrogen dioxide requires = 33.84 kJ/mol2.50 mol of nitrogen oxide will give = \(33.84 \times 2.50 \)Energy released = 84.6 energy releasedThus, the enthalpy change for the reaction will be 84.6 energy released.
Learn more about enthalpy change here:
https://brainly.com/question/25912483
Answer:
C. 84.6 kJ of energy released
Explanation:
edg 22
I need to figure out the volumes for a serial dilution. The volumes are small and I cannot measure anything less than 1µL. Please show your work clearly
The initial concentration is 14.2mM. The final concentrations are 10µM, 5µM, 2.5µM, 1µM, 750nM, 500nM, 250nM, 100nM, 50nM, 10nM in 1mL of stock media.
Answers
By following serial dilution method, you can achieve the desired concentrations using small volumes while ensuring accurate dilution ratios. It is essential to handle the small volumes carefully and accurately to maintain the desired concentrations throughout the dilution process.
To perform a serial dilution with small volumes, such as in this case where measuring less than 1µL is not possible, we can use a stepwise dilution approach.
Start with the initial concentration of 14.2mM in 1mL of stock media.
To prepare the first dilution of 10µM, transfer 1µL from the stock solution and add it to 99µL of a diluent (such as water or buffer). This results in a 100µL solution with a concentration of 10µM.
For subsequent dilutions, repeat the same process. Take 1µL from the previous dilution and add it to 99µL of diluent.
Repeat step 3 for each desired concentration. For example, to obtain a concentration of 5µM, take 1µL from the 10µM solution and add it to 99µL of diluent.
Continue this stepwise dilution process until you reach the final desired concentrations: 2.5µM, 1µM, 750nM, 500nM, 250nM, 100nM, 50nM, and 10nM.
Learn more about dilution here:
https://brainly.com/question/31521767
#SPJ11
What coefficients would balance the following equation?
__Al + __O2 → __Al2O3
A. 2Al + 3O2 → 1Al2O3
B. 2Al + 3O2 → 2Al2O3
C. 3Al + 3O2 → 2Al2O3
D. 4Al + 3O2 → 2Al2O3

Answers
Answer is D
give the systematic name for the compound ba no3 2
Answers
The systematic name for the compound Ba(NO3)2 is barium nitrate. Barium nitrate is an inorganic salt with the chemical formula Ba (NO3)2. It is a colorless, odorless, and crystalline solid that is highly soluble in water. The compound is formed by combining one atom of barium and two ions of nitrate.
The name “barium” comes from the Greek word “barys,” which means “heavy,” and is a reference to its high density. The term “nitrate” refers to the polyatomic ion NO3-, which is composed of one nitrogen atom and three oxygen atoms. Barium nitrate is commonly used in pyrotechnics, as it is a powerful oxidizing agent that produces a bright green flame when ignited.
The systematic naming of inorganic compounds is based on the rules set out by the International Union of Pure and Applied Chemistry (IUPAC). The name of an ionic compound is composed of the cation name followed by the anion name. In the case of barium nitrate, “barium” is the name of the cation, while “nitrate” is the name of the anion.
Therefore, the systematic name for the compound Ba(NO3)2 is barium nitrate.
Know more about systematic name here:
https://brainly.com/question/1787202
#SPJ11
the radioactive element carbon-14 has a half-life of 5750 years
Answers
Answer:
What are we solving?
Explanation:
what is the chemical formula for Sulphate...
Answers
Answer:
SO4²
Explanation:
Formula and structure: The sulfate ion formula is SO42- and the molar mass is 96.06 g mol-1. This salt is formed by one sulfate center to which 4 atoms of oxygen are attached, 2 of these atoms are forming S=O.
Answer:
\(so _{4} ^{2 - } \)
Explanation:
sulfate is consisting of sulfur element with 4 oxygen as the structure in the pic given and this group is 2- charged

Mendeleev's fixed an apparent discrepancy in the order of his periodic table by reversing the placement of which two elements
Answers
In Mendeleev's tables, iodine should be listed before tellurium. Iodine, however, has chemical characteristics with bromine and chlorine. Mendeleev modified the placements of iodine and tellurium.
Why is it called a periodic table?Because of the orderly arrangement of the elements, it is known as the periodic table. They're arranged in rows and columns, as you'll see. Periods and Groups are the names given to the horizontal rows that run from left to right and indeed the vertical columns that run from top to bottom, respectively.
Which periodic table was the original?Mendeleev's stoichiometry was a vertical diagram that included 63 known elements in order of atomic weight and was first published in 1869. Components with comparable characteristics were arranged in horizontal rows.
To know more about Periodic table visit:
https://brainly.com/question/11155928
#SPJ4
someone help please i cant figure this out
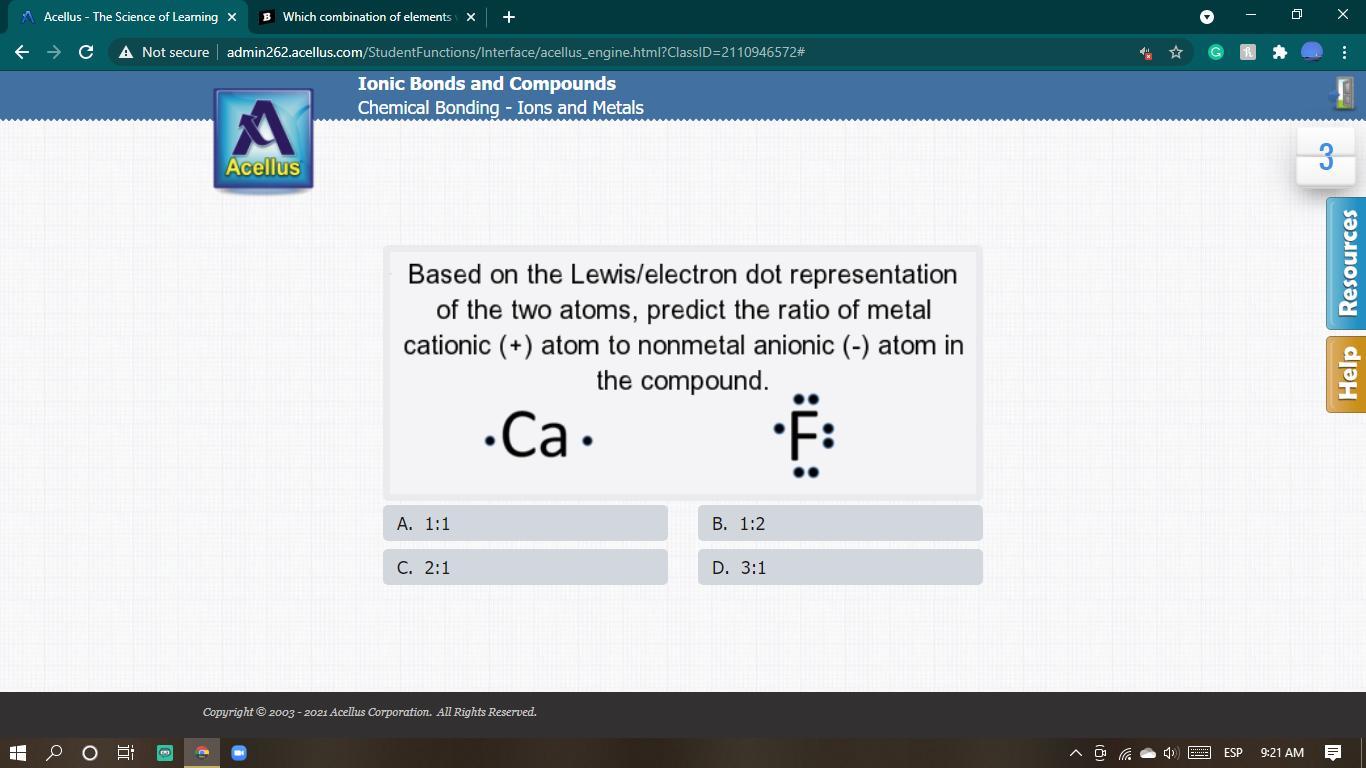
Answers
Answer: 2 : 1
Explanation:
Cation :
Ca - calcium = atomic number = 20
Electron dot configuration : 2, 8, 8, 2
Ca losses 2 electrons in its outermost shell and thus has a charge of 2+ in other to attain a stable octet state.
Anion:
F - has 7 valence electrons and thus needs 1 electron to achieve a stable octet state, hence it accepts one electron has has a charge of (-1)
Therefore,
Ratio of cation (+) to negative (-) = 2 :1
How many moles of H atoms are in 15.2 grams of pure ice?
How many moles of O atoms are in 15.2 grams of pure ice?
Answers
The number of moles of H atoms in 15.2 grams of pure ice is 0.850 moles
The number of moles of O atoms in 15.2 grams of pure ice is 13.50 moles
What is an atom?An atom is a smallest indivisible particle of a chemical element that is capable of independent existence.
Pure ice contains the H2O molecules and the molar mass of H2O is 18.02 g/molUsing the relation:
number of moles = mass/molar massThe number of moles of H2O = 15.2 grams/18.02 g/mol
number of moles of H2O = 0.8435 moles
If the atomic mass of H and O are as follow:
the atomic mass of H atom is = 1.00784 g/molthe atomic mass of O atom is = 15.999 g/molThen:
the number of H atoms in 15.2 grams of pure ice = mass of H atom × molar mass of H2O.
the number of H atoms = 0.8435 mol × 1.00784 g/mol
the number of H atoms = 0.850 moles
the number of O atoms in 15.2 grams of pure ice = 0.8435 mol × 15.999 g/mol
the number of O atoms = 13.50 moles
Learn more about atoms here:
https://brainly.com/question/25832904
the symbol for the element in period 2 group 13 is
Answers
Answer:
Boron
Explanation:
Using the periodic table we can see that Period 2 represents the second row. Group 13 indicates the 13th column.
Which has a greater energy, a photon of yellow light, or a photon of green light
Answers
Answer:
A photon of green light has a higher energy since it has a higher frequency than the yellow photon. And we can see that on the picture representation of the light spectre.
Please answer this for me

Answers
For ¹⁴₆C²⁻ ; Atomic number = 6; Mass number = 14
Number of electrons = 8; D. Number of protons = 6; E. Number of neutrons = 8.
How do we identify the atomic and mass number?The mass number represents the sum of protons and neutrons in the nucleus of an atom or ion.
In this case, the ion is represented by ²⁻, indicating that it has gained two extra electrons.
Since the number of protons remains the same, the mass number can be calculated by adding the protons and neutrons. Therefore, the mass number for ¹⁴₆C²⁻ is 14.
The atomic number represents the number of protons in the nucleus of an atom or ion. In this case, the atomic number is 6.
Find more exercises on mass number;
https://brainly.com/question/31864264
#SPJ1
If you mix 100 grams of sugar into a 1,000ml beaker, and the sugar
dissolves so you can no longer see it, how much sugar is in the beaker?
Answers
Answer:
100g
Explanation:
The amount of sugar in the beaker still remains 100g. There is no addition or loss of any quantity of sugar unit.
Physical and chemical changes obey and complies with the law of conservation of matter.
The law suggests that matter is neither created nor destroyed but can be changed from one form to another.
In this process, if we do a reverse reaction by evaporating the solution in the dish, we would get back 100g of sugar.