X-rays are often used in medical settings to create images of the body's internal structures such as bones. This is made possible by the
fact that X-rays are able to pass through the body's softer tissues without being absorbed.
Radio waves are also able to pass through the body's softer tissues without being absorbed. Why are radio waves not used to generate
medical images?
OA. The electrons in most atoms are not in high enough energy states to absorb the photons of radio waves.
OB. Radio waves tend to bend too much when they encounter solid materials to be used for generating accurate images,
OC
The frequency of most radio waves is too low to allow them to pass through bones or other solid materials.
OD.
Radio waves carry so little energy that they tend to pass through most atoms without an interaction taking place.
Answers
Answer:D
Explanation:I did it on study island.
Radio waves are not used to generate medical images because as per the electromagnetic spectrum ,the radio waves carry little energy that they pass through most atoms without interaction.
What is an electromagnetic spectrum?The electromagnetic spectrum consists of radiation which consists of waves made up of electromagnetic field which are capable of propogating through space and carry the radiant electromagnetic energy.
The radiation are composed of electromagnetic waves which are synchronized oscillations of electric and magnetic fields . They are created due to change which is periodic in electric as well as magnetic fields.
In vacuum ,all the electromagnetic waves travel at the same speed that is with the speed of air.The position of an electromagnetic wave in an electromagnetic spectrum is characterized by it's frequency or wavelength.They are emitted by electrically charged particles which undergo acceleration and subsequently interact with other charged particles.
Learn more about electromagnetic spectrum,here:
https://brainly.com/question/15576247
#SPJ2
Related Questions
If plant a, the parent plant, died from a new disease, what might happen to plant B?
Answers
What formula for a molecule formed from P and Cl would be? PCl 5 PCl 2 P 3Cl PCl PCl 3
Answers
The question requires us to identify the molecular formula of a molecule formed between phosphorus (P) and chlorine (Cl).
The first step to solve this question is write the electronic configuration of both atoms involved (P and Cl) and, from the number of valence electrons, identify how many bonds each one should make.
P has atomic number 15, thus its electronic configuration is: 1s2 2s2 2p6 3s2 3p3
Cl has atomic number 17, thus its electronic configuration is: 1s2 2s2 2p6 3s2 3p5
Note that P has 5 valence electrons (3s2 and 3p3) while Cl has 7 valence electrons (3s2 3p5).
For P, we should expect 3 bonds to other atoms in order to reach the octet, while for Cl, we should expect 1 bond to another atom to achieve the octet.
With that in mind, we can say that each Cl will bond to one P, and we need 3 Cl. Therefore, the molecular formular is PCl3 (the last option).I
two isotopes of potassium are k-37 and k- 42
Answers
In the ground state, it should be noted that the the number of valence electrons the K-42 isotope has is only one valence electron.
How to illustrate tye valence electrons?In the first group of the periodic table is potassium. It has a valence shell with one electron.
We can assume that potassium only has one electron on its valence shell because it belongs to group 1. The isotope is irrelevant because the only distinction between isotopes is the number of neutrons present in their nuclei.
In summary, it should be noted that K-42 only possesses one valence electron.
Learn more about potassium on;
https://brainly.com/question/2460164
#SPJ1
Two isotopes of potassium are K-37 and K-42. How many valence electrons are in an atom of K-42 in the ground state?
What is osmotic pressure of a solution that contains 13.7 g of propyl alcohol (C3H7OH) dissolved in enough water to make 500 mL of solution at 27C ?
Answers
Answer:
11.23 atm
Explanation:
Given
Mass = 13.7 g
Volume = 500mL = 0.5 L
Molar concentration = \(\frac{\text{Moles}}{\text{Volume}}\\\)
Moles =\(\frac{\text{MassC3H7OH }}{\text{Molar mass C3H7OH }}\) = \(\frac{13.7}{0.5}\)= 0.2279534 moles
Molar concentration =\(\frac{0.2279534}{0.5}\) = 0.4559 M
π = icRT
where
Osmotic pressure = π
Van't Hoff factor (i) = 1
Molar concentration of solute (c) = 0.4559 M
Ideal gas constant (R) = 0.0821 L.atm/K.mol
Kelvin Temperature (T) = 273 + 27 = 300 K
\(\pi\) = 1 * 0.4559 * 0.0821 * 300
= 11.23 atm
The value of osmotic pressure is 11.23 atm.
Equation of osmotic pressure:-\(pi= icRT\)......(1)
where Osmotic pressure = pi
Van't Hoff factor=i=1
Ideal gas constant =R= 0.0821 L.atm/K.mol
Temerature=T,(273 + 27) = 300 K
Concentration=c
Given:-
Mass = 13.7 g
Volume = 500mL = 0.5 L
Moles = \(\frac{Mass}{Molar mass} =\frac{13.7g}{60.09g/mol} =0.2279534\ mol\)
Molar concentration = \(\frac{0.2279\ mol}{0.5\ L}\) = 0.4559 M
Molar concentration of solute (c) = 0.4559 M
Substitute all the values in equation (1) as follows:-
\(pi = 1 * 0.4559 * 0.0821 * 300= 11.23 atm\)
Find more information about Van't Hoff factor here
brainly.com/question/22047232
Elements that have atoms with full outer shells of electrons___
A) will form many compounds.
B) will normally form anions.
C) will normally form cations.
D) frequently form hydrogen bonds.
E) are inert gases
Answers
E) are inert gases. Inert gases have full outer shells of electrons, making them unreactive and unlikely to form compounds.
Elements that have full outer shells of electrons, also known as noble gases, are highly unreactive and unlikely to form compounds. This is because their electrons are fully occupied in the outermost energy level, so they have no desire to bond with other elements. As a result, they are referred to as "inert gases". Examples of inert gases include helium, neon, argon, krypton, xenon, and radon. Inert gases are often used in a variety of applications due to their stability and lack of reactivity, such as filling the space inside light bulbs, in cooling and refrigeration systems, and as shielding gases in welding.
Learn more about inert gases here:
https://brainly.com/question/14234951
#SPJ4
Examples of solutions can be:
O A. a solid dissolved in a liquid
O B. gas mixed with a liquid
O C. liquid mixed with a liquid
OD. all of the the above
Answers
Answer:
B. gas mixed with a liquid
Explanation:
because:
A. a solid can't dissolve in a liquid.
C. two liquids mixed isn't a solution
D. Not All of the above for sure.
Examples of solutions can be all of the above. The correct option is D.
What is a solution?A solution is made when a solute is mixed with a solvent. The solution can be a liquid, solid, or gas. The solution is made when the solute is fully mixed with the solvent. The solute is the substance that gets mixed, and the solvent is the base in which the solute gets dissolved.
So, a solid dissolved in a liquid, gas mixed with a liquid, or a liquid mixed with a liquid, the energy statement is true for a solution.
Examples of a solution are sugar dissolved in water when water mix with soda and state gets mixed with water or another gas.
Thus, the correct options are D. all of the the above.
Learn more about the solution, here:
https://brainly.com/question/1416865
#SPJ2
A 50.0 gram sample of NaOH is dissolved in 0.600 L of water. What volume of this solution would be needed to create a 1.50 L solution that is 0.200 M NaOH?
Answers
Answer:
0.144 L (144 mL).
Explanation:
First, let's calculate the molarity of the solution of 0.600 L of water with 50.0 g of NaOH. But first, let's calculate the number of moles of 50.0 g of NaOH using its molar mass which is 40 g/mol (you can calculate the molar mass of a compound using the periodic table):
\(50.0\text{ g NaOH}\cdot\frac{1\text{ mol NaOH}}{4\text{0 g NaOH}}=1.25\text{ moles NaOH.}\)The next step is to use the formula of molarity:
\(Molarity\text{ \lparen M\rparen=}\frac{mole\text{s of solute}}{liter\text{s of solution}}=\frac{mol}{L}.\)And replace the given data (moles of solute = 1.25, liters of solution = 0.600 L):
\(Molarity=\frac{1.25\text{ moles}}{0.600\text{ L}}=2.08\text{ M.}\)We want to find the volume that is needed to create a 1.50 L (volume) of 0.200 M NaOH. We have to use the formula:
\(C_1V_1=C_2V_2.\)Where C indicates the concentration in M and V the volume in L. In this case, our unknown value can be V1 but remember that the concentration of this solution (C1) was 2.08 M and we have to equal this to the concentration (0.200 M) and volume (1.50 L) of the wanted solution. It will look like this:
\(V_1=\frac{C_2V_2}{C_1}=\frac{0.200\text{ M}\cdot1.50L}{2.08\text{ M}}=0.144\text{ L.}\)The volume required would be 0.144 L (144 mL).
Here is a second order reaction A→ P. If the initial concentration of A 0.0818 M goes down 30.0% in 3.15 minutes, what is the rate constant for the reaction?
Answers
The rate constant of the second-order reaction is 0.111 M^-1 min^-1.
The given data represents a second-order reaction where the rate of the reaction is proportional to the square of the concentration of A.
The integrated form of the second-order reaction is:
1/[A]t = kt + 1/[A]0
where [A]t and [A]0 are the concentrations of reactant A at time t and time zero, respectively, k is the rate constant.
We can use the given information to calculate the rate constant (k) of the reaction for the given half-life (t1/2) of 3.15 minutes:
t1/2 = (1 / k[A]0)
Using the percentage decrease in concentration and the given initial concentration, we can calculate the concentration of A at time t:
[A]t = [A]0 - 0.30[A]0 = 0.57126 M
Substituting the given values, we get:
3.15 min = (1 / k)(0.0818 M) / (0.0818 M - 0.57126 M)
Simplifying the equation above, we can solve for k:
k = 0.111 M^-1 min^-1
Therefore, the rate constant of the second-order reaction is 0.111 M^-1 min^-1.
For such more questions on constant
https://brainly.com/question/3159758
#SPJ11
What is the role of calcium ions in the release of a neurotransmitter substance?
Answers
The emission of a transmitter is caused by the action of calcium ions, which also cause synaptic vesicle exocytosis, which releases the neurotransmitters inside the vesicles and starts synaptic transmission.
What functions does calcium ion serve in the body?Nearly all bodily biological processes, including heart and muscle pulses, neurotransmission of information, memories and learning baby creation, cell proliferation, and Calcium ions enter the cytoplasm of organelles through calcium channels.
Why are calcium ions necessary for the brain?Calcium plays a critical role in the brain's regulation of synaptogenesis and memory formation. This process activates certain calmodulin signal transmission pathways and involves important protein effectors such CaMKs, MAPK/ERKs, or CREB.
To know more about Calcium ions visit:
https://brainly.com/question/28186243
#SPJ4
List the 2 pKa's for H2SO4
Answers
10. Although lidocaine is marketed as its hydrochloride salt, it doesn’t exhibit the same level of physiological activity as the free amine. The free amine is more lipophilic and diffuses across a neuron cell membrane more rapidly than the ionic salt, resulting in a more rapid onset of anesthesia. Therefore, sodium bicarbonate (NaHCO3) is added to a solution of lidocaine prior to injection. How does the addition of sodium bicarbonate promote a faster anesthetic effect?
Answers
Answer:
Bicarbonate neutralizes the acidity of Lidocaine and hence reduce the pain
Explanation:
Alkalinization by addition of sodium bicarbonate causes buffering of local anesthetics and hence produce faster anesthetic effect such as pain control, pain reduction while injecting the patient and faster onset of local anesthetics.
Lidocaine along with epinephrine results into acidic compound wit respect to subcutaneous tissue. Hence, when bicarbonate is added, it neutralizes the acidity of Lidocaine and hence decrease the pain.
Why are the components of the mixture separated ? Give any 6 reasons........
Answers
We need to separate different components of a mixture to separate the useful components from the non-useful or some harmful components.
Examples:
(a) Tea leaves are separated from tea.
(b) Pebbles are separated form rice and pulse.
Explanation:
The mixtures are separated into their components for the following reasons:
1. To remove an undesirable component - Tea is made by boiling tea leaves in water and then adding milk and sugar. After the tea has been made, the used tea leaves become the undesirable component of the mixture 'tea' and are removed from it by using a tea strainer.
2. To remove a harmful component - Foodgrains like rice, wheat, pulses etc. usually contain small pieces of stones and some insects etc. These are harmful to us and are hence removed from the foodgrains before using them.
3. To obtain a pure sample of a substance - Tap water contains some dissolved salts in it so it is an impure mixture. This water is made free of dissolved salts or impurities by the process of distillation and as a result, we get a pure sample of water.
4. To obtain a useful component - Milk is a mixture from which useful component 'butter' is separated.
Given that an IV is mixed so that each 150. mL of the solution contains 500. mg
of the drug lidocaine and the rate of infusion is set at 300. mL/hr. How many minutes will
it take for 0.750 g of lidocaine to be administered?
Answers
Answer:
First, we need to convert 0.750 g of lidocaine into milligrams (mg):
0.750 g = 750 mg
Next, we can calculate the total volume of the solution needed to administer 750 mg of lidocaine. Since each 150 mL of the solution contains 500 mg of lidocaine, we can set up the following proportion:
(750 mg) / (500 mg) = (x mL) / (150 mL)
Cross-multiplying:
500x = 750 * 150
500x = 112,500
x = 112,500 / 500
x = 225 mL
Now, we know that it will take 225 mL of the solution to administer 750 mg of lidocaine.
To find the time it takes to infuse 225 mL at a rate of 300 mL/hr, we can set up another proportion:
(225 mL) / (300 mL/hr) = (y min) / (60 min)
Cross-multiplying:
300y = 225 * 60
300y = 13,500
y = 13,500 / 300
y = 45 min
Therefore, it will take 45 minutes to administer 0.750 g (or 750 mg) of lidocaine at a rate of infusion of 300 mL/hr.
It will take approximately 75 minutes for 0.750 g of lidocaine to be administered through the IV at a rate of 300 mL/hr.
To calculate the time it takes to administer 0.750 g of lidocaine, we need to use the information provided.
Given:
Each 150 mL of the solution contains 500 mg of lidocaine.
The rate of infusion is set at 300 mL/hr.
First, we need to determine the amount of lidocaine administered per mL of the solution:
500 mg of lidocaine per 150 mL of solution = 500 mg / 150 mL ≈ 3.33 mg/mL
Next, we can convert the amount of lidocaine to be administered (0.750 g) to milligrams:
0.750 g * 1000 mg/g = 750 mg
Now, we can calculate the total volume of the solution needed to administer 750 mg of lidocaine:
750 mg / 3.33 mg/mL ≈ 225.23 mL
Finally, we can find the time it takes to administer 225.23 mL of the solution at a rate of 300 mL/hr.:
Time (in hours) = Volume (mL) / Rate (mL/hr.)
Time (in hours) = 225.23 mL / 300 mL/hr ≈ 0.75077 hr.
Converting hours to minutes:
Time (in minutes) ≈ 0.75077 hr * 60 min/hr ≈ 45.05 min
Therefore, it will take approximately 75 minutes for 0.750 g of lidocaine to be administered.
To learn more about lidocaine here
https://brainly.com/question/33427933
#SPJ2
When zinc reacts with copper sulfate solution, zinc sulfate solution and copper are formed.(i) An experiment was carried out to measure the temperature change when zinc powder reactswith copper sulfate solution.initial temperature of copper sulfate solution = 20 °Cfinal temperature of mixture after the reaction = 46 °CExplain what the temperature readings show about the type of heat change that occurs duringthis reaction.
Answers
The temperature increase from 20 °C to 46 °C indicates that the reaction between zinc and copper sulfate solution is exothermic, with heat being released into the surroundings.
In the given reaction between zinc and copper sulfate solution, the temperature change can provide insights into the type of heat change occurring during the reaction. Based on the provided information, the initial temperature of the copper sulfate solution was 20 °C, and the final temperature of the mixture after the reaction was 46 °C.
The temperature increase observed in this reaction indicates an exothermic heat change. An exothermic reaction releases heat energy into the surroundings, resulting in a temperature rise. In this case, the reaction between zinc and copper sulfate solution is exothermic because the final temperature is higher than the initial temperature.
During the reaction, zinc displaces copper from copper sulfate to form zinc sulfate and copper metal. This displacement reaction is known as a single displacement or redox reaction. Zinc is more reactive than copper and therefore replaces copper in the compound.
The formation of new chemical bonds during the reaction releases energy in the form of heat. This energy is transferred to the surroundings, leading to an increase in temperature. The heat released is greater than the heat absorbed, resulting in a net increase in temperature.
The exothermic nature of this reaction can be explained by the difference in bond energies between the reactants and products. The breaking of bonds in the reactants requires energy input, while the formation of new bonds in the products releases energy.
In this case, the energy released during the formation of zinc sulfate and copper metal is greater than the energy required to break the bonds in copper sulfate and zinc.
For more such question on temperature visit:
https://brainly.com/question/4735135
#SPJ8
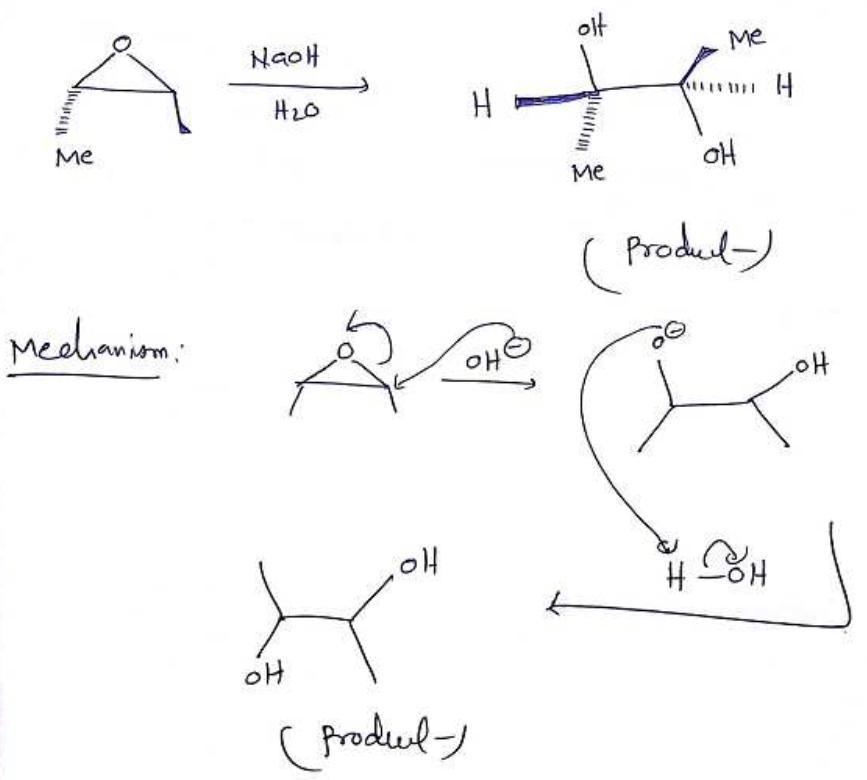
When the following chiral epoxide is treated with aqueous sodium hydroxide, only one product is obtained, and that product is achiral.
Answers
When the chiral epoxide is treated with aqueous sodium hydroxide, only one product is obtained is OH - OH.
A kind of molecule known as a chiral molecule possesses an unsuperposable mirror counterpart. The existence of an asymmetric carbon atom is the characteristic that causes chirality in compounds most frequently. The description of an object that cannot be superposed on its mirror counterpart is referred to as "chiral" in general.
The opposite of chiral is achiral. If a molecule or ion may be superimposed on its mirror counterpart, it is said to be achiral. They either have a symmetry plane or a symmetry centre.
Meso molecules are achiral molecules with a stereocenter.
Learn more about Achiral:
https://brainly.com/question/5838915
#SPJ4
Which of the following is considered to be matter?
O A. Water
O B. Facts
O C. Energy
D. Theories
Answers
bcz the defination of matter is that
anything which occupy space
water can occupy space so it is matterfact :something that you know has happened or is true which doesn't occupy any space so this option is falseenergy : energy is the quantitative property that must be transferred to a body or physical system to perform work on the body, or to heat it. it doesn't occupy any space so this option is falseA theory is a well-substantiated explanation of an aspect of the natural world that can incorporate laws, hypotheses and facts.--it doesn't occupy any space so this option is falseHence , option a is correct
Which type of rock forms when an existing rock changes under extreme pressure and intense heat?
A
igneous
B
sedimentary
C
metamorphic
Answers
Answer:
c
Explanation:
metamorphic rocks
Answer:
C. Metamorphic
Explanation:
What is it?? plzzzz help

Answers
A symmetrical molecule cancels out the effects of polar bonds.
When we combine particles of different
substances together, they sometimes end up
forming particles of a new substance. What is
happening at a particle level that could help
explain how this is possible?
Answers
Answer:
Explanation:
When particles of different substances are combined, they may interact with one another in various ways, including bonding, reactions, and phase transitions. At a particle level, these interactions may involve the transfer or sharing of electrons, the breaking and forming of chemical bonds, or the rearrangement of atoms or molecules. These processes can lead to the formation of new substances with different physical and chemical properties than the original substances. For example, when two atoms of hydrogen combine with one atom of oxygen, they form a molecule of water, which has different properties than the hydrogen and oxygen atoms on their own.
acetyl-coa brings carbon atoms to the citric acid cycle. these carbon atoms are passed through many intermediary steps. for example, there are
Answers
Acetyl-CoA brings carbon atoms to the citric acid cycle. These carbon atoms are passed through many intermediary steps. For example, there are 4 carbon atoms (C) present in oxaloacetate.
About Acetyl-coaA molecule important in metabolism and is useful in many biochemical reactions is called Acetyl-coa . The main function of this molecule is to provide as many carbon atoms which are in the acetyl group to the citric acid cycle to be oxidized to obtain energy, and synthesize a neurotransmitter named acetylcholine which is obtained by a chemical reaction with the help of the enzyme choline acetyltransferase and a by-product in the form of coenzyme A.
Generally, the metabolism of fatty acids will produce acetyl-CoA for the citric acid cycle. In the liver, when the circulation of fatty acids is too high, the production of acetyl CoA from reduction of fat will exceed the energy needed by the cells of the body and will form a ketone group. The high ratio of ketone groups in the blood circulation can cause ketosis or ketoacidosis.
Learn more about acety-coa at https://brainly.com/question/16000193.
#SPJ4
VERY URGENT! IB CHEMISTRY
0.0810 g of a group 2 metal iodide, MI2, was dissolved in water and made up to a total volume of 25.00 cm3.
Excess lead(II) nitrate solution (Pb(NO3)2(aq)) was added to the MI2 solution to form a precipitate of lead(II)
iodide (PbI2). The precipitate was dried and weighed and it was found that 0.1270 g of precipitate was obtained.
a Determine the number of moles of lead iodide formed.
b Write an equation for the reaction that occurs.
c Determine the number of moles of MI2 that reacted.
d Determine the identity of the metal, M.
Answers
The number of moles of lead iodide formed is 0.000275 moles
The equation of the reaction is as follows:
MI₂ (aq) + Pb(NO₃)₂ (aq) ----> M(NO₃)₂ (aq) + PbI₂ (s)
The number of moles of MI₂ that reacted is 0.000275 moles
The group 2 metal is Calcium whose molar mass is 40.0 g
a. From the given values:
Mass of lead iodide precipitated = 0.1270 g
molar mass of lead iodide = 461 g/mol
number of moles = mass / molar mass
number of moles of lead iodide formed = 0.1270 g / 461 g /mol
number of moles of lead iodide formed = 0.000275 moles
b. The equation of the reaction shows the reactants as well as the products formed after reaction.
The general molecular equation is given as :
MI₂ (aq) + Pb(NO₃)₂ (aq) ----> M(NO₃)₂ (aq) + PbI₂ (s)
The net ionic equation is given as:
Pb²⁺ (aq) + 2 I⁻ (aq) ---> PbI₂ (s)
c. 1 mole of MI₂ reacts with 1 mole of Pb(NO₃)₂ to produce 1 mole of M(NO₃)₂ and 1 mole PbI₂
Since 0.000275 moles of PbI₂ was formed, it would require 0.000275 moles MI₂ to be formed.
Number of moles of MI₂ that reacted = 0.000275 moles
d. Mass of 0.000275 moles of MI₂ = 0.0810 g
mass of 1 mole of MI₂ = 0.0810 / 0.000275 =294.5 g
In 294.5 g of MI₂, mass composition of Iodide = 127 * 2 = 254 g
Therefore mass of the metal = mass of compound - mass of iodine in the compound
mass of metal, M = 294.5 - 254 = 40.5 g
The group 2 metal is Calcium whose molar mass is 40.0 g
Learn more at: https://brainly.com/question/18176315\
Learn more at: https://brainly.com/question/25670663
Which statements describe the nature of science? (Select 5)
1. Scientists engage in peer reviews to avoid bias.
2. Science is a blend of logic and innovation.
3. Scientific ideas are not durable and cannot adjust to change as new data is collected.
4. Science is not observational .
5. Science is a complex social endeavor.
6. Natural world is understandable.
7. Scientists try to remain objective.
Answers
Answer:
2
Explanation:
Scientists engage in peer reviews to avoid bias, Science is a blend of logic and innovation and Scientific ideas are not durable and cannot adjust to change as new data is collected. The correct options are 1,2, and 7.
Peer reviews are used by scientists to ensure objectivity and reduce prejudice in their study. To create original ideas and hypotheses, scientists need both logical reasoning and creative thinking.
As scientists frequently interact, exchange ideas, and build on one another's work, it is a complex social endeavour. Understanding and making sense of the natural world is the central tenet of science.
Finally, scientists separate their personal beliefs from empirical evidence in order to stay as objective as possible in their research.
Thus, Scientists engage in peer reviews to avoid bias, Science is a blend of logic and innovation and Scientific ideas are not durable and cannot adjust to change as new data is collected. The correct options are 1,2, and 7.
Learn more about Nature, refer to the link:
https://brainly.com/question/30406208
#SPJ7
Which conditions produce the largest ocean waves?
Question 3 options:
strong winds that blow for a long time over a great distance
weak winds that blow for short periods of time with a short fetch
strong winds that blow for short periods of time with a short fetch
weak winds that blow for long periods of time with a long fetch
Answers
Answer:
Strong winds that blow for a long time over a great distance.
Explanation:
Generally, the biggest and most powerful wind-generated waves are produced by strong storms that blow for a sustained period over a large area. Huge and big waves, or swells, can travel over long distances. The size of the wave depends on wind speed, wind duration, and the area over which the wind is blowing. If The speed of the wind is more, it stays for along time and it covers a larger distance then the waves produced will be very powerful and large.
Allow the system to reach thermal equilibrium (constant temperature). Use the concentration values to determine K. Now go to the thermal properties, change the temperature and click on the thermally isolated system option. Determine the new k at the new temperature. From the new K at the new temperature, determine if the system is endothermic or exothermic. Record your data in the table below: Temperature Observations [Co(H2O).2) [CoCl4) K Endothermic or Exothermic Reaction? : Show all your calculations for K below.
Answers
The reaction is exndthermic as there's the
An endothermic response is any chemical response that absorbs warmness from its environment.
The absorbed power gives the activation power for the response to occur. A hallmark of this form of response is that it feels cold.
The generic cost for water’s regular boiling factor is 373.2 K (100.0 °C), and so this calculation is in affordable agreement. And so, announcing a procedure is spontaneous at “high” or “low” temperatures method the temperature is above or below, respectively, that temperature at which ΔG for the procedure is zero.
As cited earlier, this situation describes a gadget at equilibrium.Combustion procedures are exothermic (ΔH < 0> 0). The response is consequently spontaneous (ΔG < 0>).
Read more about the temperature:
https://brainly.com/question/24746268
#SPJ4
Element widths in responsive designs are often specified in percentages with the size of each element relative to ___ .
Answers
Element widths in responsive designs are often specified in percentages with the size of each element relative to the width of its parent.
Responsive design refers to an approach of web page creation that involves use of flexible layouts, flexible images, and cascading style sheet media queries. The objective of responsive design is to develop web pages that detect the visitor's screen size and orientation and change the layout accordingly. In other words, responsive web design refers to a method that simply reflows, adjust, reposition, resize overall content and images according to width of browser or screen size. Responsive websites are designed to provide accessibility across all devices regardless of size of device screen. Element widths in responsive designs are often represents a percentage value which is often used to define a size as relative to an element's parent object.
Learn more about Responsive design:
https://brainly.com/question/16805626
#SPJ4
2K + MgCh2 -> 2KCI + Mg
Which of the following statements best describes the reaction?
O This reaction is a double replacement reaction because K replaced Mg and a redox reaction because the ion charges of the elements changed from a 0 charge on K to +1 charge, and a +2 charge on Mg to a 0 charge.
• This reaction is a double replacement reaction because K replaced Mg and not a redox reaction because the ion charges of the elements remained the same.
This reaction is a single replacement reaction because K replaced Mg and a redox reaction because the ion charges of the elements changed from a 0 charge on K to +1 charge, and a +2 charge on Mg to a 0 charge.
O This reaction is a single replacement reaction because K replaced Mg and not a redox reaction
because the ion charges of the elements remained the same.
Answers
Answer:
The third option
Explanation:
This reaction is a single replacement reaction because K replaced Mg and a redox reaction because the ion charges of the elements changed from a 0 charge on K to +1 charge, and a +2 charge on Mg to a 0 charge.
How many moles of copper must react to form 0.854 mol Ag?
mol Cu
Answers
As per the given balanced chemical equation, one mole of copper metal reacts to form 2 moles of Ag. Hence, the number of moles of Cu to form 0.854 moles of Ag is 0.427.
What is copper ?Copper is a transition metal in d block of periodic table. Copper forms ionic compounds by losing electrons. As other transition metals copper shows variable oxidation states.
The given reaction is a single displacement reaction between two metals. Cl group exchanges between the metals Cu and Ag. In the given reaction, one mole of Cu reacts with 2 moles of AgCl giving two moles of Ag and one mole of CuCl₂.
The number of moles of Cu required to produce 0.854 mol of Ag is calculated as:
no.of moles of Cu = 0.854 / 2 = 0.427 moles.
Hence, 0.427 moles of Cu is needed.
Find more on copper:
https://brainly.com/question/14913590
#SPJ1
Your question is not complete. But your complete question probably was :
Consider the reaction given below:
\(\rm Cu + 2 AgCl \rightarrow CuCl_{2} + 2 Ag\)
How many moles of copper must react to form 0.854 mol Ag?
mol Cu.
Suppose you buy some inflated party balloons that are at room temperature (about 20°C). What will happen to those
balloons if you take them outside on a cold day? Explain
Answers
Answer: Balloons taken outside on a cold day will shrink because, as the temperature decreases, the pressure and volume decrease also.
Explanation:
How many grams are in 1.48x x 10
Answers
The answer is 14.8 grams, which is option (d).
How many grammes of Sodium chloride are there in 7.8 moles?Therefore keep in mind that we are using molecular masses here. Therefore, 58 0.5 g of sodium chloride and one more will be obtained. Divide the mass of Sodium chloride by its molar mass to determine the number of moles: 0.941 moles are equal to 55 g/58.44 g/mol. 55 g of Sodium chloride is therefore equal to 0.941 moles of Sodium chloride.
How much is 1 gramme?A unit of mass is a gramme. One kilogramme weighs one thousandth of a gramme. The weight of a cube of pure water that is one centimetre in size and four degrees Celsius was the original definition of a gramme. The gramme has the sign g.
To know more about grams visit:-
https://brainly.com/question/30526303
#SPJ1
Question:
How many grams are in 1.48 x10⁷μg?
1.48 x103³
1.48 x10¹³
1.48
14.8
none of the above
Why would a line graph be better to show the relationship between predator and prey birds in an ecosystem than a circle graph?
A. A circle graph would be better to show this relationship.
B.The number of the two classes stays the same over time.
C.The number of the two classes changes over time.
D.None of the above.
Answers
Answer:
C
Explanation:
Line graphs are better to show the relations between over time, as circle shows the amount or present of something in a cert time