write the reaction for the dehydration of 3 – hexanol in the presence of heat and an acid catalyst, h2so4.
Answers
The reaction for dehydration of 3-hexanol in the presence of heat and an acid catalyst, H₂SO₄, can be written as
3-hexanol + H₂SO₄ → 3-hexene + H₂O + H₂SO₄
In this reaction, 3-hexanol (C₆H₁₄O) is dehydrated to form 3-hexene (C₆H₁₂) by the removal of a molecule of water (H2O). The reaction is catalyzed by H₂SO₄, which helps to break the O-H bond in the alcohol group (-OH) of 3-hexanol. The acid protonates the -OH group, making it a better leaving group. This leads to the formation of a carbocation intermediate, which can undergo elimination of a proton (H+) to form the alkene (3-hexene).
The reaction takes place under high temperatures (usually around 160-180°C) to provide the activation energy required for the reaction to occur. The H₂SO₄ catalyst also speeds up the rate of reaction by lowering the activation energy required for the reaction to take place.
In conclusion, the dehydration of 3-hexanol in the presence of heat and H₂SO₄ is a chemical reaction that involves the removal of a molecule of water (H₂O) from the alcohol group of 3-hexanol to form 3-hexene. The acid catalyst, H₂SO₄, plays a crucial role in the reaction by breaking the O-H bond and speeding up the rate of reaction.
To know more about reaction for dehydration, click here
https://brainly.com/question/29644505
#SPJ11
Related Questions
Isotope what dose it mean
Answers
Answer:
each of two or more forms of the same element that contain equal numbers of protons but different numbers of neutrons in their nuclei, and hence differ in relative atomic mass but not in chemical properties; in particular, a radioactive form of an element.
"some elements have only one stable isotope"
Explanation:
The percent composition of carbon in acrylonitrile is
a 5.690%
b 67.89%
С
47.90%
d 22.646%
Answers
Answer:
67.89
Explanation:it’s right
The percent composition of carbon in compound of acrylonitrile is 22.646%.
What is percent composition?Percent composition is defined as a convenient way to record concentration of solution.It is a expression which relates mass of element to mass of compound as,mass of element/mass of compound×100.
Percent composition helps in elemental analysis of compound which is useful in analysis of ores also. It helps in making solutions while performing the titrations. It helps in prediction of chemical formula which can be predicted by mass and molar mass of an element.
The compound acrylonitrile has a molar mass 53.06 g/mole and that of carbon is 12 g/mole thus, percent composition of carbon in compound of acrylonitrile is 12/53.06×100=22.646%.
Thus, the percent composition of carbon in compound of acrylonitrile is 22.646%.
Learn more about percent composition,here:
https://brainly.com/question/17505281
#SPJ6
which group of mamaals has the most mebers
Answers
Infraclass: Placentals
Placental mammals are the most diverse group of mammals with about 4000 discovered species.
Help me with the periodic table and balancing chemical equations, image attacked.

Answers
If 1.0 mole of C8H18 are available to completely react
with enough O2, how many moles of H20 can be produced?
Answers
Answer: 1 mol C8H18 produces 9 mol H2O
Explanation: reaction : C8H18 + 12.5 O2 ⇒ 8 CO2 + 9 H2O
20. Given the formula:
HHH
H-C-C-C-0-H
HHH
The bond between which two atoms has the
greatest degree of polarity?
A. C and C
B. C and O
C. H and C
D. H and O
Answers
Answer:
H and O
Explanation:
H and O because the difference in electronegativity is 1.4 which makes the bond polar.
The bonds that haver the greatest polarity is H and O. Option D
What is a polar bond?We know that a polar bond is the kind of bond that would exists between two compounds that have a high magnitude of difference in electronegativity. Now in this case, we can see that there are different magnitudes of electronegativities of the compound.
Let us recall that hydrogen is a member of group one and oxygen is a member of group six and then we can see that these compounds would not have the same polarity.
Learn more about polar bonds:https://brainly.com/question/10777799
#SPJ1
Requires a medium to travel (travel fastest through solid-like metal train tracks A Waves B Sound Waves C Light Waves D matter
Answers
Answer:
b
Explanation:
becuase of sound waves
The distance between two adjacent peaks on a wave is called the wavelength.
(a) The wavelength of a beam of ultraviolet light is 247 nanometers (nm). What is its wavelength in meters?
(b) The wavelength of a beam of red light is 6760 pm. What is its wavelength in angstroms (A)?
Answers
(a) The wavelength of the ultraviolet light beam is 2.47 × 10⁻⁷ meters.
(b) The wavelength of the red light beam is 676 Å (angstroms).
(a) To convert the wavelength of ultraviolet light from nanometers (nm) to meters, we need to divide the value by 10⁹ since there are 10⁹ nanometers in a meter.
Given that the wavelength is 247 nm, the conversion to meters can be done as follows:
Wavelength in meters = 247 nm / 10⁹
= 2.47 × 10⁻⁷ meters
Therefore, the wavelength of the ultraviolet light beam is 2.47 × 10⁻⁷ meters.
(b) To convert the wavelength of red light from picometers (pm) to angstroms (Å), we need to divide the value by 10 since there are 10 picometers in an angstrom.
Given that the wavelength is 6760 pm, the conversion to angstroms can be done as follows:
Wavelength in angstroms = 6760 pm / 10
= 676 Å
Therefore, the wavelength of the red light beam is 676 Å.
Learn more about Wavelength from the link given below.
https://brainly.com/question/31143857
#SPJ4
Use the drop-down menus to name these
structures.
cis-3-decene
cis-3-nonene
trans-3-decene
trans-3-nonene
Answers
Using drop-down menu , IUPAC name is as follows :
cis-3-decene: (Z)-3-decene , cis-3-nonenetriene: (Z,Z,Z)-3-nonenetriene
trans-3-decene: (E)-3-decene , trans-3-nonene: (E)-3-nonene
cis-3-decene is an alkene with a double bond between carbon atoms 3 and 4, and both alkyl groups (attached to the double bond) on the same side of the double bond. Therefore, its IUPAC name is (Z)-3-decene.
cis-3-nonenetriene is a triene with three double bonds. The double bonds are between carbon atoms 3 and 4, 6 and 7, and 9 and 10. Since all the alkyl groups attached to the double bonds are on the same side of the double bonds, the compound is named as (Z,Z,Z)-3-nonenetriene. trans-3-decene is an alkene with a double bond between carbon atoms 3 and 4, and both alkyl groups (attached to the double bond) on opposite sides of the double bond. Therefore, its IUPAC name is (E)-3-decene. trans-3-nonene is an alkene with a double bond between carbon atoms 3 and 4, and both alkyl groups (attached to the double bond) on opposite sides of the double bond. Therefore, its IUPAC name is (E)-3-nonene.
For more such questions on IUPAC name
https://brainly.com/question/31569541
#SPJ8
pyridine (c5h5n) is a base with a kb of 1.7 x 10–9. what is the ph of 0.10 m pyridine?
Answers
To solve this problem, we need to use the equilibrium expression for the base dissociation reaction of pyridine:
C5H5N + H2O ↔ C5H5NH+ + OH-
where Kb is the base dissociation constant for pyridine, defined as:
Kb = [C5H5NH+][OH-]/[C5H5N]
We can use the Kb value to determine the concentration of hydroxide ions produced when pyridine dissolves in water. We can assume that the initial concentration of pyridine is equal to the final concentration of pyridine, since pyridine is a weak base and only partially dissociates in water.
We can also assume that the concentration of hydroxide ions produced is much smaller than the initial concentration of pyridine, so we can neglect its contribution to the total concentration of the solution.
First, we can calculate the concentration of hydroxide ions produced by pyridine:
Kb = [C5H5NH+][OH-]/[C5H5N]
1.7 x 10^-9 = [x][x]/[0.10-x]
where x is the concentration of hydroxide ions produced by pyridine.
Simplifying the expression, we get:
x^2 / (0.10 - x) = 1.7 x 10^-9
Since x is much smaller than 0.10, we can assume that (0.10 - x) is approximately equal to 0.10:
x^2 / 0.10 = 1.7 x 10^-9
Solving for x, we get:
x = sqrt(1.7 x 10^-9 x 0.10) = 1.2 x 10^-5 M
Therefore, the concentration of hydroxide
To know more about dissociation refer here
https://brainly.com/question/30961097#
#SPJ11
51 kJ heat is transferred to a pistoncylinder system that loses 12 kJ and the piston produces work. Calculate the amount of work in kJ produced by the system.
Answers
In the given scenario, a piston-cylinder system receives 51 kJ of heat and loses 12 kJ. The system produces work, and To calculate work we can use W = Q - ΔU formula
The first law of thermodynamics states that the change in internal energy of a system is equal to the heat added to the system minus the work done by the system. Mathematically, this can be represented as:
ΔU = Q - W
Where ΔU is the change in internal energy, Q is the heat added to the system, and W is the work done by the system.
In this case, the system receives 51 kJ of heat (Q = 51 kJ) and loses 12 kJ (Q = -12 kJ). We need to calculate the work done by the system (W).
Using the first law of thermodynamics equation, we can rearrange it to solve for W:
W = Q - ΔU
Since the change in internal energy (ΔU) is not given, we cannot directly calculate the work done. Additional information about the change in internal energy or any other relevant parameters would be required to determine the amount of work produced by the system.
Learn more about work here: https://brainly.com/question/19382352
#SPJ11
the energy required to change a solid into a liquid is known as the:_____.
Answers
Answer:
Melting
Explanation:
Because the solid is being liquified
Determine the pressure change when a constant volume of gas with an initial pressure of 10 am is cooled from 40 degrees Celsius to 20 degrees Celsius.
Answers
Answer:
To determine the pressure change when a constant volume of gas is cooled from 40°C to 20°C, we can use the ideal gas law, which relates the pressure, volume, temperature, and number of moles of a gas:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
Since we are dealing with a constant volume of gas, we can simplify the ideal gas law to:
P1/T1 = P2/T2
where P1 and T1 are the initial pressure and temperature, and P2 and T2 are the final pressure and temperature.
Converting the temperatures to Kelvin:
T1 = 40°C + 273.15 = 313.15 K
T2 = 20°C + 273.15 = 293.15 K
Substituting the values into the equation:
10 atm / 313.15 K = P2 / 293.15 K
Solving for P2:
P2 = (10 atm / 313.15 K) x 293.15 K
P2 = 9.354 atm
Therefore, the pressure of the gas decreases from 10 atm to 9.354 atm when cooled from 40°C to 20°C at constant volume.
Why is familiarizing and determining the reactants and products of a chemical reaction important?
Answers
Familiarizing and determining the reactant and product of a chemical reaction is important because reactant and product are the two main component of the chemical reaction.
All the chemical reactions involves the reactant and product. chemical reactions are comprised of reactant and product. Reactants are substances that start a chemical reaction. Products are substances that are produced in the reaction. The relationship between reactants and products in a chemical reaction can be represented by a chemical equation that has this general form,
Reactants → Products
The chemical formula allows us to give the scientific name to the substance. The chemical formula allows us to predict the nature and properties of the substance. The chemical formula allows us to write balanced chemical equations.
To learn more about Chemical reaction please visit:
https://brainly.com/question/11231920
#SPJ4
Why are noble gases called "safe" gases?
Answers
Answer:
Noble gases are called "safe" gases because they are inert.
General Formulas and Concepts:
Reading a Periodic TableChemical Properties of Noble GasesExplanation:
Because Noble Gases have filled their outer shell electrons fully, they tend not to form compounds with other elements or react with others to produce another compound.
Take Ne (neon) for instance. It's electron configuration is 1s²2s²2p⁶. The outer most shell is the 2p sublevel, and according quantum numbers, the 2p sublevel only has 3 spaces for 2 electrons each that it can hold. Therefore, Ne has a full outer shell and no electron spots to create a bond with other elements.
Which of the following best describes a vacuum
Answers
Answer:
it sucks up stuff,cleans around the house,and can be emptied
Explanation:
because its what it is spose to do
Answer:
a space entirely devoid of matter.
Explanation:
No links no bots please
which element is most likely to react with Br
Sr
Ar
K
O
Answers
There are two types of chemical compound one is covalent compound and another is ionic compound in chemistry. In ionic bonds, electrons are completely transferred. The correct option is option C.
What is chemical Compound?Chemical Compound is a combination of molecule, Molecule forms by combination of element and element forms by combination of atoms in fixed proportion.
Covalent compounds are formed by covalent bond and ionic compounds are formed by ionic bond. Covalent bond is formed by sharing of electron and ionic bond are formed by complete transfer of electron. Ionic bonds are stronger than covalent bonds. The melting and boiling points are higher in ionic compounds.
Ionic bonds are formed by elements whose electronegativity difference is very large. Since K has high electronegativity among all given elements so K is most likely to react with Br.
Therefore, the correct option is option C.
To learn more about chemical compound, here:
https://brainly.com/question/26487468
#SPJ2
2.F is a solution containing O.122mol/dm3 of HCl.G contains 7.0g of Y0H per dm3.Assuming at the end of titration exercise,28.00cm3 of the acid neutralized 25.00cm3 of the base.Calculate the, i.Concentration of G in mol/dm3 ii.Molar mass of YOH iii.Percentage by mass of Y in YOH The equation for the reaction HCl + YOH YCl + H20
Answers
i) The concentration of G in mol/dm3 is also 0.003416 mol/dm³.
ii) Molar mass of YOH is 204.49 g/mol
iii) The concentration of G is 0.003416 mol/dm³, the molar mass of YOH is 204.49 g/mol, and the percentage by mass of Y in YOH is 91.63%.
To solve this problem, we'll use the given information and the equation for the reaction: HCl + YOH → YCl + H2O
i. Concentration of G in mol/dm³:
From the given information, we know that 28.00 cm³ of the acid (F) neutralizes 25.00 cm³ of the base (G). This means the stoichiometric ratio between HCl and YOH is 1:1. Therefore, the number of moles of HCl neutralized by 28.00 cm³ of F is:
n(HCl) = concentration of F × volume of F in dm³
= 0.122 mol/dm³ × 28.00 cm³ / 1000 cm3/dm³
= 0.003416 mol
Since the stoichiometric ratio is 1:1, the concentration of G in mol/dm³ is also 0.003416 mol/dm3.
ii. Molar mass of YOH:
To calculate the molar mass of YOH, we need to know the mass of YOH used in the reaction. From the given information, we know that 7.0 g of YOH is present in 1 dm3 of G. Therefore, the molar mass of YOH can be calculated as:
Molar mass of YOH = Mass of YOH / Number of moles of YOH
= 7.0 g / 0.003416 mol
= 204.49 g/mol (rounded to two decimal places)
iii. Percentage by mass of Y in YOH:
The molar mass of Y in YOH can be calculated by subtracting the molar mass of OH from the molar mass of YOH:
Molar mass of Y = Molar mass of YOH - Molar mass of OH
= 204.49 g/mol - 17.01 g/mol
= 187.48 g/mol
The percentage by mass of Y in YOH can be calculated as:
Percentage by mass of Y = (Molar mass of Y / Molar mass of YOH) × 100%
= (187.48 g/mol / 204.49 g/mol) × 100%
= 91.63% (rounded to two decimal places)
Therefore, the concentration of G is 0.003416 mol/dm3, the molar mass of YOH is 204.49 g/mol, and the percentage by mass of Y in YOH is 91.63%.
for more questions on concentration
https://brainly.com/question/28564792
#SPJ8
Which of the following indicators is the best choice for this titration? a) Methyl orange (pH range 3.2 – 4.4) b) Methyl red (pH range 4.6 – 6.0) c) Phenolphthalein (pH range 8.2 - 10) d) Bromomethyl blue (pH range 6.1 – 7.6)
Answers
The required correct answer is c) Phenolphthalein (pH range 8.2 - 10).
Explanation : In order to determine which indicator is the best choice for the titration, we need to know the pH range of the equivalence point of the acid and base involved. For example, if the pH range of the equivalence point is 3.2 – 4.4, we would choose an indicator with a pH range close to that.
Each indicator changes color at a specific pH value. Phenolphthalein is the best choice for this titration because its pH range is closest to the equivalence point which is around pH 9.3 for the titration of strong base and weak acid. This is within the pH range of phenolphthalein (8.2 – 10).
In other words, phenolphthalein changes color around the pH where the equivalence point of the titration will occur. Therefore, Phenolphthalein is the best choice for this titration. The correct option is c) Phenolphthalein (pH range 8.2 - 10).
Learn more about titration here https://brainly.in/question/6652836
#SPJ11
how many grams of mgso 4 4 should be used if you desire to make 1.0 grams of mgcl 2 2 ? do not include units in your answer. make sure your answer has at least 3 significant digits
Answers
The mass (in grams) of MgSO₄ That should be used is 1.26 g
How to determine the mass of MgSO₄We'll begin by writing the balanced equation for the reaction. This is given below:
MgSO₄ + 2HCl —> MgCl₂ + H₂SO₄
Molar mass of MgSO₄ = 24 + 32 + (16 × 4) = 120 g/molMass of MgSO₄ from the balanced equation = 1 × 120 = 120 gMolar mass of MgCl₂ = 24 + (35.5 × 2) = 95 g/mol Mass of MgCl₂ from the balanced equation = 1 × 95 = 95 gFinally, we shall determine the mass of MgSO₄ needed for the reaction as follow:
From the balanced equation above,
95 g of MgCl₂ were produced from 120 g of MgSO₄
Therefore,
1 g of MgCl₂ will be produce from = (1 × 120) / 95 = 1.26 g of MgSO₄
Thus, the mass of MgSO₄ needed is 1.26 g
Learn more about stoichiometry:
https://brainly.com/question/2621383
#SPJ1
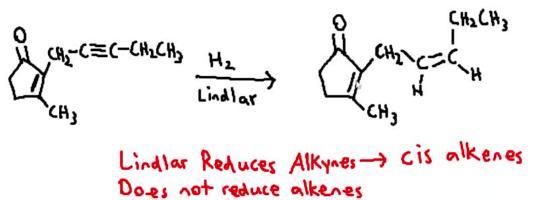
Draw the structure of cis−jasmone, a natural product isolated from jasmine flowers, formed by treatment of alkyne a with h2 in the presence of the lindlar catalyst.
Answers
I can outline how to acquire cis-jasmone: Start with the triple-bonded alkyne A, which has two carbons. In the presence of the Lindlar catalyst, add hydrogen gas (H2).
The jasmone family includes the naturally occurring chemical molecule cis-jasmone. It is an odourless liquid that is present in many plants, especially jasmine blooms, and has a lovely flowery aroma. It is created by hydrogenating cis-3-hexyn-1-ol and then oxidising the resultant alcohol. It has a cis-configuration of the double bond. Cis-jasmone is used in a wide range of products, such as perfumes, soaps, and cosmetics. Both the fragrance and agricultural businesses are interested in this substance because it has potential uses in agriculture as a plant growth regulator and an insect attractant.
Learn more about cis-jasmone here:
https://brainly.com/question/30663933
#SPJ4
Scientists use a star's color to calculate its _____
Answers
Scientists use a star's color to calculate its temperature.
The color of a star is directly related to its temperature, with cooler stars appearing redder and hotter stars appearing bluer. By observing the color of a star, scientists can calculate its temperature and learn more about its properties and behavior.
The color of a star is determined by the blackbody radiation that it emits. The hotter the star, the more energetic the blackbody radiation it emits, which appears more blue. The cooler the star, the less energetic the blackbody radiation it emits, which appears more red.
By measuring the intensity of the radiation emitted at different wavelengths, scientists can determine the temperature of a star.
Learn more about star's color at: https://brainly.com/question/15441904
#SPJ11
An unknown element X forms a compound with chlorine: XCL2. Predict the chemical formula of the compound that element X makes with oxygen. Justify your answer.
Answers
What is the mass of 3.50 mol of Zn atoms? A. 18.7g. B. 229g. C. 0.0535g
Answers
Answer:
B; 229g of Zn
Explanation:
To convert mol of Zn, we must find the atomic mass of Zn. This is 65.38.
Multiply 3.5 mol of zn by 65.38.
3.50 mol of Zn*65.38g = 228.83g of Zn
Therefore, the mass = 229g of Zn
how are compounds Similar to elements and how are they different
Answers
Answer:
A compound contains atoms of different elements chemically combined together in a fixed ratio. An element is a pure chemical substance made of same type of atom. Compounds contain different elements in a fixed ratio arranged in a defined manner through chemical bonds. They contain only one type of molecule.
Step 7: Determine the Limiting Reactant (Trial 2)
50
Analysis: The limiting reactant(s) appeared to be
because ...
Answers
Answer:
aluminum
no aluminum is left over
Explanation:
the next answers for step 7 are 0.019, 0.0093, aluminum
Answer:
Aluminum because no aluminum is left over
Explanation:

An amide that has a molecular ion with an m/z value of 129.
Express your answer as a molecular formula. Enter the elements in the order: C, H, N, O.
Answers
An amide that has a molecular ion with an m/z value of 129.
The molecular formula is C₉H₁₃NO.
To determine the molecular formula of the amide with an m/z value of 129, we need to consider the possible combinations of carbon (C), hydrogen (H), nitrogen (N), and oxygen (O) that would yield that molecular mass.
The m/z value of 129 indicates the mass-to-charge ratio of the molecular ion. Since we're dealing with a neutral molecule, we can assume a charge of +1 for the molecular ion. Therefore, the molecular mass would be equal to 129.
To find the molecular formula, we can consider different combinations of elements that sum up to a molecular mass of 129. Here are a few possibilities:
1. C₈H₁₁NO: In this case, the sum of the atomic masses is (8 × 12.01) + (11 × 1.01) + 14.01 + 16.00 = 128.09, which is close to the desired molecular mass but not exactly 129.
2. C₈H₁₀N₂O: In this case, the sum of the atomic masses is (8 × 12.01) + (10 × 1.01) + (2 × 14.01) + 16.00 = 128.14, which is also close to 129 but not exact.
3. C₉H₁₃NO: In this case, the sum of the atomic masses is (9 × 12.01) + (13 × 1.01) + 14.01 + 16.00 = 129.12, which is very close to 129.
Therefore, the molecular formula that best fits the given m/z value of 129 is C₉H₁₃NO.
To know more about m/z value here
https://brainly.com/question/31482540
#SPJ4
Which is the most likely way an automotive engineer would use chemistry?
A. By testing the maximum speed and stopping distance of a new
car
B. By doing market research to determine which types of cars
consumers want
C. By designing cars that are more aerodynamic
D. By making parts that reduce the pollutants cars emit
Answers
Answer:
by making parts... emit
Barbara completed an assignment for extra credit in science class. She used a pH meter to find the pH of different substances.
After she finished measuring the pHs, she created a diagram that showed the substances arranged in order from the most acidic to the most basic.
If Barbara added lemon juice—pH 2.2—to her investigation, where should it appear in her diagram?
A.
between milk and water
B.
to the left of cola
C.
between soap and bleach
D.
between water and blood

Answers
Answer:
if ur here for the coffee one its between cola and acid rain
Explanation:
ur mom
Which substance has the highest boiling point, CH3OH, CO, N2? CH3OH, because it has hydrogen bonding, dipole-dipole forces, and dispersion forces!
Answers
CH3OH has the highest boiling point of the three substances, with a boiling point of 64.7°C.
What is Hydrogen bonding?
Hydrogen bonding is a type of chemical bond that occurs when a hydrogen atom is covalently bonded to a highly electronegative atom, such as oxygen or nitrogen. This type of bond is stronger than a typical covalent bond and can result in the formation of water molecules, DNA, and proteins. Hydrogen bonds are responsible for many of the properties of water and other molecules, such as their boiling points.
The highest boiling point among the three given substances is CH3OH with a boiling point of 64.7 °C. This is due to the presence of strong intermolecular hydrogen bonding forces between the molecules. The other two substances, CO and N2, do not engage in hydrogen bonding and have much lower boiling points of -191.5 °C and -195.8 °C respectively.
What are dipole-dipole forces?
Dipole-dipole forces are a type of intermolecular force that results from the electrostatic attraction between two molecules that have permanent dipole moments. This type of force occurs when the positive end of one dipole is attracted to the negative end of another dipole. Dipole-dipole forces are relatively weak compared to other intermolecular forces such as ion-dipole forces and hydrogen bonding.
What are dispersion forces?
Dispersion forces, also known as London forces, are weak intermolecular forces that occur when temporary dipoles form in molecules. These forces are usually present in non-polar molecules, and are caused by fluctuations in electron density across the molecule. Dispersion forces are the weakest of the intermolecular forces, but are still important for determining physical properties such as boiling points, vapor pressures, and solubility.
To know more about Dipole-dipole forces,
https://brainly.com/question/9929584
#SPJ4