Answers
Explanation:
Magnesium chloride = MgCl₂
aluminum nitrate= Al(NO₃)₃
ammonium sulfate=( NH₄)₂SO₄
Related Questions
which of the following would result if a covalent bond is formed between the two atoms

Answers
Answer:
\(A\)Explanation:
Here, we want to get what will happen in a covalent relationship between the two atoms
In a covalent bond, the electrons are shared between the two atoms.
In the ordinary covalent type, the two atoms would contribute electrons which is then shared by them.
The set of electrons shared is controlled by the nuclei of both atoms
g Arrange the following compounds in order of acidity (highest to lowest): H2O, H3O , HCl A. CH3COOH > HCl > H2O B. H2O > CH3COOH > HCl C. HCl > H2O > CH3COOH D. HCl > CH3COOH > H2O
Answers
Answer:
Arrange the following compounds in order of acidity (highest to lowest): H2O, CH3COOH , HCl
A. CH3COOH > HCl > H2O
B. H2O > CH3COOH > HCl
C. HCl > H2O > CH3COOH
D. HCl > CH3COOH > H2O
Explanation:
The given substances are acetic acid, hydrochloric acid, and water.
Since HCl is a strong acid and it undergoes complete ionization.
CH3COOH acetic acid is a weak acid and it undergoes partial dissociation in water.
Pure water is a neutral substance.
Hence, the order of acidity is shown below:
HCl > CH3COOH > H2O.
Among the given options, option D is the correct answer.
Sherman suggests that reproduction always creates individuals with adaptive traits. Does this seem correct? Why or why not?
Answers
If Sherman suggests that reproduction always creates individuals with adaptive traits, then he/she is not correct because variation may lead to non-adaptive phenotypes.
What is the presence of genetic variation in individuals of a given population?The presence of genetic variation in individuals of a given population is not adaptive per se, but instead, it provides the raw material for natural selection that leads to the differential survival and reproduction of the most adaptive phenotypes.
The genetic variation in individuals of a given population does not crate adaptive traits and it may derive from sexual reproduction.
Therefore, with this data, we can see that the presence of genetic variation in individuals of a given population may or not be adaptive in the function of the environment in which the individual produced by means of sexual reproduction is developed and therefore it is an advantage for the population but the individual may be harmful.
Learn more about adaptive genetic variation here:
https://brainly.com/question/29313212
#SPJ1
H3PO4 + Ca(OH)2 ----> H2O + Ca3(PO4)2 If 10.3g of Ca(OH)2 reacts , How much water is made
Answers
Taking into account the reaction stoichiometry, if 10.3 g of Ca(OH)₂ reacts, 5.01 grams of H₂O are formed.
Reaction stoichiometryThe balanced reaction is:
2 H₃PO₄ + 3 Ca(OH)₂ → 6 H₂O + Ca₃(PO₄)₂
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
H₃PO₄: 2 molesCa(OH)₂: 3 molesH₂O: 6 molesCa₃(PO₄)₂: 1 moleThe molar mass of the compounds is:
H₃PO₄: 98 g/moleCa(OH)₂: 74 g/moleH₂O: 18 g/moleCa₃(PO₄)₂: 310 g/moleBy reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
H₃PO₄: 2 moles ×98 g/mole= 196 gramsCa(OH)₂: 3 moles ×74 g/mole= 222 gramsH₂O: 6 moles ×18 g/mole= 108 gramsCa₃(PO₄)₂: 1 mole ×310 g/mole= 310 gramsMass of water formedThe following rule of three can be applied: if by reaction stoichiometry 222 grams of Ca(OH)₂ form 108 grams of H₂O, 10.3 grams of Ca(OH)₂ form how much mass of H₂O?
mass of H₂O= (10.3 grams of Ca(OH)₂ ×108 grams of H₂O)÷ 222 grams of Ca(OH)₂
mass of H₂O= 5.01 grams
Finally, 5.01 grams of H₂O are formed.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
#SPJ1
Calculate the vapor pressure at 85.0°C of a solution prepared by dissolving 0.300 mol of liquid dibromoethane (C₂H4Br₂, Pº=127 torr)
in 1.80 mol of liquid dibromopropane (C3H6Br2, P=173 torr).
torr
Answers
The vapor pressure at 85.0°C of a solution prepared by dissolving 0.300 mol of liquid dibromoethane the vapor pressure at 85.0°C of a solution prepared by dissolving 0.300 mol of liquid dibromoethane is 164.83 torr.
What is vapor pressure ?The term vapor pressure is defined as the tendency of a material to change into the vapour state, and it increases with temperature.
For calculating mole fraction of C₂H₄Br₂ as follows
X C₂H₄Br₂ = moles of C₂H₄Br₂ / moles of C₂H₄Br₂ + moles of C₃H₆Br₂
= 0.3 / 0.3 + 1.80
= 0.14
For calculating mole fraction of C₃H₆Br₂ as follows:
XC₃H₆Br₂ = moles of C₃H₆Br₂ / moles of C₂H₄Br₂ + moles of C₃H₆Br₂
= 1.80 / 2.1
= 0.85
For calculating total vapor pressure as follows:
P total = [ ( 0.14 × 127) + (0.85 × 173) ]
= 17.78 + 147.05
= 164.83 torr
Thus, The vapor pressure at 85.0°C of a solution prepared by dissolving 0.300 mol of liquid dibromoethane the vapor pressure at 85.0°C of a solution prepared by dissolving 0.300 mol of liquid dibromoethane is 164.83 torr.
To learn more about the vapor pressure, follow the link;
https://brainly.com/question/11864750
#SPJ1
What is the main product of cellular respiration which cells need for energy?
sunlight
ATP
carbon dioxide
ACP
Answers
Answer:
ATP
Explanation :
Because the energy is released during the process of glycolysis in cellular respiration and then the molecule is captured by the energy carrying molecule which is the ATP (adenosine triphosphate).
WORTH 35 POINTS! MY LIFE DEPENDS ON IT HELP!
When naming ionic compounds, always
1. use prefixes
2. write the metal first
3. write the nonmetal first
4. capitalize the metal and nonmetal
Answers
Answer
2.Write the metal first ( if I am right) as it is ionised
What volume of CO2(g), measured at STP is produced if 15.2 grams of CaCO(s) is heated?
Answers
Answer:
Volume = 3.4 L
Explanation:
In order to calculate the volume of CO₂ produced when 15.2 g of CaCO₃ is heated, we need to first write out the balanced equation of the thermal decomposition of CaCO₃:
CaCO₃ (s) + [Heat] ⇒ CaO (s) + CO₂ (g)
Now, let's calculate the number of moles in 15.2 g CaCO₃:
mole no. = \(\mathrm{\frac{mass}{molar \ mass}}\)
= \(\frac{15.2}{40.1 + 12 + (16 \times 3)}\)
= 0.1518 moles
From the balanced equation above, we can see that the stoichiometric molar ratios of CaCO₃ and CO₂ are equal. Therefore, the number of moles of CO₂ produced is also 0.1518 moles.
Hence, from the formula for the number of moles of a gas, we can calculate the volume of CO₂:
mole no. = \(\mathrm{\frac{Volume \ in \ L}{22.4}}\)
⇒ \(0.1518 = \mathrm{\frac{Volume}{22.4}}\)
⇒ Volume = 0.1518 × 22.4
= 3.4 L
Therefore, if 15.2 g of CaCO₃ is heated, 3.4 L of CO₂ is produced at STP.
Parts of a neuron include:
A. dendrites.
B. hormones.
C. cortexes.
D. collosums.
Answers
The parts of the neuron include (A) dendrites.
What are dendrites?Dendrites are appendages that are designed to receive communications from other cells. They take the shape of projections with a tree-like structure that are triggered by other neurons and carry the electrochemical charge to the cell body. These occupy a large surface area of a neuron.These little appendages convey electrical stimulation to the soma and take in information from neighboring neurons. Synapses are also found on dendrites.Dendrites are also called the "arms" of a neuron.As it is given in the description, Synapses are also found on dendrites.
Therefore, the parts of the neuron include (A) dendrites.
To learn more about dendrites, click on the link:
https://brainly.com/question/11364230
#SPJ13
Consider these equations: 2S(s)+3O2(g)→2SO3(g) , ΔH=−792kJ 2S(s)+2O2(g)→2SO2(g) , ΔH=−594kJ 2SO2(g)+O2(g)→2SO3(g) , ΔH=? What is the missing ΔH ? −294kJ −198kJ +198kJ +294kJ
Answers
Hess law can help us to obtain the enthalpy of a series of reactions by summation. The missing enthalpy of this reaction is −198kJ.
What is Hess law?According to the Hess law of constant heat summation, the enthalpy of a sequence of reactions can be obtained as the sum of the enthalpies of all the reactions.
Now looking at all the reavtion written here in order to obtain the missing enthalpy, we conclude that the missing enthalpy is −198kJ.
Learn more about enthalpy: https://brainly.com/question/3393755
2SO2 (g) + O2 (g)→2SO3 (g), ΔH = −198 kJ.
1. A sample of commercial concentrated hydrochloric acid is 11.8 M HCl and has a density of 1.190 g/mL. Calculate (a). the mass % of HCI (b). the molality of HCI (c). the mole fraction of HCI
Answers
(a) The mass percent of HCl in the solution is approximately 36.1%.
(b) The molality of HCl in the solution is approximately 15.5 mol/kg.
(c) The mole fraction of HCl in the solution is approximately 0.218.
(a) To calculate the mass percent of HCl, we need to determine the mass of HCl in a given volume of the solution.
Given: Concentration of HCl = 11.8 M
Density of the solution = 1.190 g/mL
First, we need to calculate the mass of the solution. Since density is mass per unit volume, the mass of 1 mL of the solution is 1.190 g.
Next, we need to calculate the mass of HCl in 1 mL of the solution. Since the concentration is given in moles per liter (M), and the molar mass of HCl is 36.46 g/mol, we can calculate the mass of HCl in 1 mL as follows:
Mass of HCl = concentration × volume × molar mass
= 11.8 mol/L × 0.001 L × 36.46 g/mol
= 0.430 g
Now, we can calculate the mass percent of HCl using the following formula:
Mass percent = (mass of solute ÷ mass of solution) × 100
= (0.430 g ÷ 1.190 g) × 100
≈ 36.1%
(b) The molality of HCl is calculated by dividing the moles of solute (HCl) by the mass of the solvent (water) in kilograms.
Since the density of the solution is given as 1.190 g/mL, the mass of 1 mL of the solution is 1.190 g. However, we need to consider the density of the solvent (water) to calculate the mass of water in the solution.
Assuming the density of water is 1 g/mL, the mass of water in 1 mL of the solution is (1.190 g - 0.430 g) = 0.760 g.
To calculate the molality of HCl, we need to convert the mass of water to kilograms:
Mass of water (kg) = 0.760 g ÷ 1000 = 0.000760 kg
The molality (m) is calculated using the formula:
Molality = (moles of solute ÷ mass of solvent in kg)
= (11.8 mol/L × 0.001 L) ÷ 0.000760 kg
≈ 15.5 mol/kg
(c) The mole fraction (X) of HCl is calculated by dividing the moles of HCl by the total moles of all components in the solution.
To calculate the mole fraction, we need to consider the volume of the solution and convert it to liters.
Given: Concentration of HCl = 11.8 M
Volume of the solution = 1 mL
Volume of the solution (L) = 1 mL ÷ 1000 = 0.001 L
To calculate the mole fraction of HCl, we need to calculate the moles of HCl and the moles of water (solvent) in the solution.
Moles of HCl = concentration × volume
= 11.8 mol/L × 0.001 L
= 0.0118 mol
Moles of water = mass of water ÷ molar mass of water
= 0.760 g ÷ 18.015 g/mol (molar mass of water)
= 0.0422 mol
Total moles in the solution = moles of HCl + moles of water
= 0.0118 mol + 0.0422 mol
= 0.054 mol
Mole fraction of HCl = moles of HCl ÷ total moles
= 0.0118 mol ÷ 0.054 mol
≈ 0.218
For such more questions on molality
https://brainly.com/question/14366957
#SPJ8
at 25c, a 0.0100m ammonia solution is 4.1% ionized. calculate (a) the concentration of the oh- and nh4+ ions, (b) the concentration of molecular ammonia, (c) the ionization constant of aqueous ammonia, (d) [oh-] after 0.0090mol of nh4cl is added to 1l of the above solution
Answers
A)Concentration of NH4+ = 0.041 * 0.0100 M = 0.00041 M
B)Concentration of molecular NH3 = 0.0100 M - 0.00041 M = 0.00959 M
C)Ka = (0.00041 M)(0.00041 M) / 0.00959 M = 1.76 x 10^-5
D)[OH-] after NH4Cl addition = 0.00041 M - 0.0090 M = -0.00859 M (negative value indicates that OH- is completely consumed)
To calculate the values requested, we can use the given information and the concept of ionization of aqueous ammonia. The ionization reaction is as follows:
NH3 + H2O ⇌ NH4+ + OH-
(a) To determine the concentration of NH4+ and OH- ions:
Given that the ammonia solution is 4.1% ionized, this means that 4.1% of the initial concentration of NH3 has ionized.
Concentration of NH4+ = 0.041 * 0.0100 M = 0.00041 M
Concentration of OH- = 0.00041 M
(b) To find the concentration of molecular ammonia:
Concentration of molecular NH3 = initial concentration - concentration of NH4+
Concentration of molecular NH3 = 0.0100 M - 0.00041 M = 0.00959 M
(c) To calculate the ionization constant (Ka) of aqueous ammonia:
Ka = [NH4+][OH-] / [NH3]
Ka = (0.00041 M)(0.00041 M) / 0.00959 M = 1.76 x 10^-5
(d) When 0.0090 mol of NH4Cl is added to 1 L of the solution, it reacts with OH- to form NH3 and H2O. The NH4Cl dissociates fully, providing 0.0090 mol of NH4+ and Cl- ions.
Since NH4+ reacts with OH-, the concentration of OH- will decrease by 0.0090 M.
[OH-] after NH4Cl addition = 0.00041 M - 0.0090 M = -0.00859 M (negative value indicates that OH- is completely consumed)
Please note that the negative value for [OH-] is not physically meaningful in this context, as the actual concentration cannot be negative. It suggests that all OH- ions have been consumed in the reaction, and the solution is now acidic.
For more question on Concentration
https://brainly.com/question/28564792
#SPJ8
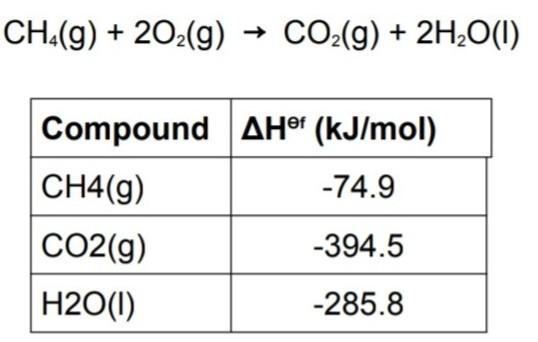
Use the information in table to find standard enthalpy of the combustion of methane and also create a hess enthalpy diagram for reaction.
Answers
The combustion of methane can be represented by the equation CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)We are to find the standard enthalpy of combustion of methane.
We are also to create a Hess enthalpy diagram for the reaction. Standard enthalpy of combustion is the amount of energy released when one mole of a substance is completely burned in excess oxygen at standard temperature and pressure. We can use the data in the table to calculate the standard enthalpy of combustion of methane.The table gives us the standard enthalpies of formation of the reactants and products. The standard enthalpy of formation is the amount of energy change that occurs when one mole of a compound is formed from its constituent elements in their standard states (usually at 25°C and 1 atm).We can use the standard enthalpies of formation to calculate the standard enthalpy of combustion of methane. The standard enthalpy of combustion is given by the following equation:ΔH°c = ΣΔH°f(products) – ΣΔH°f(reactants)We can calculate the standard enthalpy of combustion of methane using the standard enthalpies of formation from the table as follows:ΔH°c = [ΔH°f(CO2(g)) + 2ΔH°f(H2O(g))] – [ΔH°f(CH4(g)) + 2ΔH°f(O2(g))]Substituting the values from the table:ΔH°c = [(–393.5 kJ/mol) + 2(–241.8 kJ/mol)] – [(–74.8 kJ/mol) + 2(0 kJ/mol)]ΔH°c = (–890.2 kJ/mol) – (–74.8 kJ/mol)ΔH°c = –815.4 kJ/mol. Therefore, the standard enthalpy of combustion of methane is –815.4 kJ/mol.We can create a Hess enthalpy diagram for the reaction as shown below: The Hess enthalpy diagram is used to show how the standard enthalpy of the reaction can be calculated using the standard enthalpies of formation of the reactants and products. The diagram shows the two steps involved in the reaction. The first step involves the breaking of the bonds in methane and oxygen to form the reactants. The second step involves the formation of the products from the reactants. The standard enthalpy of combustion is the difference between the standard enthalpies of formation of the products and reactants. The Hess enthalpy diagram shows that the same standard enthalpy of combustion can be obtained regardless of the pathway taken to get from reactants to products.
for such more questions on equation
https://brainly.com/question/26694427
#SPJ8
A factory uses a water tank to cool one of its pieces of machinery. To keep the water cold, fresh water enters the tank at a rate of 4 kilograms per second. Water also flows out of the tank at the same rate. The tank holds 8,000 kilograms of water.
Answers
The residence time for the average water molecule in that water tank is 2,000 seconds.
What is residence time?
The amount of material in the reservoir divided by the inflow or outflow constitutes the residence time, according to definition (they are equal when the reservoir is at equilibrium). If there are several inflows or outflows, we calculate the residence time using the sum of the inflows or outflows.
In one second, 4 kilograms will be added to the tank. In other words, you divide 8,000 kilograms of water by 4, which gets you 2,000.
Therefore, the residence time for the average water molecule in that water tank is 2,000 seconds.
To learn more about residence time from the given link.
https://brainly.com/question/9300464
#SPJ1
Bronsted lowry base that are not considered Arrhenius bases
Answers
Answer: An example is sodium ethoxide (NaOCH2CH3) dissolved in ethanol (CH3CH2OH).
Hope this helps!
A thermally insulated system consists of 1.00 mol of a diatomic gas at 148 K and 2.00 mol of a solid at 178 K that are separated by a rigid insulating wall. Find the equilibrium temperature of the system after the insulating wall is removed, assuming that the gas obeys the ideal-gas law and that the solid obeys the Dulong-Petit law. HINT: the gas does no work during the expansion, so Qgas = AEint = nc', AT. K Submit
Answers
169.2K is the equilibrium temperature of the system after the insulating wall is removed.
What is equilibrium?Generally speaking, a condition of equilibrium is one in which nothing is changing. A body in equilibrium won't undergo any energy exchanges, either positive or negative. Equilibrium is defined significantly differently in biology, physics, and chemistry.
Yet the underlying idea is the same. A body in balance will be least affected by outside influences. Even when external pressures are present, the opposing forces often have a balanced impact on the item under consideration.
for gas, n1=1mol
T1= 148K
for solid,n2=2mol
T2=178K
for conservation of energy, ΔQ= Qgas+ Qsolid=0
Q= CvΔT
0=Cvgas(Teql-148) + Cvsolid(Teq-178)
0= 5/2×1×R(Teql-148) + 3×2×R(Teq-178)
Tequi= 169.2K
Therefore, 169.2K is the equilibrium temperature of the system after the insulating wall is removed.
To learn more about equilibrium, here:
https://brainly.com/question/16989820
#SPJ1
Identify each layer of Earth as a solid or a liquid.
crust
mantle
outer core
inner core

Answers
Two solutions, one with a mass of 450 g and the other with a mass of 350 g, are mixed. A chemical reaction occurs and 125 g of solid crystals are produced that settle on the bottom of the container. What is the mass of the remaining solution?
Answers
475 g is the correct response to the query. This is true because the combined mass of two solutions with masses of 450 g and 350 g equals 800 g. 125 g of solid crystals are created and fall to the bottom of the container as a result of a chemical reaction.
As a result, the mass of the residual solution is equal to 475 g, or 800 g less 125 g. It is significant to observe that the masses of the two solutions that were combined originally do not equal those of the solid crystals or the leftover solution.
This is because the two solutions' chemical reaction when combined results in a transition of the matter that is resulting in the production of a new substance.
Learn more about mass at:
https://brainly.com/question/11954533
#SPJ1
Elisa feels tired because she has a condition that affects whether the right molecules are getting to her cells. If her body were functioning correctly, this is what would happen with oxygen:
Answers
Answer:
It would be working properly and providing her energy for her daily activities.
Explanation:
Hello!
In this case, since her oxygen is actually working properly, it is going to be travelling throughout her entire body (via blood) and therefore carbon dioxide would be released as the exhalation product due to cellular respiration carried out in the alveoli which provides energy for her daily activities.
However, the intrusion of different molecules to oxygen into her cells may affect the rate at which cellular respiration is carried out and therefore she may feel tired because such process provides energy to the body.
Best regards!
Which of the following numbers is in scientific notation?
A~ 13.5 x 10^14
B~ 1.35 x 10^14
C~ 1.35 x 9^14
D~ 13.5 x 9^14
Answers
Identify the compound with the lowest dipole moment. Identify the compound with the lowest dipole moment. CH3CH2CH3 CH3OCH3 CH3CHO CH3OH CH3CN
Answers
Answer:
CH3CH2CH3
Explanation:
Dipole moment is the measure of the polarity of a chemical bond. It is the extent of charge separation in a molecule.
Dipole moment is the product of the magnitude of charge and the distance separating the charges from each other.
The molecule having the lowest dipole moment among the options is the molecule that has the least polarity. The least polar molecule among the options is CH3CH2CH3, it has no polar bonds in its structure.
We have that for the Question "Identify the compound with the lowest dipole moment. Identify the compound with the lowest dipole moment. CH3CH2CH3 CH3OCH3 CH3CHO CH3OH CH3CN "
it can be said that
\(CH_3CH_2CH_3 have the lowest dipole moment\)From the question we are given
CH3CH2CH3
CH3OCH3
CH3CHO
CH3OH
CH3CN
Generally alkanes have the lowest dipole moment, they have C-H bond which are non polar.
Therefore,
\(CH_3CH_2CH_3\) have the lowest dipole moment
For more information on this visit
https://brainly.com/question/17756498
9. Ibuprofen contain which of the following two functional groups: (1 point)
A) benzene
B) halogen
C) carboxyl
D) hydroxyl
Answers
Answer:
A and C
I hope this helps you:)
which of the following choices provides the most probable values for the first four ionization energies of aluminum
Answers
The most probable values for the first four ionization energies of the aluminum are : IE₁ = 578 kJ/mol, IE₂ = 1817 kJ/mol, "IE₃ = 2745 kJ/mol", IE₄ = 11577 kJ/mol.
The ionization energy is the amount of the energy required to remove the electron from an atom. The electronic configuration of Al is given as :
Al electronic configuration = 1s² 2s² 2p⁶ 3s² 3p¹. The fourth ionization energy is comparatively high because the 2p subshell is fully filled and the energy required to remove the electron from the stable electronic configuration will be high. Therefore, the fourth ionization energy is high.
To learn more about ionization energy here
https://brainly.com/question/29629534
#SPJ4
If 6.000 g of sugar is mixed with 9.000 g of water, what is the
concentration in weight percent?
Answers
The concentration in weight percent is 40%
Data obtained from the questionThe following data were obtained from the question:
Mass of sugar = 6.000 gMass of water = 9.000 gTotal mass = 6.000 + 9.000 = 15.000Concentration in weight percent = ?How to determine the concentration in weight percentConcentration in weight percent = (mass of sugar / total mass) × 100
Concentration in weight percent = (6.000 / 15.000) × 100
Concentration in weight percent = 0.4 × 100
Concentration in weight percent = 40%
Learn more about percentage composition:
https://brainly.com/question/23158390
#SPJ1
Describe the trend of the reactivity of the elements in group VII
Answers
The non-metal elements in Group 7 – known as the halogens – get less reactive as you go down the group
Answer & Explanation:
The reactivity of elements in Group VII, also known as Group 17, decreases with increasing atomic radius. This is because halogens have high electronegativities and a proclivity to gain electrons in noble gas configurations. Myths are traditional stories or beliefs that explain cultural or societal beliefs, customs, or natural phenomena. They can be passed down through generations and can be based on true or fictitious events. Mythology, on the other hand, is the collection of myths associated with a specific culture or religion. Mythology can be amplified through retelling, incorporation into religious practices; association with significant events or figures, and adaptation into other media forms such as literature, film, or art.
we notice that co2 changes over the course of the year that passes which is regular but what are most likely caused by?A. burning of fossil fuels B. seasonal trends in tourismC. forest firesD. photosynthesis and respiration
Answers
Answer
D. photosynthesis and respiration
Explanation
Levels of carbon dioxide in the atmosphere rise and fall each year as plants, through photosynthesis and respiration, take up the gas in spring and summer and release it in fall and winter. Now the range of that cycle is expanding as more carbon dioxide is emitted from burning fossil fuels and other human activities.
What is the name of the compound shown here?
СН3
CH,
1
CH3 -CH=CH-
CH-CH-CH=CH,
SH

Answers
Answer:
3, 5–dimethyl–4–heptanethiol.
Explanation:
To name the compound given in question above, we must:
1. Determine the longest continuous carbon chain. This gives the parent name of the compound.
2. Determine the functional group of the compound and give it the lowest possible count.
3. Identify the substituent group attached.
4. Give the substituent the lowest possible count.
5. Combine the above to obtain the name of the compound.
Now, let us name the compound given in question with the above ideas in mind. This can be achieved as shown below:
1. The longest continuous carbon chain is 7 i.e heptane.
2. The functional group is –SH i.e thiol. We shall add thiol to end end of heptane making the parent to be heptanethiol. The functional group is at carbon 4.
3. The substituent attached is CH3 i.e methly.
4. There are two CH3 attached to compound and they are at carbon 3 and 5 respectively.
5. The name of the compound is:
3, 5–dimethyl–4–heptanethiol.
Someone help me out pls

Answers
The specific heat capacity and molar heat capacity of the metal cannot be determined due to missing values.
However, the values can be obtained using the formula below:
ΔH₁ + ΔHcalorimeter + ΔH₂ = 0What is the specific heat capacity and molar heat capacity of the metal?The specific heat capacity and molar heat capacity of the metal are calculated using the formula below:
ΔH₁ + ΔHcalorimeter + ΔH₂ = 0
where:
ΔH₁ is the heat change of the water = m₁Cwater(Tfinal - T1)
ΔHcalorimeter is heat change of the = Ccalorimeter(Tfinal - T1)
ΔH₂ is the heat change of the metal = m₂c₂(Tfinal - T2)
ΔH₁ = 99.45 * 4.184 * (23.9 - 19.5)
ΔH₁ = 1830.83 J
ΔHcalorimeter = Ccalorimeter * 23.9 - 19.5
The heat capacity of the calorimeter is not given, therefor the specific heat capacity and molar heat capacity of the metal cannot be determined.
Learn more about specific heat capacity and molar heat capacity at: https://brainly.com/question/15080895
#SPJ1
At standard temperature and pressure. 0.500 mole of xenon gas occupies
Answers
Answer:
0.500 mole of Xe (g) occupies 11.2 L at STP.
General Formulas and Concepts:
Gas Laws
STP (Standard Conditions for Temperature and Pressure) = 22.4 L per mole at 1 atm, 273 KStoichiometry
Mole ratioDimensional AnalysisExplanation:
Step 1: Define
Identify.
0.500 mole Xe (g)
Step 2: Convert
[DA] Set up: \(\displaystyle 0.500 \ \text{mole Xe} \bigg( \frac{22.4 \ \text{L Xe}}{1 \ \text{mole Xe}} \bigg)\)[DA] Evaluate: \(\displaystyle 0.500 \ \text{mole Xe} \bigg( \frac{22.4 \ \text{L Xe}}{1 \ \text{mole Xe}} \bigg) = 11.2 \ \text{L Xe}\)Topic: AP Chemistry
Unit: Stoichiometry
What happens when two cars converge