Write the balanced equation for the ionization of the weak base pyridine, c5h5n , in water, h2o.
Answers
The equation for the ionization of the weak base pyridine in water is:
C5H5N + H2O ⇌ C5H5NH+ + OH-
Explanation:
Pyridine is a weak base, so it will react with water to create hydroxide (OH-) ions, along with the conjugate acid of pyridine, which is called pyridinium ion (C5H5NH+). The overall equation for the reaction can be written as:C5H5N + H2O ⇌ C5H5NH+ + OH-In this equation, the arrows indicate that the reaction is reversible, meaning that the products can also react to form the reactants. Therefore, the concentration of each species in the reaction mixture will be related by an equilibrium constant (K).
The balanced equation for the ionization of the weak base pyridine in water is C5H5N + H2O ⇌ C5H5NH+ + OH-.
To know more about ionization visit:
brainly.com/question/1602374
#SPJ11
Related Questions
Part C
Bends in rivers are called meanders. Meanders in rivers tend to bend more sharply over time due to uneven erosion on the shoreline at the locations where the river bends. Use your model to investigate this phenomenon. Is there more erosion on the inside or the outside of the curve in the riverbed as water flows through it? Draw an image of your river, and label the location along each curve where the most erosion seems to occur.
Answers
Based on the principles of fluid mechanics and erosion, the water in the river flows faster on the outside of the bend and slower on the inside.
What is the about?Meandering rivers are formed due to the lateral erosion of the riverbank, which creates a curvy path for the water to flow. This is caused by the differences in water velocity between the outside and inside bends of the river.
The above difference in velocity causes the water on the outside to exert more erosive force on the bank, leading to more erosion on the outside of the curve. The slower-moving water on the inside, by contrast, tends to deposit sediment, which can result in the formation of sandbars and other features.
Therefore, it is likely that there is more erosion on the outside of the curve in the riverbed as water flows through it.
Read more about erosion here:
https://brainly.com/question/17905503
#SPJ1
Sixty-three grams of copper reacted with 32 grams of sulfur. this chemical reaction produced the compound copper sulfide. what was the mass of this compound?
Answers
The mass of, \(Cu_2S\), compound formed is 77.9g
62 grams of copper reacted with 32grams of sulfur to form copper sulfide.
\(Cu + S_2 \rightarrow CuS_2\)
stoichiometry of Cu to S is 2:1
We need to find the limiting reactant
Molar mass of copper = 63.5 g/mol
Molar mass of Sulfur = 32 g/mol
Number of moles of Copper = \(\dfrac{mass} {molar mass } = 0.99mole\)
Number of moles of Sulfur = \(\dfrac{mass} {molar mass} = \dfrac{32g} {32g/mol} = 1 mole\)
Since copper have lesser number of moles, therefore the limiting reagent is copper so the amount of product formed depends on amount of Cu present
stoichiometry of Copper to \(Cu_2S\) is 2:1
0.99 mol of Copper forms = \(\dfrac{0.99} {2}\) = 0.49 mol of \(Cu_2S\)
Mass of \(Cu_2S\) produced = Number of moles \(\times\) Molar mass
Mass of \(Cu_2S\) produced = 0.49 mol \(\times\) 159 g/mol = 77.9g
Learn more about limiting reagent here-
https://brainly.com/question/9913056
#SPJ4
The electron configuration of an element is 1s22s22p¹. How many valence electrons does the element have
a) 1
b) 2
c)3
d)4
Answers
Answer:
\(\huge\boxed{\sf 3 \ electrons}\)
Explanation:
Electronic configuration:\(1s^22s^22p^1\)
The outermost/valence shell is the second one, seeing from the electronic configuration.
Valence electrons:= 2 + 1 = 3 (electrons are raised to the power in the electronic configuration)
Since, the valence shell here is 2, the electrons of second shell will be counted as valence electrons.
\(\rule[225]{225}{2}\)
what does Le châteliers principle state?
Answers
Hope this helps!
On a hot summer day you and your friend decided to make a drink by mixing ice cubes water and sugar and koolaid mix . What was the type of change that occurred
Answers
Answer:
thats when you mix so that's friction
Explanation:
1 lemonade+1 lemonade=2 lemonaede
and i hope the lemonade tast good! :D
The type of change that has occurred on mixing ice cubes, water ,sugar, koolaid mix is a physical change.
What is a physical change?Physical changes are defined as those changes which affect only the form of a substance but not it's chemical composition. They are used to separate mixtures in to chemical components but cannot be used to separate compounds to further simpler compounds.
Physical changes are always reversible by using physical means and involve a change in the physical properties.Examples of physical changes include melting,boiling , change in texture, size,color,volume and density.Magnetism, crystallization, formation of alloys are all reversible changes and hence physical changes.
They involve only rearrangement of atoms and are often characterized to be changes which are reversible.
Learn more about physical change,here:
https://brainly.com/question/17931044
#SPJ6
Graphing data can help you to recognize
Answers
Answer:
Graphing data can help you to recognize changes in what your doing.
Explanation:
Answer:
Explanation:
To identify trends, make predictions, and recognize anomalous data. Helps you understand what your data means or a picture of your graph. A graph wich the points form a straight line.
why does aluminium form at negative electrode during electrolysis
Answers
Answer:
The negative electrodes (cathodes ) and the positive electrodes (anodes ) are made of graphite, a form of carbon. During electrolysis: positively charged aluminium ions gain electrons from the cathode, and form molten aluminium. oxide ions lose electrons at the anode, and form oxygen molecules.
can there be 4 electrons in the first energy level
Answers
where are the reproductive parts of plants located?leaves,flower,roots, stem
Answers
The reproductive part of plants is located in flowers.
The flowers are the reproductive part of a plant.
Stamens are the male reproductive part.
The pistil is the female reproductive part.
Process of reproduction in a plant:
Pollen is transferred from one flower to another by insects or the wind. Pollination is the name given to this procedure.When pollen enters a fresh flower, it moves to the ovary where it fuses the ovules, the egg cells that become seeds, to produce seeds.Animals or the wind can disperse the seeds.To know more about reproduction in a plant refer to:
https://brainly.com/question/24437919
Air is cooling at night. The frost point (temperature at which RH with respect to ice reaches 100%) is reached at T = -10 degree Celsius. a) What is the RH (normal RH with respect to liquid water) at this point? b) Upon further cooling the air reaches a temperature of T =-11 degree Celsius Kaolinite particles of 200 nm diameter are present. Do you expect ice particles to form? If yes, do they form via deposition nucleation or condensation of droplets followed by freezing? Briefly explain your answer. c) Upon even further cooling the air reaches a temperature of T = -12 degree Celsius. Same question as before: Do you expect ice particles to form now? If yes, do they form via deposition nucleation or condensation of droplets followed by freezing? Briefly explain your answer. Equilibrium vapor pressures may be calculated or taken from the table below. t/°C 0 -1 -2 -3 -4 -5 -6 -7 -8 -9 - 10 -11 -12 -13 T/ Keow /Pa 273.15 611.2 272.15 568.2 271.15 527.9 270.15 490.2 269.15 454.8 268.15 421.8 267.15 390.9 266.15 362.1 265.15 335.1 264.15 310.0 263.15 286.5 262.15 264.7 261.15 244.3 260.15 225.4 259.15 207.8 258.15 191.4 e oi/Pa 611.2 562.7 517.7 476.1 437.5 401.8 368.7 338.2 310.0 283.9 259.9 237.7 217.3 198.5 181.2 165.3 - 14 - 15 Equilibrium vapor pressures with respect to water (eow) and with respect to ice (coi).
Answers
The equilibrium vapor pressure with respect to water (eow) is 259.9 Pa. assume that saturation vapor pressure is same as equilibrium vapor pressure.
Therefore, the RH at the frost point is
RH = (eow / saturation vapor pressure) × 100
= (259.9 Pa / 259.9 Pa) × 100
= 100%
b) At T = -11 °C, we need to compare the equilibrium vapor pressure with respect to water (eow) and the equilibrium vapor pressure with respect to ice (coi) to determine if ice particles will form. From the given table, at T = -11 °C, the equilibrium vapor pressure with respect to water (eow) is 237.7 Pa, and the equilibrium vapor pressure with respect to ice (coi) is 165.3 Pa.
The air is supersaturated with respect to ice, and the presence of Kaolinite particles can provide surfaces for water droplets to condense onto, leading to the formation of ice particles.
c) At T = -12 °C, we compare the equilibrium vapor pressure with respect to water (eow) and the equilibrium vapor pressure with respect to ice (coi). From the given table, at T = -12 °C, the equilibrium vapor pressure with respect to water (eow) is 217.3 Pa, and the equilibrium vapor pressure with respect to ice (coi) is 181.2 Pa.
Learn more about equilibrium vapor here
https://brainly.com/question/15629887
#SPJ11
Why do chemical equations need to be balanced?
Group of answer choices
to ensure that the reactants have more atoms than the products
Because otherwise the reaction won't work
because matter, such as atoms, cannot be created or destroyed
The reaction is unstable otherwise
Answers
what kind of chemical bond is formed by transfer of electrons between alkali metals and halogens?
Answers
The transfer of electrons between alkali metals and halogens results in the formation of a strong ionic bond characterized by the attraction between positively charged alkali metal cations and negatively charged halogen anions.
The transfer of electrons between alkali metals and halogens leads to the formation of an ionic bond. An ionic bond is a type of chemical bond that occurs when there is a complete transfer of one or more electrons from an atom of one element (in this case, the alkali metal) to an atom of another element (the halogen).
Alkali metals, such as sodium (Na) and potassium (K), have one valence electron in their outermost shell, while halogens, such as chlorine (Cl) and iodine (I), require one electron to complete their outermost shell. In the process of bond formation, the alkali metal readily donates its valence electron(s) to the halogen, resulting in the formation of positively charged ions known as cations (e.g., Na+ and K+), and negatively charged ions known as anions (e.g., Cl- and I-).
The resulting electrostatic attraction between the oppositely charged ions leads to the formation of a strong ionic bond. This bond is characterized by the attraction between the positively charged alkali metal cations and the negatively charged halogen anions. Ionic bonds are typically strong and have high melting and boiling points, as well as good solubility in polar solvents.
To learn more about electrons
https://brainly.com/question/12001116
#SPJ4
Explain how soil contributes to biodiversity, fighting disease and agriculture.
Answers
Answer:
In order to promote biodiversity, fight disease, and advance agriculture, soil is essential in the following ways:
Biodiversity: A variety of microbes, insects, and plants that make up intricate food webs live in soil. The diversity of the soil fosters the growth of plants, which in turn provide habitat and food for other organisms, and contributes to the stability of the ecosystem.
Fighting disease: Soil microorganisms compete with pathogenic organisms for resources and space and also produce antimicrobial chemicals, which helps to avoid plant diseases. Additionally, minimising tillage and increasing organic matter are two soil management techniques that can help lower the prevalence of plant diseases.
Agriculture: Soil supports crops physically, supplies them with vital nutrients, and controls the flow of air and water. Crop yields and quality can both rise with healthy soil and a balanced soil microbiota. Furthermore, sustainable agriculture can be supported by soil management techniques like cover crops and decreased tillage that preserve soil resources and maintain soil health.
The soil is home to a wide range of bacteria, insects, or plants that support complex food webs.
What is soil?The bioactive, porous media that has grown in the top layer of the Earth's crust is known as soil. Being a source of water as well as nutrients, a filter for harmful wastes, a site for their breakdown, and a collaborator in the cycle of carbon as well as other substances through the planet's ecosystem, soil constitutes one of the main substrates of life on Earth.
In the following way soil contributes to biodiversity, fighting disease and agriculture. Biodiversity: The soil is home to a wide range of bacteria, insects, or plants that support complex food webs. Disease prevention: Soil microorganisms create antimicrobial compounds and compete against pathogenic organisms for nutrients and space, which helps prevent plant diseases. Agriculture: Soil provides crops with physical support, essential nutrients, and control over air and water flow.
Therefore, the soil is home to a wide range of bacteria, insects, or plants that support complex food webs.
To know more about soil, here:
https://brainly.com/question/27588666
#SPJ2
What do all volcanos emit?
Answers
Answer:
By far the most abundant volcanic gas is water vapor, which is harmless. However, significant amounts of carbon dioxide, sulfur dioxide, hydrogen sulfide and hydrogen halides can also be emitted from volcanoes.
In the image, which reactant is limiting?
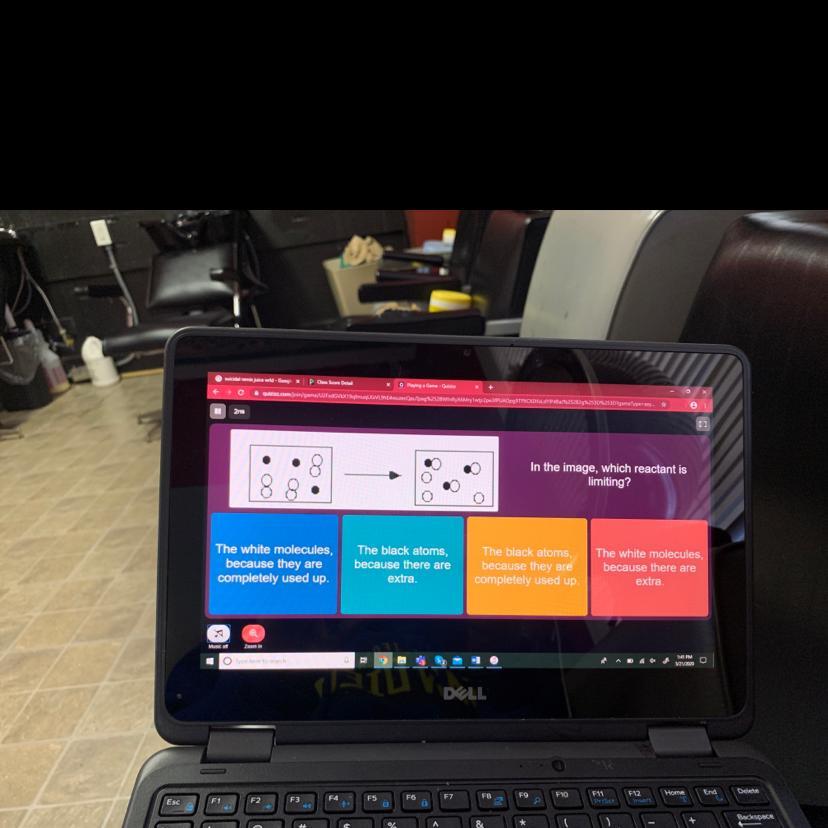
Answers
Answer:
The black atoms
Explanation:
Please Help
All matter has physical and chemical properties. These properties can be used to identify the type of matter. Which of these statements describes a chemical property?
Select one:
a. A metal is added to a beaker of water, a gas is formed, and the beaker explodes.
b. A certain heavy metal turns to a liquid at room temperature.
c. A 2-feet-long metal bar has a mass of only 176 g.
d. A particular substance evaporates at 30 °C.
Answers
A chemist divides a large sample of a mixture into three smaller portions. each of these portions contains different ratios of the component substances that make up the mixture. which description best fits the mixture? a. compound b. element c. heterogeneous mixture d. homogeneous mixture e. pure substance
Answers
A heterogeneous mixture is not uniform in composition. Hence, option C is correct.
What is a heterogeneous mixture?A heterogeneous mixture is a mixture in which the composition is not uniform throughout the mixture.
This substance is not an element or compound, because different components could be observed in the substance.
A pure substance is made up of only one type of atom (element) or only one type of molecule (compound), mixtures and solutions are made from two or more types of pure substances.
For example, aluminium is an element and ammonia is a compound.
This substance is not a homogeneous mixture, because different samples of the substance appeared to have different proportions of the components.
Hence, this substance is a heterogeneous mixture.
Learn more about the heterogeneous mixture here:
https://brainly.com/question/11315552
#SPJ1
Answer:
heterogeneous mixture
Explanation:
why does the hydrogen bonded to an sp hybridizied orbital of terminal alkyne, appear at a lower delta value than a hydrogen bonded to an sp2 carbon of an alkene
Answers
The hydrogen bonded to an sp hybridized orbital of a terminal alkyne appears at a lower delta value than a hydrogen bonded to sp2 carbon of alkene because the sp orbital has more s-character than the sp2 orbital.
In valence bond theory, hybridization is a mixing of atomic orbitals to form new orbitals with different shapes and energies. The sp hybrid orbital has 50% s-character and 50% p-character, while the sp2 hybrid orbital has 33% s-character and 66% p-character. This means that the sp orbital has more s-character and therefore more electron density than the sp2 orbital. The greater electron density in the sp orbital makes the hydrogen atom more acidic, and therefore the C-H bond more polar. This increased polarity leads to a lower delta value for the hydrogen bonded to an sp hybridized orbital of a terminal alkyne.
To learn more about valence bond theory here brainly.com/question/29075903
#SPJ11
Below is a statement of assertion followed by a statement of reason. Choose the correct answer out of the following choices. Assertion: Sodium alginate is insoluble in water Reason: Ionic salts are soluble in water Both assertion and reason are correct statements, but reason is not the correct explanation of the assertion. Both assertion and reason are wrong statements. Assertion is correct, but reason is wrong statement. Both assertion and reason are correct statements, and reason is the correct explanation of the assertion Assertion is wrong but reason is correct statement
Answers
Assertion is correct, but reason is wrong statement(d) is a statement of assertion followed by a statement of reason.
The assertion that sodium alginate is insoluble in water is correct. However, the reason given that ionic salts are soluble in water is not a correct explanation.
While most ionic salts are indeed soluble in water, there are exceptions such as insoluble ionic salts that do not dissolve in water. Sodium alginate is one such exception. It is a salt of alginic acid, which is a polysaccharide, and the sodium ions are not easily dissociated in water. Hence, sodium alginate is insoluble in water.
For more questions like Assertion click the link below:
https://brainly.com/question/30443467
#SPJ11
what is the atomic number of this atom???

Answers
The atomic number of this atom is 3.
Atomic Number =Number of Protons .
Here protons=3, Neutrons=4 and Electrons=3. So, The atomic number of this atom is 3.
What is Atomic Number?The charge number of an atomic nucleus is the chemical element's atomic number, also known as nuclear charge number (symbol Z). This is equivalent to the proton number (np), or the number of protons present in the nucleus of each atom of that element, for conventional nuclei.
Ordinary chemical elements can be uniquely identified by their atomic number. The atomic number and the number of electrons are both equal in a regular, uncharged atom.
The atomic mass number A of a regular atom is calculated by adding its neutron number N and neutron number Z. Since the mass of electrons is negligible for many uses, protons and neutrons have roughly equal masses, thus the mass defect of a nucleon.
To learn more about Atomic Number refer to:
brainly.com/question/1805828
#SPJ1
if the volume of the system is reduced to one half of its original volume and the system is allowed to restablish equilibrium at 298k, what will be the pressure in atm, of the water vapor at the new volume
Answers
According to ideal gas law ;
PV = nRT where, P refers to the pressure of the ideal gas, V is the volume of the ideal gas, n is the total amount of ideal gas that is measured in terms of moles, R is the universal gas constant, and T is the temperature.
If Volume of system is reduced to half = V/2
then; P = 2(nRT/V), Pressure will be doubled to reestablish the equilibrium.
What is Chemical equilibrium?
Chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and products occurs. A reversible chemical reaction is one in which the products, as soon as they are formed, react to produce the original reactants.
Learn more about Equilibrium here ; https://brainly.com/question/4289021?referrer=searchResults
#SPJ4
lead can react with oxygen gas. If lead (IV) oxide is the product of the reaction, how would the reaction be classified
Answers
Answer:
this reaction is an oxidation reaction
The chemical reactions involving both oxidation and reduction are known as redox reactions. The oxidation involves the loss of electrons. The reaction of lead with oxygen is an example of oxidation reaction.
What is oxidation reaction?According to the classical concept, oxidation is defined as a process which involves the addition of oxygen or electronegative element or the removal of hydrogen or electropositive element.
During electron transfer, the species which loses electrons is said to be oxidized and the species which gains electrons is said to be reduced.
The lead (IV) oxide is the formula of PbO₂. Here the oxidation state of lead is +4. The lead (IV) oxide is used in manufacturing dyes and also used to make explosives. It is a corrosive outcome that can evolve in lead pipes that are used for drinking water.
The formation of lead (IV) oxide is given as:
Pb (s) + O₂ (g) → PbO₂ (g)
Thus the reaction is an oxidation reaction.
To know more about oxidation reaction, visit;
https://brainly.com/question/29218241
#SPJ2
what is the answer to a blank cell has pairs of chromosomes
Answers
Each cell has 23 pairs of chromosomes.
What are chromosomes?The nucleus of each cell contains the chromosome, and it can be defined as packaged into thread-like structures. Each chromosome is structurally composed of DNA that is tightly coiled around special proteins. The chromosomes are typically not visible under the microscope.
All living organisms possess chromosomes. The human body has 23 pairs of chromosomes. Out of the 23 pairs of chromosomes, 22 pairs of these chromosomes are known as autosomes.
These are identical in both males and females while the 23rd pair of chromosomes are known as allosomes. This pair differs between the sexes. Females have two copies of the “X” chromosome while males have one “X” and “Y” chromosome.
Learn more about chromosomes, here:
https://brainly.com/question/1596925
#SPJ1
A gas mixture has three components: N2, F2, and He. Their partial pressures are 0.50 atm, 0.16 atm,
and 0.18 atm, respectively. What is the pressure of the gas mixture?
Enter your answer in the provided box.
atm
Answers
Answer:
What????????????????????????????
Explanation:
???
Many common substances may be classified as acids or bases. Which of
the following statements best describes the materials shown?*

Answers
Answer:
Bleach is more basic while human blood is slightly basic
Explanation:
The higher the number on the pH scale, the more basic. Therefore, since bleach's number is higher than the blood, it is more basic.
2. CCC Patterns Use the figure to compare the melting points of the metals in Groups 1
and 2. Describe the general pattern in the relationship between a metal's position in
these two groups and its melting point.
Answers
In Groups 1 and 2 of the periodic table, the melting points of metals generally decrease as you move down the group. This trend is known as a general pattern in the relationship between a metal's position in these groups and its melting point.
Group 1 consists of alkali metals (Li, Na, K, etc.), and Group 2 consists of alkaline earth metals (Be, Mg, Ca, etc.). As we move down these groups, the number of electron shells increases, and the atomic radius of the metals also increases. This increase in atomic radius leads to weaker metallic bonding between the atoms.
The melting point of a metal is influenced by the strength of the metallic bonds. Metallic bonding occurs when metal atoms share their outer electrons freely, forming a "sea" of delocalized electrons. These delocalized electrons are responsible for the high electrical conductivity and malleability of metals. The stronger the metallic bonding, the higher the melting point of the metal.
As we move down Groups 1 and 2, the increased atomic radius results in a greater distance between the metal ions in the crystal lattice. This increased distance weakens the metallic bonding, making it easier to break the bonds and convert the solid metal into a liquid state. Therefore, metals lower in Groups 1 and 2 have lower melting points compared to metals higher up in the groups.
Additionally, the increased number of electron shells also leads to greater shielding of the outer electrons from the positive charge of the nucleus. This reduced attraction between the outer electrons and the nucleus further contributes to the weaker metallic bonding and lower melting points as we move down the groups.
In summary, the general pattern in the relationship between a metal's position in Groups 1 and 2 and its melting point is that the melting points decrease as we move down the groups due to the increasing atomic radius, weaker metallic bonding, and reduced attraction between the outer electrons and the nucleus.
for more questions on periodic table
https://brainly.com/question/15255548
#SPJ8
Define ionization energy ?
Answers
Answer:
to remove an electron
Explanation:
the amount of energy required to remove an electron from an isolated atom
Ionization energy, also known as ionization energy, is the minimal amount of energy needed to free the electron with the loosest bond from an isolated gaseous atom, positive ion, or molecule in physics and chemistry.
What is ionization energy?The energy necessary to release an electron from a gaseous atom or ion is known as the ionization energy (ie), or more precisely, the ionization enthalpy. Since the ion's charge has increased, each consecutive ionization energy has been higher than the one before it.An element's capacity to engage in chemical processes requiring the creation of ions or the donation of electrons is measured by the ionization energy. In general, it also has to do with how the chemical bonds between the components in the compounds they produce are constructed. Likewise, see electron affinity and binding energy.For more information on ionization energy kindly visit to
https://brainly.com/question/28385102
#SPJ4
What do you notice when you get into a car that has been sitting in the sun for a while?
Answers
When you get into a car that has been sitting in the sun for a while, there are several noticeable things that may occur. Here are some of the common observations:
1. Heat: One of the first things you'll notice is the intense heat inside the car. This is because the sun's rays have been absorbed by the car's exterior and trapped inside, creating a greenhouse effect. The temperature inside the car can become significantly higher than the temperature outside.
2. Hot Surfaces: The surfaces inside the car, such as the seats, dashboard, steering wheel, and metal parts, can become extremely hot to the touch. This is due to the absorption of heat from the sun. It's important to be cautious and avoid direct contact with these hot surfaces to prevent burns or discomfort.
3. Odor: The interior of the car may have a distinct smell when it has been sitting in the sun for a while. This is often referred to as the "hot car smell." It is caused by the combination of materials, such as upholstery, plastic, and carpet, heating up and emitting a specific odor.
4. Fading or Discoloration: Prolonged exposure to sunlight can cause fading or discoloration of materials inside the car. For example, the upholstery, dashboard, and other surfaces may gradually lose their original color and become faded or discolored over time.
5. Glare: When you first enter a car that has been sitting in the sun, you may notice a strong glare from the sunlight reflecting off the windshield and other glass surfaces. This glare can make it difficult to see clearly and may require the use of sunglasses or adjusting the sun visors to minimize the brightness.
It's important to note that these observations may vary depending on factors such as the intensity of the sunlight, the duration the car has been in the sun, and the materials used in the car's interior. Regular maintenance and taking precautions, such as using sunshades or parking in shaded areas, can help minimize some of these effects.
to know more about the greenhouse effect here:
brainly.com/question/31595505
#SPJ11
Plzz giving brainlist to first answer!Plss fill in the blanks!!ASAP.Modeling Our Solar System
The planets follow these ____________ paths as they travel around the Sun. The Sun’s ______________
makes the planets move in this way. It pulls on the planets, keeping them in motion in their ____________.
Modeling is a way to ________________ or _____________ a particular idea or
concept.
Models do have some ______________________.
Often a model is made with certain _______________________.
A model of the solar system may contain all of the planets and the Sun, but it may not include the planets’
individual moons or every __________ and ________________ in the solar system.
need to be updated regularly with new information
Example: Older models of the solar system may show ________ planets, which we now know is inaccurate.
can draw by hand or even use computer software
Creating a Model
Question 1
Part A
The table shows the diameters of the planets in our solar system.
1. Find the scale (ratio) between Jupiter and the basketball.
2. Then use this ratio to find the scaled diameter of the other planets.
3. Enter these numbers into the table.
4. Finally, choose a real-world spherical or nearly spherical object that matches the scaled diameter of eac
Answers
Answer:
The planets follow these orbit paths as they travel around the Sun. The Sun’s gravity
makes the planets move in this way. It pulls on the planets, keeping them in motion in their orbit.
Modeling is a way to see ideas or have a particular idea or
concept.
Models do have some lines .
Often a model is made with certain things.
A model of the solar system may contain all of the planets and the Sun, but it may not include the planets’
individual moons or every planit and rock in the solar system.
Explanation: if you think about it and and look at pics it is easy
hope this helps
Analog and digital waves both need to be amplified after traveling a certain distance. Digital waves are amplified by a device known as a digital repeater. Analog waves are amplified by a device known as an amplifier.
A digital repeater works by reconstructing a new square wave wave from the incoming wave, even if the incoming wave includes
noise. An analog amplifier simply amplifies all of the information it receives, including noise. The diagram below illustrates how the resulting waves might appear.
The example above suggests that?
А. there is no clear advantage to transmitting digital waves over analog waves.
B. digital waves cannot have noise or other distortions removed from them.
C. noise is less of a problem for digital waves than for analog waves.
D. analog waves do not experience as much noise as digital waves.

Answers
Answer:
C. noise is less of a problem for digital waves than for analog waves.
Explanation:
Answer: C
Explanation: Study Island