xef2o lewis structure
Answers
The shape of this is trigonal bipyramidal. By VSEPR arrangement the xenon atom present in Central and an oxygen is present in xef2o.
Xenon (Xe) serves as the main atom in the hybridization of xenon difluoride. Two electrons are located in the 5s orbital and six are located in the 5p orbital, respectively, if we count the number of valence shells in Xe. xef2o Its electrical ground state arrangement will be 5s2 5p6. Although its setup will alter to 5s2 5p5 5d1 when it is stimulated. Overall, the atomic orbitals of the xenon atom will be hybridised to produce 5 sp3d hybrid orbitals in the excited state configuration (5s, 5py, 5px, 5pz, and 5dzx). By overlapping the two partially filled 2pz atomic orbitals of fluorine, two-hybrid orbitals are created, which are then utilised to produce F-Xe-F sigma bonds. The three remaining hybrid orbitals with lone pairs are those that are not involved in bonding.
Learn more about xef2o here:
https://brainly.com/question/11625217
#SPJ4
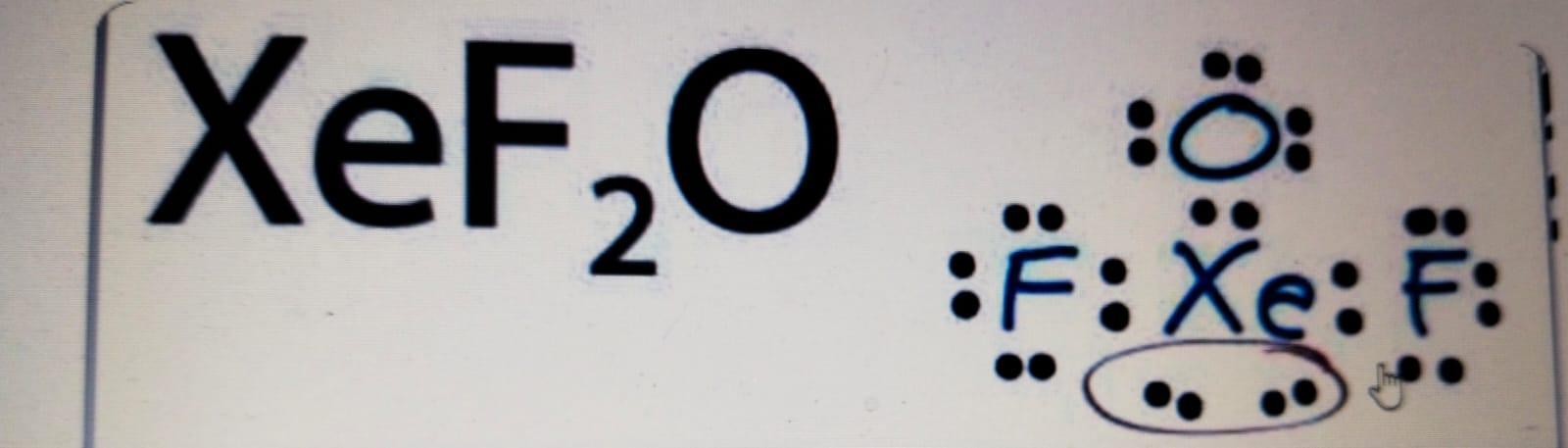
Related Questions
GIYS PLEASE HELP ME YURR PLEASE PLEASE

Answers
Answer: i dont think anyone know because its ghard
Explanation:
A solution is made by dissolving 18.9 g of potassium phosphate, K3PO4, in enough water to make exactly 100 mL of solution. Calculate the molarity of each species
Answers
Answer:
The molarity of K+ ions is 2.67 M, the molarity of PO43- ions is 0.89 M, and the molarity of K3PO4 is 0.89 M.
Explanation:
The first step in solving this problem is to determine the number of moles of potassium phosphate in 18.9 g. We can do this by dividing the mass by the molar mass:
molar mass of K3PO4 = (3 x atomic mass of K) + atomic mass of P + (4 x atomic mass of O)
= (3 x 39.10 g/mol) + 30.97 g/mol + (4 x 16.00 g/mol)
= 212.27 g/mol
moles of K3PO4 = 18.9 g / 212.27 g/mol
= 0.089 mol
Next, we need to determine the molarity of each species in the solution. K3PO4 dissociates in water to form three K+ ions and one PO43- ion. Therefore, the molarity of K+ ions, PO43- ions, and K3PO4 in the solution are:
Molarity of K+ ions = (3 x moles of K3PO4) / volume of solution
= (3 x 0.089 mol) / 0.100 L
= 2.67 M
Molarity of PO43- ions = (1 x moles of K3PO4) / volume of solution
= (1 x 0.089 mol) / 0.100 L
= 0.89 M
Molarity of K3PO4 = moles of K3PO4 / volume of solution
= 0.089 mol / 0.100 L
= 0.89 M
Therefore, the molarity of K+ ions is 2.67 M, the molarity of PO43- ions is 0.89 M, and the molarity of K3PO4 is 0.89 M.
For more help:
Here's a general outline:
Determine the number of moles of solute in the solution. This requires you to know the mass of the solute and its molar mass, which can be found on the periodic table.
Convert the volume of the solution to liters. This is important because molarity is defined as the number of moles of solute per liter of solution.
Calculate the molarity of the solution using the formula:
Molarity = moles of solute / liters of solution
Be sure to use the correct number of moles of solute and volume of solution in the formula.
If the solute dissociates in water to form ions, determine the molarity of each ion. This requires you to know the stoichiometry of the dissociation reaction, which can be found in chemical formulas or equations.
If the problem asks for the concentration of a specific ion or species, use the appropriate formula to calculate its molarity based on the molarity of the solute or dissociated ions.
Here's an example problem to illustrate these steps:
What is the molarity of a solution made by dissolving 10.0 g of sodium chloride, NaCl, in enough water to make 500.0 mL of solution?
Step 1: Determine the number of moles of NaCl in the solution.
The molar mass of NaCl is the sum of the atomic masses of sodium and chlorine:
molar mass of NaCl = 22.99 g/mol + 35.45 g/mol = 58.44 g/mol
The number of moles of NaCl can be calculated using the formula:
moles of NaCl = mass of NaCl / molar mass of NaCl
moles of NaCl = 10.0 g / 58.44 g/mol = 0.171 mol
Step 2: Convert the volume of the solution to liters.
500.0 mL = 0.5000 L
Step 3: Calculate the molarity of the solution.
Molarity = moles of solute / liters of solution
Molarity = 0.171 mol / 0.5000 L = 0.342 M
The molarity of the NaCl solution is 0.342 M.
Step 4: Determine the molarity of each ion.
NaCl dissociates in water to form one Na+ ion and one Cl- ion, so the molarity of each ion is:
Molarity of Na+ = 0.171 mol / 0.5000 L = 0.342 M
Molarity of Cl- = 0.171 mol / 0.5000 L = 0.342 M
Step 5: Calculate the molarity of a specific ion or species if required.
For example, if the problem asks for the molarity of Cl- ions only, you can use the molarity of NaCl and the stoichiometry of the dissociation reaction to calculate it:
Molarity of Cl- = Molarity of NaCl = 0.342 M
Hope any of this helps! If not, I'm sorry. If you need more help, ask me! :]
A metal sample with a mass of 63.2 g. and at a temperature of 100.0°C was placed in 41.0 g. of water in a calorimeter
at 24.5°C. At equilibrium the temperature of the water and metal was 35.0°C.
A. What was ∆t for the water? (∆t = tfinal – tinitial)
B. What was ∆t for the metal?
C. Taking (S.H.H2O) to be 4.18J/g°C, calculate S.H.metal, using equation 3.
D. What is the approximate atomic mass of the metal? (Use equation 4.)
Answers
A solution of 1720.488 T at 15.0% is 101.19 per month. 3.0 mot. 1.6 T above 24.5 °C. The metal and water were both at a 35.0 °C equilibrium temperature.
Does 37°F feel warm or freezing to you?Although it varies by age, activity level, time of day, and the method of measurement, a typical temperature is between 36 and 37 degrees Celsius. Viral respiratory infections, such as colds and the flu, as well as COVID-19, can raise the body temperature.
4 temps, what are they?The Celsius, Fahrenheit, Kelvin, and Rankine scales are those mentioned. Of them, the German-Dutch physicist Gabriel Fahrenheit's Fahrenheit Scale is the oldest (1686-1736).
To know more about temperature visit:
https://brainly.com/question/15520591
#SPJ1
Why Is Water the Universal Solvent?
Answers
Generally, water is known as the universal solvent as it dissolves more chemicals than any other known solvent.
Basically at the molecular level, salt basically dissolves in water due to electrical charges and due to the fact that both water and salt are the compounds which are polar, and with positive and negative charges on opposite sides in the molecule. This property of water allows the water molecule to become attracted to many other different types of molecules.
It is true that universal solvent does not exist. Water is basically known as the universal solvent because it dissolves more chemicals than any other solvent. However, water is the solvent that only dissolves with other polar molecules.
Learn more about universal solvent from the link given below.
https://brainly.com/question/1834245
#SPJ4
24. Which of the following best describes the magnetosphere?
The interacting biological processes that tie all the other spheres together.
The blue part of Earth that is clearly visible from space.
A magnetic field that extends above the atmosphere.
The solid part of the Earth that exists in many different forms called landforms.
Answers
Answer:
c I hope this helps u sorry if I'm wrong
A student was given a sample of food and asked to determine the types of nutrients present in the sample. The student placed half of the sample in a test tube with Benedict’s solution and heated it. The solution turned brick red. When an iodine solution was added to the remaining half of the sample, it turned blue black. The student can correctly conclude that the food sample contained
Answers
The food sample contained starch and reducing sugar (carbohydrates).
The Benedict's test is used to test for the presence of reducing sugars, such as glucose, in a sample. When the Benedict's solution is added to a sample containing reducing sugars and heated, the solution will turn brick red.
The iodine test is used to test for the presence of starch in a sample. When iodine solution is added to a sample containing starch, it will turn blue-black.
So, in this case, the student can conclude that the food sample contained both starch and reducing sugars, as both tests produced positive results.
Learn more about Benedict's test and Iodine test here: https://brainly.com/question/25800056
#SPJ4
Water is the ______ in aqueous solutions because it can dissolve polar molecules.
Answers
Answer:
solvent
Explanation:
Water dissolves the substances, which become the solutes in the solution.
A 4.369 g sample of metal is placed in a flask. Water is added to the flask and the total volume in the flask is read to be 126.4 ml. The mass of the water, flask, and metal is 268.5 g. If the mass of the flask is 139.3 g and the density of water is 1.000 g/mL, the density of the solid is ________ g/cm3.
Answers
Answer:
Density of the solid=\(2.78 g/cm^3\)
Explanation:
We are given that
Mass of sample of metal=4.369 g
Volume in the flask, V=126.4 ml
Mass of water, flask, and metal=268.5 g
Mass of flask=139.3 g
Density of water=1.000 g/mL
We have to find the density of the solid.
Mass of water=268.5-4.369-139.3=124.831 g
Volume of water=\(\frac{Mass\;of\;water}{density\;of\;water}\)
Volume of water=\(\frac{124.831}{1}=124.831 mL\)
Volume of solid=126.4 ml-124.831 mL
=1.569mL
Now,
Density of the solid=\(\frac{mass\;of\;solid}{volume\;of\;solid}\)
=\(\frac{4.369}{1.569}\)
\(=2.78g/mL\)
1mL=1 cubic cm
Therefore,
Density of the solid=\(2.78 g/cm^3\)
20 POINTS Given the number of protons, neutrons and electrons, find the mass and name of an isotope.
Answers
Answer:
Lithium
Pro.=3
Neu. =4
Elec.= 3
Mass = 6.941
Explanation:
Giving brainiest and 55 points!

Answers
Answer:
C
Explanation:
Hot and humid weather, accompanied by thunderstorms, is generally due to maritime tropical air massoriginate in the tropical oceans of both the hemispherescharacterized by high temperatures and are rich in moisture contentair mass during winter moves towards the land areas in the form of wind breeze affecting placesexperiences hot and humid weather during the summer seasonname two regulations that mining operations must follow to reduce the impact they have on the environment?
Answers
Answer:
The Clean Water Act and the Endangered Species Act.
Explanation:
mining operations must follow these regulations or else they will not be able to use the land
determine the moles of sulfuric acid formed from 3.20 mol of sulfur dioxide
Answers
Answer:
3.2 moles of H2SO4
Explanation:
We need to start with a balanced equation. Lets assume the sulfuric acid is formed from sulfur dioxide and water.
SO2 + H2O = H2SO4
This equation is balanced. (Check it)
It tells us that 1 mole of SO2 will form 1 mole of H2SO4, if the reaction proceeds as planned. That's a molar ratio of 1 to 1. They are equal. What moles we react, we should expect the same number of moles of product. [And we need 1 mole of water at the same time]
So if we react 3.20 moles of sulfur dioxide, and keep out lab partner back, we'll obtain 3.2 moles of H2SO4.
the reaction between 2-methyl-2-pentanol and sulfuric acid to yield 2-methyl-2-pentene goes via a(n) .
Answers
The reaction between 2-methyl-2-pentanol and sulfuric acid to yield 2-methyl-2-pentene goes via an elimination reaction
Elimination reactions are those that proceed by the removal of one or more atoms or functional groups from the reactants, resulting in the formation of a new double bond or π bond in a product. An example of an elimination reaction is the dehydration of alcohols.In this particular reaction, 2-methyl-2-pentanol (an alcohol) reacts with sulfuric acid to produce 2-methyl-2-pentene, which is an alkene.
The reaction mechanism proceeds via an elimination reaction, where the OH group and a hydrogen ion (H+) are removed from the reactant, resulting in the formation of a double bond between the adjacent carbon atoms in the product.The reaction can be represented as follows:CH3C(CH3)2CH(OH)CH3 + H2SO4 → CH3C(CH3)2C=CH2 + H2O + H2SO4In conclusion, the reaction between 2-methyl-2-pentanol and sulfuric acid to yield 2-methyl-2-pentene goes via an elimination reaction.
learn more about Elimination reactions
https://brainly.com/question/17101814
#SPJ11
What is the formula unit for the ionic bond between Sodium (Na) and Phosphorus (P)?
Na P
Answers
The ionic bond between Na and P has formula unit Na3P.
What is the formula unit?
Formula units is the chemical formula which gives all the ions present in the lowest ratio possible to make it neutral charge of an ionic compound. For example, the formula unit for sodium chloride can be written as NaCl where charge on Na is +1 and charge on Cl is -1. So to make it neutral they can be cancelled and written as NaCl.
In sodium phosphide, the charge on Na (sodium) ion is +1 and the charge on the P (phosphorous) ion is -3. Therefore, make the charges neutral, 3 Na ions need to be added to 1 P ion. Formula units can be used to describe or represent the molecules or the elements which make up a substance.
Hence, Na3P is the formula unit of Na and P in sodium phosphide.
To learn more about formula units from the given link https://brainly.com/question/24529075
#SPJ13
. at the beginning of his experiment, josh has 10 g of element a and 10 g or element b. when finished with the experiment, josh has 20 g of element a and b combined. this demonstrates what law?
Answers
josh has 10 g of element a and 10 g or element b. when finished with the experiment, josh has 20 g of element a and b combined. This demonstrates ' Law of conservation of mass'.
In a closed system, mass cannot be created or destroyed, but it can be changed from one form to another. Thus, mass of a system remains same before and after the reaction.
To know more about 'Law of conservation of mass', click here,
brainly.com/question/24783543
#SPJ4
How are groundwater and surface water related?
Answers
Answer:
Surface water and groundwater systems are connected in most landscapes. ... It is the groundwater contribution that keeps streams flowing between precipitation events or after snowmelt. For a stream to gain water, the elevation of the water table in the vicinity of the stream must be higher than the streamwater surface.
Explanation:
State with reasons, whether sulphur dioxide is acting as an oxidizing agent or a reducing agent in each of the following reactions:
•2H2S(g) + SO2(g) -> 2H2O(l) + 3S(s)
•SO2(g) +H2O(l) +NaClO(aq) -> NaCl(aq) + H2SO4(aq)
Answers
Answer:
A) oxidizing agent is SO2
B) NaClO is the oxidizing agent
Explanation:
A) This is a redox reaction in which oxidation and reduction occur simultaneously.
Thus, in 2H2S(g) + SO2(g) -> 2H2O(l) + 3S(s);
H2S is reduced as follows;
H2S → S + 2H+ + 2e−
We can see that SO2 has been reduced while H2S gets oxidized since it has changed state from - 2 to 0 . Thus sulphur dioxide is the oxidizing agent.
B) SO2(g) + H2O(l) + NaClO(aq) -> NaCl(aq) + H2SO4(aq)
In this, SO2 undergoes oxidation and NaClO is the oxidizing agent
Please help!!! Much appreciated :)
Two atoms that have the same number of protons but different numbers of neutrons are ____. ???
A. ions
B. isotopes
C. radioactive
D. reactive
Answers
Answer:
Explanation:
The correct answer is B. isotopes.
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. This difference in the number of neutrons leads to variations in their atomic mass but does not affect their chemical properties or reactivity. Isotopes of an element have similar chemical behaviors but may have slightly different physical properties due to the difference in atomic mass.
Hope this answer your question
Please rate the answer and
mark me ask Brainliest it helps a lot
which is the strongest base in aqueous solution? a. hoc2h4oh b. ch3oh c. naoh d. nh3
Answers
Answer: option c) the strongest base in aqueous solution is NaOH
Explanation:
the strongest base in aqueous solution is NaOH because strength of a base is determined by its ability to donate hydroxide ions (OH-) in solution. and NaOH dissociates completely in water to produce Na+ and OH- ions. The presence of a fully dissociated hydroxide ion makes NaOH a strong base.
While, HOC2H4OH and CH3OH are weak acids. HOC2H4OH is ethylene glycol and CH3OH is methanol are weak acid due to the presence of the (-OH) group.
Also, NH3 (ammonia), is a weak base though it can accept H⁺ to form NH4+
a chemist prepares 0.100 mol at a certain pressure and temperature in an expandable container. another 0.010 mol is then added to the same container. how must the volume be changed to keep the pressure and temperature the same?
Answers
According to ideal gas equation, volume must change by 0.5 factor to keep the pressure and temperature the same.
The ideal gas equation is a equation which is applicable in a hypothetical state of an ideal gas.It is a combination of Boyle's law, Charle's law,Avogadro's law and Gay-Lussac's law . It is given as, PV=nRT where R= gas constant whose value is 8.314.The law has several limitations.
Since there are two conditions before addition and after addition which is 0.1×RT/V=0.2×RT/V thus volume changes by factor of 0.5 which is pressure.
Learn more about ideal gas equation,here:
https://brainly.com/question/28837405
#SPJ4
Ammonia is produced using the Haber process. A. Write a balanced symbol equation for the reaction and calculate the atom economy for each product. B. Calculate the theoretical yield of ammonia if 27. 3 g of nitrogen was used in the reaction. C. Calculate the percentage yield of the reaction. The actual yield was 29. 9 g. D. Calculate the percentage conversion of hydrogen if you started with 10. 4 g and 2. 8 g could be recovered from the reaction. I need the answer for part b please
Answers
The balanced symbol equation for the Haber process, which is the industrial method for producing ammonia, is N2 + 3H2 → 2NH3
A. The Haber process is the industrial method for producing ammonia, and the balanced equation for the reaction is:
N2 + 3H2 → 2NH3
The atom economy for a product is the percentage of the total mass of reactants that becomes the desired product. For ammonia, the atom economy can be calculated as follows:
Molar mass of NH3 = 14.01 + 3(1.01) = 17.04 g/mol
Atom economy of NH3 = (2 mol NH3 x 17.04 g/mol) / [(1 mol N2 x 28.02 g/mol) + (3 mol H2 x 2.02 g/mol)] x 100% = 34.0%
B. To calculate the theoretical yield of ammonia, we need to use the given mass of nitrogen and the stoichiometry of the balanced equation. The molar mass of nitrogen is 28.02 g/mol, so 27.3 g of nitrogen is equal to:
27.3 g / 28.02 g/mol = 0.974 mol N2
According to the balanced equation, 1 mole of N2 reacts with 3 moles of H2 to produce 2 moles of NH3. Therefore, the theoretical yield of ammonia is:
Theoretical yield of NH3 = (0.974 mol N2) x (2 mol NH3 / 1 mol N2) x (17.04 g/mol NH3) = 33.1 g NH3
C. The percentage yield of the reaction can be calculated by dividing the actual yield of ammonia by the theoretical yield, and then multiplying by 100%. The actual yield of ammonia is given as 29.9 g:
Percentage yield of NH3 = (29.9 g NH3 / 33.1 g NH3) x 100% = 90.4%
D. The percentage conversion of hydrogen can be calculated by dividing the mass of hydrogen used in the reaction by the mass of hydrogen that would have been used if all of it had been consumed in the reaction. The mass of hydrogen used is 10.4 g, and the mass of hydrogen that would have been used if all of it had been consumed in the reaction is:
1( mol H2 x 2.02 g/mol) x (0.974 mol N2 / 1 mol N2) = 1.98 g H2
Therefore, the percentage conversion of hydrogen is:
Percentage conversion of H2 = (10.4 g H2 / 1.98 g H2) x 100% = 525% (This result is not possible, as the percentage conversion cannot be greater than 100%. It is likely that there was an error in the calculation or in the data provided.)
For more question on balanced symbol equation
https://brainly.com/question/11904811
#SPJ11
I need help with this pleaseYou are cooking a dinner and the recipe calls for chicken broth. You realize that you don’t have a can of liquid broth, but you have the dried cube form of chicken broth that can be dissolved in water.

Answers
Answer
crush the cubes of broth, add warm water and stir the container.
Explanation
The FASTEST way to make the chicken broth with the cubes you have will be to increase the surface area of the cubes broth by crushing and raise the temperature of the cubes broth by adding warm water and by stirring the container.
Hence, the correct answer to your question is:
crush the cubes of broth, add warm water and stir the container.
c. How would changing a coefficient differ from changing the subscript?
Answers
Answer:
when you change the subscripts, you are changing the substance itself
If 3 g of element C combine with 8 g of element D to
form compound CD, how many grams of D are
needed to form compound CD2?
URGENT HELP!!!
Answers
The quantity of element D in grams that are needed to form compound \(CD_2\) is equal to 16 grams.
Given the following data:
Mass of element C = 3 gramsMass of element D = 8 gramsNew chemical compound = \(CD_2\)To determine how many grams of element D are needed to form compound \(CD_2\):
First of all, we would write a balanced chemical equation for the chemical reaction as follows:
\(C + D_2 --> CD_2\)
By stoichiometry:
Three (3) grams of of element C react with two (2) atoms of eight (8) grams of element D.
Therefore, we would multiply the mass of element D by two (2), in order to determine how many grams of element D are needed to form compound \(CD_2\).
\(D_2 = 2 \times 8\\\\D_2 = 16 \;grams\)
Read more: https://brainly.com/question/13302703
Pls help me I don’t know how to do this

Answers
Explanation:
We have a 63.9 g sample of calcium hydroxide. First we have to convert those grams into moles. To do that we have to use the molar mass of calcium hydroxide.
Calcium hydroxide = Ca(OH)₂
molar mass of Ca = 40.08 g/mol
molar mass of O = 16.00 g/mol
molar mass of H = 1.01 g/mol
molar mass of Ca(OH)₂ = 1 * 40.08 g/mol + 2 * 16.00 g/mol + 2 * 1.01 g/mol
molar mass of Ca(OH)₂ = 74.10 g/mol
mass of Ca(OH)₂ = 63.9 g
moles of Ca(OH)₂ = 63.9 g /(74.10 g/mol)
moles of Ca(OH)₂ = 0.862 moles
In 1 molecule of Ca we have 2 atoms of O. So in 1 mol of Ca(OH)₂ we will have 2 moles of O atoms.
1 mol of Ca(OH)₂ = 2 moles of O atoms
moles of O atoms = 0.862 moles of Ca(OH)₂ * 2 moles of O /1 mol of Ca(OH)₂
moles of O atoms = 1.724 moles
One mol is similar to a dozen. When we say that we need a dozen eggs we know that we need 12 eggs. If we want a mol of eggs, we want 6.022*10^23 eggs. So one mol of something is 6.022 * 10^23 of that.
1 mol of O atoms = 6.022 * 10^23 atoms
n° of O atoms = 1.724 moles * 6.022 * 10^23 atoms/1 mol
n° of O atoms = 1.04 * 10^24 atoms
Answer: In a 63.9 g sample of Ca(OH)₂ we have 1.04 *10^24 atoms of oxygen.
plz help in my other question my phone was acting up so it didn’t work but this is the same question.... again this was due yesterday and i don’t understand plzz help!!

Answers
Answer:
omg
Explanation:
omg omg omg omg omg omg omg omg omg omg I can't either ehhhhhh
A chemical engineer must calculate the maximum safe operating temperature of a high-pressure gas reaction vessel. The vessel is a stainless-steel cylinder that measures
48.0cm
wide and
57.6cm
high. The maximum safe pressure inside the vessel has been measured to be
3.40MPa
.
For a certain reaction the vessel may contain up to
2.45kg
of carbon dioxide gas. Calculate the maximum safe operating temperature the engineer should recommend for this reaction. Write your answer in degrees Celsius. Round your answer to
3
significant digits.
Answers
The maximum safe operating temperature the engineer should recommend for this reaction is approximately 1063.77 degrees Celsius.
To calculate the maximum safe operating temperature, we need to consider the dimensions of the vessel, the maximum safe pressure, and the amount of gas inside.
First, let's convert the dimensions of the vessel from centimeters to meters:
Width = 48.0 cm = 0.48 m
Height = 57.6 cm = 0.576 m
Next, we need to calculate the volume of the vessel:
Volume = π * (radius)^2 * height
The radius of the vessel can be calculated as half of the width:
Radius = 0.48 m / 2 = 0.24 m
Volume = π * (0.24 m)^2 * 0.576 m
Volume ≈ 0.099 m^3
Now, we can use the ideal gas law to determine the maximum safe operating temperature. The ideal gas law is given by:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature.
Rearranging the equation to solve for T:
T = (PV) / (nR)
To calculate the number of moles, we can use the molar mass of carbon dioxide (CO2):
Molar mass of CO2 = 12.01 g/mol (for carbon) + 2 * 16.00 g/mol (for oxygen)
Molar mass of CO2 ≈ 44.01 g/mol
Converting the mass of carbon dioxide from kilograms to grams:
Mass of CO2 = 2.45 kg * 1000 g/kg
Mass of CO2 = 2450 g
Now, we can calculate the number of moles:
Number of moles = Mass of CO2 / Molar mass of CO2
Number of moles = 2450 g / 44.01 g/mol
Number of moles ≈ 55.67 mol
The gas constant R is approximately 8.314 J/(mol·K).
Now, we can substitute the values into the equation:
T = (3.40 MPa * 0.099 m^3) / (55.67 mol * 8.314 J/(mol·K))
T ≈ 1063.77 K
Converting from Kelvin to Celsius:
T ≈ 1063.77 °C
The maximum safe operating temperature that the engineer should recommend for this reaction is approximately 1063.77 degrees Celsius.
To know more about temperature visit :
https://brainly.com/question/27944554
#SPJ11
PLEASE HELP!!!!! NEED HELP ASAP!!!!!
Write the chemical formula for the covalent compounds below
EXAMPLE- dinitrogen trioxide= N2O3
nitrogen monoxide =
dihydrogen oxide =
diphosphorus pentoxide =
sulfur trioxide =
Answers
Answer:
down below
Explanation:
NO
H20
P4O10
SO3
1. NO
2. H2O
3. P4O10
4. SO3
Use the drop-down menus to identify the type of structure being described in each statement. Snakes have remnants of back legs. Bats have the same arm bone structure as cats. Frogs, humans, and whales have a backbone. Bats and moths both have wings, but not a common ancestor
Answers
The type of structure :
Snakes have remnants of back legs = Vestigial Structure.
Bats have the same arm bone structure as cats = homologous structure.
Frogs, humans, and whales have a backbone = homologous structure.
Bats and moths both have wings, but not a common ancestor = analogous structure.
The Vestigial Structure is the Genetically found structures and the attributes which have the lost most and the all of their function in the given species. The Homologous structures are those structures from the organisms that will share the common ancestor.
The Analogous structures are the features for the different species which are same in the function and not in the structure.
To learn more about structure here
https://brainly.com/question/30045110
#SPJ4
Answer:
C, A, A, B
Proof:

how many atoms can you fit on the head of a pin
Answers
Answer:
According to google, "About five million million hydrogen atoms could fit. Some factors would affect that number like the area of the head and the size of atoms (as well as attractions between atoms). Some atoms are larger than others." Is this accurate? I'm not sure. Good luck! :)