YALL HELP ASAP
1) If big molecules can't get absorbed in the small intestine, why aren't there other big molecules besides fiber, like complex carbohydrates, coming out in the poop of healthy people?
2) What's happening to the other big molecules like complex carbohydrates? How can we explain why the amount of complex carbohydrates could be decreasing as food travels through the digestive system?
WHATS THE ANSWER TO THESE PLS HELPME
Answers
1) The reason why other big molecules, such as complex carbohydrates, don't usually come out in the feces of healthy people is because they are broken down into smaller, absorbable units during the digestive process.
If big molecules can't get absorbed in the small intestine, why aren't there other big molecules besides fiber, like complex carbohydrates, coming out in the poop of healthy people:
Complex carbohydrates are broken down into simple sugars like glucose through the action of enzymes such as amylase, which is present in saliva and pancreatic secretions. These simple sugars can then be absorbed by the small intestine and used by the body for energy. In contrast, fiber cannot be broken down by human digestive enzymes, so it remains undigested and is eliminated in the feces.
2) What's happening to the other big molecules like complex carbohydrates? How can we explain why the amount of complex carbohydrates could be decreasing as food travels through the digestive system?
As food travels through the digestive system, complex carbohydrates are gradually broken down into smaller, absorbable units. This process begins in the mouth with the action of salivary amylase, which starts breaking down the complex carbohydrates into smaller units. As the food continues to the stomach and then to the small intestine, more enzymes, like pancreatic amylase, are secreted to further break down the complex carbohydrates into simple sugars. These simple sugars are then absorbed by the small intestine and enter the bloodstream, where they can be used for energy or stored for later use. This is why the amount of complex carbohydrates decreases as food travels through the digestive system.
To know more about digestive system:
https://brainly.com/question/29694477
#SPJ11
Related Questions
Shown above is information about the dissolution of AgCl(s) in water at 298K. In a chemistry lab a student wants to determine the value of s, the molar solubility of AgCl, by measuring [Ag+] in a saturated solution prepared by mixing excess AgCl and distilled water. How would the results of the experiment be altered if the student mixed excess AgCl with tap water (in which [Cl−]=0.010M) instead of distilled water and the student did not account for the Cl− in the tap water?
Answers
Answer:
Value for K would be too small. Less AgCl would dissolve due to the common ion effect due to the presence of Cl- in the water.
Explanation:
Think of this through the lenses of a shifting problem. Cl- ions are a product in this situation and increasing its concentration would shift the reaction back to the solid AgCl. In this specific case, due to Cl- ions, AgCl would dissolve less to maintain equilibrium and as a result, the concentrations of Ag+ and Cl- ions would be lower than normal making a smaller K value.
In the solution of AgCl in tap water, the dissociation constant K has been decreased.
The dissolution of silver chloride in water results in the formation of silver ions and chloride ions.
The dissociation constant has been the amount of compound that has been dissociated into the constituent ions at equilibrium.
Dissociation constant for AgClThe dissociation constant has been dependent on the number of ions in the solution that has been present.
The common ion effect has been defined as the change in the dissociation constant for the compound with the presence of common ions in the solution.
The dissociation of AgCl in tap water has been resulted with presence of Cl ions in the solution. There has been early reach to the equilibrium in tap water.
Thus, with the solution of AgCl in tap water, the dissociation constant K has been decreased.
Learn more about dissociation constant, here:
https://brainly.com/question/7145687
2. How many grams of nitric acid are required to produce 8.75 g dinitrogen monoxide?
4Zn + 10 HNO3 → 4Zn(NO3)2 + N2O + 5 H2O
Answers
Answer:
125 grams of HNO3
Explanation:
(8.75g N2O) x (1/44g N2O) x (10mol HNO3) x (63.02g HNO3/ 1mol HNO3) = 125g HNO3
In this exercise we have to use our knowledge of chemistry to calculate the final amount in grams for nitrogen monoxide, as:
125 grams of HNO3
Thus, to make these calculations it will be necessary to observe the given equation:
\(4Zn + 10 HNO3 \rightarrow 4Zn(NO3)2 + N2O + 5 H2O\)
So after performing the stoichiometry we can perform the calculations as follows:
\((8.75g N2O) * (1/44g N2O) *(10mol HNO3) * (63.02g HNO3/ 1mol HNO3)\\ = 125g HNO3\)
See more about stoichiometry at brainly.com/question/9743981
draw the major organic product of the bimolecular substitution and use curved‑arrow notation to draw the mechanism. be sure to draw any non‑bonding electrons.
Answers
Here, we have to draw the main organic products of a given bimolecular substitution reaction and the curved arrow mechanism of the reaction.
(Answer is attached in the picture)
Substitution reactions involve the replacement of one functional group in a molecule by another functional group. Nucleophilic substitution reactions involve a nucleophilic reagent with a substrate that has a positively or partially positively charged portion of the molecule (electrophile).
An electrophile is an atom or molecule that lacks electrons. In organic reactions, electrophiles act as electron acceptors (Lewis's acids). These reagents can be cations or neutral molecules that have relatively positively charged atoms.
Meanwhile, a nucleophile is an atom or molecule that is rich in electrons. The nucleophile has an electron pair that can be donated (a Lewis base). Some nucleophiles are neutral molecules that have a PEB and some are negatively charged. In a chemical reaction, electrons from the nucleophile strike the electrophile center to form new bonds as a result of the reaction.
On the question:
Use of curved arrows to indicate: A broken or formed bond.
Electron layer protective layer arrow
In the reaction of this problem, I replaced Br (Substitution) and Br bonded with Na⁺.
The nucleophile "replaces" the leaving group.
Called a substitution reaction: I replace Br (change places).
Learn more about substitution reactions at https://brainly.com/question/10143438.
#SPJ4


What is the density of platinum if it crystallizes in a face-centered cubic unit cell with an edge length of 393 pm?
Answers
The density of platinum with an edge length of 393 pm in a face-centered cubic unit cell is approximately 21,340 kg/m³ or 2.134 x 10⁴ g/m³.
How to determine the density of platinumThe density of platinum can be calculated using the face-centered cubic (fcc) unit cell properties and the given edge length.
In an fcc unit cell, there are 4 atoms per unit cell. The edge length is 393 pm, and the atomic weight of platinum is 195.08 g/mol.
To calculate the density, we'll use the formula:
Density = (Mass of atoms in unit cell) / (Volume of unit cell)
First, we need to find the mass of atoms in the unit cell:
Mass of atoms = (4 atoms/unit cell) * (195.08 g/mol) / (Avogadro's number)
Mass of atoms ≈ 1.297 x 10⁻²¹g
Next, we convert the edge length to meters:
393 pm = 393 x 10⁻¹² m
Now, we calculate the volume of the unit cell:
Volume = (Edge length)³
Volume ≈ 6.077 x 10⁻²⁹ m³
Finally, we find the density:
Density = (Mass of atoms) / (Volume)
Density ≈ 2.134 x 10⁴ g/m³
Learn more about density at
https://brainly.com/question/15164682
#SPJ11
when transition metal atoms ionize, from which atomic orbitals are electrons removed first? from which atomic orbitals areelectrons removed second?
Answers
In order to determine the electronic configuration of cations, electrons are first removed from the outermost p orbital, then from the s orbital, and finally from the d orbitals.
When transition metal cations are formed, the outermost s electrons are always the first to be lost. The charge of 2+ is a highly frequent one for the ions of transition metals since the majority of them have two valence electrons. When transition metals are ionised, electrons are typically taken from the valence-shell s orbitals before they are removed from the valence d orbitals. Ionization energies drop due to the looser coupling of valence electrons further from the nucleus, which makes them easier to remove.
To learn more about electronic configuration click here https://brainly.com/question/29757010
#SPJ4
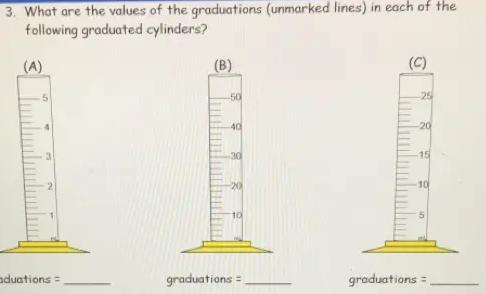
3. What are the values of the graduations (unmarked lines) in each of the
following graduated cylinders?
Q
(A)
50
3
graduations =
(B)
50
-40
-30
-20
10
graduations =
(C)
25
-20
-15
10
5
graduations =
Answers
The values of the Graduations for A , B and C will be 0.2 ml, 2ml and 1ml respectively.
Graduated Cylinder - A popular piece of scientific equipment used to measure the volume of a liquid is a graduated cylinder, sometimes referred to as a measuring cylinder or mixing cylinder. Its form is slender and cylindrical. Each marked line on the graduated cylinder indicates the volume of liquid that has been measured.
The clear graduated cylinder is widely used for volume measurements and is regarded to be more accurate than a beaker since it contains permanently marked incremental graduations.
To calculate the value of graduations-
1. Subtract the two values to get the value between the labeled graduations.
2. Determine how many spaces there are between the two graduations. Keep in mind that volume equals space.
3. Divide the number of gaps by the value between the graduations.
For cylinder A.
2 ml- 1ml = 1ml
Number of spaces = 5
∴1/ 5 = 0.2
Graduation = 0.2ml
For cylinder B -
20-10 = 10
number of spaces = 5
∴10/ 5 =2
Graduation = 2ml
For cylinder C-
10-5 = 5
Number of spaces= 5
∴5/5 = 1
Graduation = 1ml
Hence , Graduations for A , B and C will be 0.2 ml, 2ml and 1ml respectively.
To learn more about graduated cylinders refer-https://brainly.com/question/26216613
#SPJ9
Your question is incomplete. Please find the missing image below.
Calculate the frequency of visible light having a wavelength of 568.8 nm. a. 170.5 s b. 1.897 x 10 c. 1.897 x 10's d. 5.271 x 10's e. 5.271 x 104 s
Answers
The frequency of visible light will be 5.271 × 10¹⁴ s⁻¹
Frequency = speed of light / wavelength
Frequency = ( 3 • 10^8ms-1 ) / ( 568.8nm )
Frequency = ( 3 • 10^8ms-1 ) / ( 568.8 • 10^-9m )
Frequency = [ 3 / 568.8 ] • 10^17 s-1
Frequency = 5.271 × 10¹⁴ s⁻¹
The wavelength of a wave describes how lengthy the wave is. The distance from the "crest" (top) of 1 wave to the crest of the following wave is the wavelength. Alternately, we can degree from the "trough" (bottom) of 1 wave to the trough of the subsequent wave and get an equal price for the wavelength.
Wavelength: the space between one peak and the following in a chain of waves, or the distance between one trough and the following. It is also one of the “yardsticks” used to measure radiation.
The SI unit of wavelength meters. At the same time as measuring wavelength, the multiples or fractions of a meter are also used. Extensively, exponential powers of 10 are used whilst wavelengths are of huge assets.
Learn more about Wavelength here:-https://brainly.com/question/24452579
#SPJ4
8. aconitase catalyzes the ____ of citrate, followed by a ____ reaction. group of answer choices a. dehydration; hydration
b. oxidation; reduction c. reduction; oxidation d. hydration; dehydration e. isomerization; isomerization
Answers
The enzyme aconitase catalyzes the isomerization of citrate followed by a dehydration reaction.
Isomerization is a process in which a molecule undergoes a structural change, but the molecular formula remains the same. In this case, citrate is converted into isocitrate, which is an important step in the citric acid cycle.
Aconitase is a member of the iron-sulfur protein family that contains a [4Fe-4S] cluster, and it is involved in catalyzing the isomerization of citrate in the citric acid cycle. This enzyme has two active sites, one of which is responsible for the isomerization reaction, and the other is responsible for the dehydration reaction.
Aconitase works by binding to the citrate molecule and causing it to undergo a structural change. This results in the formation of an intermediate molecule called cis-aconitate. The dehydration reaction is then catalyzed by the enzyme, which removes a molecule of water from the cis-aconitate to produce isocitrate.
The reaction catalyzed by aconitase is important because it helps to generate energy for the cell. The citric acid cycle is a metabolic pathway that is used by cells to generate ATP, which is the primary source of energy for cellular processes. The isomerization of citrate is a critical step in this pathway because it helps to convert the energy stored in food molecules into a form that can be used by the cell.
Therefore, the correct answer is option e) isomerization; dehydration.
To know more about aconitase, refer here:
https://brainly.com/question/29340630#
#SPJ11
Where exactly on teh map does the size and concentration of the boxes apperar to be the highest
Answers
The concentration and size of the boxes appear to be highest near Broad Street.
What are communicable diseases?Communicable diseases are those diseases that can be transferred from one person to another and one animal to another animal or person.
The black boxes show the relative numbers of cholera-related fatalities. Because of the lack of socioeconomic development in the locations where it occurs, cholera is known as a disease of poverty.
Overpopulated camps are ideal for a cholera breakout. Other humanitarian emergencies that interrupt water and sanitation infrastructure include earthquakes, civil upheaval, and war.
Therefore, near Broad Street, both the quantity and size of the boxes seem to be at their maximum.
To learn more about communicable diseases, refer to the link:
https://brainly.com/question/27330218
#SPJ1
The question is incomplete. Your most probably complete question is given below:
The Spread of 30The Spied of- Tools Communicable diseases are spread between indivuals by different methods, but they are all caused by pathogens, which are commonly called "germs." Knowledge of pathogens and the ways in which they can be spread helps humans understand and prevent disease out Model 1 - The 1854 London Cholera Outbreak Relative number of deaths due to cholera Portland S. St. Annes Court Beoad St. Pamp 2 Peter St. Old Compton St Pamp Pump +

what is the ratio of aluminum chloride to lithium sulfate
Answers
find δg o for the following reaction, at 25°c, using δh o f and s o values. nh4cl(s) → nh3(g) hcl(g)
Answers
The value of δG° for the given reaction, at 25°C, using δH°f and ∆S° values is 107.49 kJ/mol.
The reaction is :
NH4Cl (s) → NH3 (g) + HCl (g)
The standard enthalpy of formation of NH4Cl(s),
ΔHof = -314.5 kJ/mol
The standard entropy of formation of NH4Cl(s),
ΔSof = 94.6 J/K/mol
The standard entropy of formation of NH3(g),
ΔSof = 192.5 J/K/mol
The standard entropy of formation of HCl(g),
ΔSof = 186.9 J/K/mol
∆G° can be calculated by using the formula,
∆G° = ∆H° - T∆S°
where,∆H° = Standard Enthalpy Change
∆S° = Standard Entropy Change
T = Temperature
Let's calculate ∆G°,
∆G° = {[∆Hof (NH3) + ∆Hof (HCl)] - ∆Hof (NH4Cl)} - T {[∆Sof (NH3) + ∆Sof (HCl)] - ∆Sof (NH4Cl)}
Convert all the values into J as the temperature is given in Kelvin.
∆G° = {[(-46.11 kJ/mol) + (-92.31 kJ/mol)] - (-314.5 kJ/mol)} - (298 K) {[ (192.5 J/K/mol) + (186.9 J/K/mol)] - (94.6 J/K/mol)}
∆G° = {(-138.42 kJ/mol) + 314.5 kJ/mol} - (298 K) {(379.4 J/K/mol) - (94.6 J/K/mol)}
∆G° = 176.08 kJ/mol - 68.59 kJ/mol
∆G° = 107.49 kJ/mol
Therefore, the value of δG° for the given reaction, at 25°C, using δH°f and ∆S° values is 107.49 kJ/mol.
Learn more about δG° at: https://brainly.com/question/9179942
#SPJ11
Convert 0.6330 mol of Na3PO4 to grams. Show the unit analysis by inserting the correct components into their unit- factor slots.
(see picture)

Answers
Answer:
No of moles = mass in gram / molar mass
Mass in gram = no of moles× molar mass
Mass in gram = 0.6330× 163.94
103.77 gram
Explanation:
look below for question

Answers
Calculate the temperature of 2.0 moles of a gas occupying a volume of 5.0 L at 2.46 atm. (R = 0.08125 L atm/mol L)
a. 75 K
b. 348 K
c. 198 K
d. 0.013 K
Answers
Answer:
Hi how are you doing today Jasmine
Answer: a. 75K
Explanation:
https://socratic.org/answers/200353

b) These are the parts of DNA that carry the robustness of DNA genetic code. -pyrimidines -purines -the carbohydrate -phosphate group
Answers
The parts of DNA that carry the robustness of DNA genetic code are the nitrogenous bases, which are divided into two categories: pyrimidines and purines.
Pyrimidines include thymine (T) and cytosine (C), while purines include adenine (A) and guanine (G). These nitrogenous bases pair up with complementary bases to form the rungs of the DNA ladder, and the sequence of these base pairs determines the genetic code. The nitrogenous bases are attached to a sugar molecule, which is known as the carbohydrate, and a phosphate group. Together, these three components make up a nucleotide, which is the building block of DNA.
Hence, The robustness of the DNA genetic code is primarily carried by purines and pyrimidines. Purines include adenine and guanine, while pyrimidines consist of cytosine, thymine, and uracil. These nitrogenous bases form the genetic code by pairing through hydrogen bonds, providing the foundation for DNA's stability and genetic information transmission.
To know more about bases visit
https://brainly.com/question/14115232
#SPJ11
Attributes of the genetic code include all of the following except: A. Each codon consists of 3 nucleotides. B. Each codon specifies more than one amino acid. C. Codons are non-overlapping. D. Most am
Answers
The attributes of the genetic code include all of the following except B. Each codon specifies more than one amino acid.
A. Each codon consists of 3 nucleotides: This is a correct attribute of the genetic code. Codons are made up of three consecutive nucleotides, which form the basic unit of the genetic code.
B. Each codon specifies more than one amino acid: This is incorrect. Each codon typically specifies only one amino acid. However, there are some exceptions called "ambiguous codons" where a single codon can code for more than one amino acid, but they are relatively rare.
C. Codons are non-overlapping: This is a correct attribute of the genetic code. Codons are read sequentially and are not overlapping. Each codon starts at a specific position in the DNA or mRNA sequence.
D. Most amino acids are specified by more than one codon: This is a correct attribute of the genetic code. With a few exceptions, most amino acids are encoded by multiple codons. This redundancy provides some level of error tolerance and allows for variations in the DNA sequence without affecting the encoded protein.
learn more about amino acid
https://brainly.com/question/31872499
#SPJ11
Which of the following is not a reason for extinction?
a change in climate
a change in predation rate
the ability to breed successfully
loss of habitat
Answers
Answer:
the ability to breed successfully
Explanation:
Answer:
the ability to breed successfully
Explanation:
the more offspring's an organism has, the less chance of extinction would happen. I hope that helped bestie
How is melting order related to the melting point of a substance?
Answers
solids are similar to liquids in that both are condensed states, with particles that are far closer together than those of a gas. However, while liquids are fluid, solids are not. The particles of most solids are packed tightly together in an orderly arrangement. The motion of individual atoms, ions, or molecules in a solid is restricted to vibrational motion about a fixed point. Solids are almost completely incompressible and are the densest of the three states of matter.
What is the scientific meaning of the following sentence: lemon juice is an acid
Answers
The scientific meaning of the sentence "lemon juice is an acid" is that lemon juice has a low pH level and can donate hydrogen ions (H+) in solution.
Acid is a substance that has a pH value of less than 7. The pH of pure water is 7, whereas lemon juice is an acid because it has a pH value of 2.3 to 2.5.
Lemon juice gets its acidic properties from citric acid, which makes up about 5% to 8% of its composition.Citric acid can donate protons or H+ ions, which can lower the pH of the solution. Therefore, lemon juice is an acid.
Learn more about pH at:
https://brainly.com/question/31182068
#SPJ11
how many O atoms are in 1.50 mol of sodium carbonate.
Answers
There are 2.71*10^24 atoms of oxygen.
1st) It is necessary to calculate the amount of oxygen in 1.50mol of sodium carbonate (Na2CO3).
We know that 1 mole of sodium carbonate has 3 moles of oxygen, so to calculate the moles of Oxygen in 1.50 moles of Na2CO3 we can use a mathematical Rule of Three:
\(\begin{gathered} 1molNa_2CO_3-3molO \\ 1.5molNa_2CO_3-x=\frac{1.5molNa_2CO_3\cdot3molO}{1molNa_2CO_3} \\ x=4.5molO \end{gathered}\)Now we know that there are 4.5 moles of oxygen in 1.50 moles of sodium carbonate.
2nd) Finally, we can calculate the atoms of oxygen using the Avogadro's number (6.022*10^23 atoms/mol):
\(\begin{gathered} 1\text{mol}-6.022\cdot10^{23}atoms \\ 4.5\text{mol}-x=\frac{4.5\text{mol}\cdot6.022\cdot10^{23}atoms}{1\text{mol}} \\ x=2.71\cdot10^{24}atoms \end{gathered}\)So, there are 2.71*10^24 atoms of oxygen in 1.50 mol of sodium carbonate.
A chemistry teacher adds red food coloring to water and students observed the dye spreading out to fill the container. Is the teacher
demonstrating a physical or chemical change?
Answers
Answer:
its obviously a chemical change
Explanation: Facts
The addition of the food color in water only causes the change in the cor of water but no new substance is formed. Therefore, the teacher is restarting the physical change.
What is a physical change?A physical change can occur when the characteristics of the matter change but the identity does not. Physical changes are classified as: reversible and irreversible. For example, the melting of water is reversible in nature since the melted ice cube will be refrozen.
Physical change can be described as a kind of change where only physical properties of matter such as odor, color, solubility, etc. can change. During physical changes, there is no chemical bonds are broken or formed between atoms of the substance.
The chemical composition as well as the chemical nature of the substance remains unchanged during a physical change. The molecules of matter can rearrange without changing the internal composition of matter.
Therefore, adding food color to water is a physical change as no new substance formed.
Learn more about physical change, here:
brainly.com/question/17931044
#SPJ2
What is the mass % of H in Mn(C₂H₂O₂)4?
Answers
The percent mass of H is 3.3%. This can be found from the data of the elements that we have in the problem.
How do you find the percent mass of an element in a compound?To find the percent mass of an element in a compound, you need to follow these steps:
Determine the molar mass of the compound: This is the sum of the atomic masses of all the elements in the compound. You can find the atomic masses of elements on the periodic table.
Determine the molar mass of the element: This is the atomic mass of the element as it appears in the compound.
Calculate the moles of the element in the compound: This can be done using the formula: Moles = Mass of element / Molar mass of element.
Calculate the percent mass of the element in the compound: This can be done using the formula: Percent mass = (Moles of element x Molar mass of element / Molar mass of compound) x 100%.
Molar mass of the compound = 239 g/mol
Mass of H present = 8 (1) = 8
Percent of H = 8/239* 100/1
= 3.3%
Learn more about percent mass:https://brainly.com/question/5394922
#SPJ1
PLEASE ANSWER QUICKLY
Which are characteristics of arthropods? Check all that apply.
radial symmetry
nervous system
asexual reproduction
segmented body
open circulatory system
Answers
Answer: I say its Segmented body and radial symmetry im not sure if it is right
Explanation:
Answer:
B. Nervous system
D. Segmented body
E. Open circulatory system
Explanation:
Hope this helps!
Have a nice dayy! :)
what is a lock and key model of enzymes
Answers
Answer:
states that the active site of an enzyme precisely fits a specific substrate.
Explanation:
The Avogadro constant is 6.02 x 1023 mol-¹.
Calculate the number of moles of:
a water molecules, H₂O, in 9 g of water
Answers
The number of moles 18.01528.
How to find number of moles?
In the International System of Units, the mole is the unit of substance amount. A mole of a substance is defined as a mass of material that contains exactly 12,000 g of 12C's exact number of atoms as fundamental units. One mole has 600 sextillion molecules. While employing the mole, complicated calculations are more easily understandable. To get the number of moles, divide the compound's known mass by its molar mass. Consider a scenario where your sample of Na2SO4 weighs 20 g. 20 grammes divided by 142 grammes per mole yields 0.141 moles.
To know more about moles, refer: -
https://brainly.com/question/14276478
SPJ1
Given: K for acetic acid is 1.8 X 10–5You are titrating 0.108 M NaOH into 142.0 ml of acetic acid of unknown concentration. You have an indicator that will change color when equivalence is reached. At equivalence, you have added 72.0 ml of the base. Calculate molarity of the acid. What is the pH of the solution at the equivalence point? Now that you know the molarity of the acid, find pH when you mix 50.0ml of the acid with 75.0 ml of the same NaOH solution. Now you are working with different acid and base, both weak. K for the acid is 2.25 X 10-5. You mix 63 ml of 0.275 M acid with 55.0 ml of a weak base of concentration 0.188 M. Find pH
Answers
Answer:
Explanation:0.493 M NaOH means 0.493 mol NaOH/L
mols
mols = ------ x L
L
mols = M x V
In a titration procedure, 40.57 mL of 0.493 M NaOH solution was used. How many mols NaOH did this volume of NaOH solution contain?
mols = M x V
0.493 mols NaOH
mols = ----------------------- x 0.04057 L
L
mols = 0.0200 mols NaOH
Why would it be more comfortable to walk on hot beach sand in flip-flops
than without them?
O A. Thermal insulation is blocked by the flip-flops.
B. Convection warming is blocked by the flip-flops.
C. Radiation warming is blocked by the flip-flops.
O D. Thermal conduction is blocked by the flip-flops.
Answers
Answer:
D. Thermal conduction is blocked by the flip-flops.
Explanation:
Convection is a process of heat transfer which involves fluids( liquid or gas). This however rules convection out of the answer.
Radiation involves heat transfer from the sun to bodies on earth.
Conduction involves heat transfer between two solid bodies. Hot beach sand and flip-flops are solid bodies which validates thermal(heat) conduction.
Can you provide a simple diagram that would explain (why/how)the difference in boiling temperature between an alcohol and a diol?
Answers
Explanation:
Hydrogen bonding, present in alcohols but not hydrocarbons, leads to strong intermolecular forces and increases the boiling point significantly.
For example:
Glycerol has 3 OH groups, which lead to a much more extensive hydrogen-bonding network and a higher boiling point compared to the 1 OH or 2 OH in other chains.


if the pco2 in the plasma increases, what effect will this have on plasma ph?
Answers
When the partial pressure of carbon dioxide (pCO2) in the plasma increases, this leads to a decrease in plasma pH, resulting in a more acidic environment. The relationship between pCO2 and pH is described by the Henderson-Hasselbalch equation, which helps predict the acid-base balance in the body.
An increase in pCO2 levels indicates that more CO2 is being produced or less is being eliminated. As CO2 dissolves in the plasma, it forms carbonic acid (H2CO3), which subsequently dissociates into hydrogen ions (H+) and bicarbonate ions (HCO3-). The increase in H+ ions is what causes the decrease in pH, signifying a more acidic environment.
This change in pH can disrupt the body's normal homeostasis and is commonly referred to as respiratory acidosis. The body's response to this imbalance involves various buffering systems, such as the bicarbonate buffer system, to help restore pH to a normal range.
In conclusion, an increase in plasma pCO2 levels leads to a decrease in plasma pH, creating a more acidic environment. This can disrupt the body's normal functioning and prompt compensatory mechanisms to restore the acid-base balance.
Learn more about bicarbonate here:
https://brainly.com/question/8560563
#SPJ11
from our discussion of how water and carbon dioxide interact on mars, what would happen if a large body of liquid water (a big lake perhaps) were to appear on mars?
Answers
Carbon dioxide would be absorbed by the water, lowering air pressure, resulting in evaporation or freezing of the water.
The early atmosphere of Mars and its early, universal magnetic field both had a direct and indirect impact on the presence of liquid water on the planet. The atmosphere of Mars was much thicker than it is now about 4 billion years ago. And there was a lot more carbon dioxide in there (and other gases). A greenhouse gas called carbon dioxide contributes to the planet's warming. According to two researchers from Caltech's Jet Propulsion Laboratory, a sizable store of carbon dioxide trapped in the soil of Mars may be responsible for the apparent abrupt shifts in the red planet's temperature over billions of years.
Learn more about carbon dioxide Refer:brainly.com/question/3049557
#SPJ4