Answers
Answer:
\(Pb(ClO_{3})_{4}\\Pb(MnO_{4})_{4}\\Fe(ClO_{3})_{3}\\\Fe(MnO_{4})_{3}\\\)
Explanation:
\(Pb^{4+}(ClO_{3}^{-})_{4}--->Pb(ClO_{3})_{4}\\Pb^{4+}(MnO_{4}^{-})_{4}--->Pb(MnO_{4})_{4}\\Fe^{3+}(ClO_{3}^{-})_{3}--->Fe(ClO_{3})_{3}\\\Fe^{3+}(MnO_{4}^{-})_{3}--->Fe(MnO_{4})_{3}\\\)
Related Questions
Is there other life in the univers
Answers
Answer:
nobody knows
Explanation:
maybe you'll be the one to find out
Answer:
yes. yes there is.
Explanation:
QUESTION 57 Select the diamagnetic ion.
A. Ni2+ B. Cu2+ C. Sr2+ D. Cr3+
Answers
Answer:
c
Explanation:
Describe a Mesosaurus.
Answers
Mesosaurus lived in freshwater lakes and ponds. Elongated and slim, it measured about 3.3 feet long. The skull and tail were both long and narrow, and the animal probably undulated through the water as it fed on small crustaceans and other prey with its jaws, which were full of long, thin, pointed teeth.
What is the amino group in a protein?
Answers
An amino group is a functional group consisting of an amino (-NH2) and a carboxyl (-COOH) group, which is found in proteins.
It is essential for the formation of peptide bonds between amino acids, which are the building blocks of proteins. The amino group is partially positively charged and is therefore able to interact with other molecules through hydrogen bonding and electrostatic interactions.
The amino group is also able to donate a hydrogen atom, making it an important component in many biochemical reactions.
Furthermore, it plays an important role in the folding of proteins, as the electrostatic interactions between the amino and carboxyl groups help stabilize the protein's three-dimensional structure. In addition, the presence of the amino group can influence the solubility of proteins in different solutions.
To know more about proteins, click below:
https://brainly.com/question/10058019
#SPJ4
what properties of a natural resource make it useful for humans as a materials or energy source?
Answers
The properties of a natural resource that make it useful for humans as a material or energy source is the ability to convert mass into energy and vice versa.
What are natural resources?The expression natural resources make reference to all types of matter and energy extracted from nature that can be used to produce goods and services.
Some examples of natural resources include for example irreversible resources such as fossil fuels (i.e., oil, or coal, gas, minerals such as metals, rocks, etc) as well as those based on the use of reversible energy such as eolic air energy, solar radiation or sunlight, soil and hydric resources or water.
Therefore, with this data, we can see that natural resources can be defined as any material and or energy obtained from nature that may be irreversible or reversibly used to produce goods and services.
Learn more about natural resources here:
https://brainly.com/question/24514288
#SPJ1
What else is produced when sodium carbonate decomposes?
Na2CO3 → Na2O + _________
Answers
Answer:
CO2
Explanation:
The vapor pressure of liquid X is lower than that of liquid Y at 20oC, but higher at 60oC. What can you deduce about the relative magnitude of the molar heats of vaporization of X and Y?
Hint: ln(P1/P2)= Hvap/R (1/T2-1/T1)
Answers
Vapor pressure of X is lower than Y at 20°C, therefore heat of vaporization pf X is greater than Y.
What is vapour pressure ?Vapour pressure is a measure of a material's tendency to change into a gaseous or vapour state, and it rises with temperature.
The boiling point of a liquid is the temperature at which the vapour pressure at its surface equals the pressure exerted by its surroundings.
Heat of vaporization ∝ 1 ÷ Vapour pressure
But at 60°C vapour pressure of X is greater than Y.
Thus, at this temperature heat of vaporization pf Y is greater than X
To learn more about the vapor pressure, follow the link;
https://brainly.com/question/11864750
#SPJ1
Which interactions and processes contribute to the dissolution of ionic compounds in water?
Answers
Explain which direction the molecules will move during diffusion.
Answers
Answer: During diffusion, molecules flow down their concentration gradient.
Explanation:
They're flowing from an area of high concentration to an area of low concentration. Molecules flowing down a concentration gradient is a natural process and does not require energy.
HELP I DON'T HAVE LONG LEFT AND I'M STRUGGLING SO BAD PLEASE I BEG U TO HELP

Answers
Formaldehyde has a wide range of uses, many of them in manufacturing. Its chemical formula is CH₂O. The model below represents formaldehyde.
Based on the model, which statement best describes formaldehyde molecules?
A. Formaldehyde molecules form an extended structure and cannot freely move past each other.
B. Formaldehyde molecules do not form an extended structure and can freely move past each other.
C. Formaldehyde molecules do not form a repeating pattern and cannot freely move past each other.
D. Formaldehyde molecules form a repeating pattern and can freely move past each other.

Answers
Answer: B
Explanation: Formaldehyde molecules do not form an extended structure and can freely move past each other
I NEED HELP ASAP!!!!
ITS DUE IN A FEW MINUTES!!!!

Answers
Answer:
The Sun and the planets were born from a cloud of gas and dust called the solar nebula 4.6 billion years ago. The collapse of the solar nebula was most likely triggered by a shock wave from a nearby supernova explosion. The Sun formed in the center, with the planets surrounding it in a thin disk.
Explanation:
You have a basketball at a temperature of 298K, and a pressure of 2.3 atm. You leave it outside on a cold day, and the temperature of the gas drops to 273K. What is the pressure in the basketball assuming the volume does not change?
Answers
Answer:
The pressure in the basketball is approximately 0.00045329 atm after experiencing the temperature drop.
Explanation:
Assuming that the volume of the basketball does not change, we can use the ideal gas law to calculate the pressure in the basketball:
PV = nRT
Where P is the pressure, V is the volume, n is the amount of substance (in moles), R is the gas constant, and T is the temperature.
To find the pressure, we will first need to rearrange the formula to solve for P:
P = nRT / V
Plugging in the given values, we have:
P = nRT / V
P = (m * 1.6605393 × 10^(-24) moles) * (0.082057 mol/L-atm) * (273.15 K) / (0.225 L)
P = 0.00045329 atm
So the pressure in the basketball is approximately 0.00045329 atm after experiencing the temperature drop.
someone help me please i need to finish this
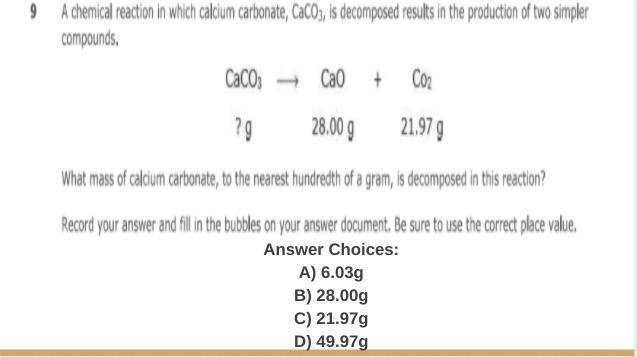
Answers
the answer is D hope it helps :)
What is the chemical formula for micas
Answers
Answer:
X2Y4–6Z8O20 (OH, F)4
Explanation:
The chemical formula for micas is X2Y4–6Z8O20 (OH, F)4, where X is K, Na, or Ca or less commonly Ba, Rb, or Cs; Y is Al, Mg, or Fe or less commonly Mn, Cr, Ti, Li, etc.; Z is chiefly Si or Al, but also may include Fe3+ or Ti1. Structurally, micas can be classed as dioctahedral (Y = 4) and trioctahedral (Y = 6)1.
The chemical formula for micas varies, but they typically have the general formula:
(K,Na,Ba,Rb,Ca)(Al,Mg,Fe)2(Si3Al)O10(OH,F)2
Where:
K, Na, Ba, Rb, and Ca represent alkali metals and alkaline earth metals that can occupy the interlayer sites. Potassium is the most common.Al and Mg represent aluminum and magnesium that occupy the octahedral sites between the silica tetrahedral sheets.Fe can substitute for Al in the octahedral sites.Si and Al occupy the tetrahedral sites within the silica sheets. The ratio of Si to Al is typically around 3:1.O represents oxygen atomsOH or F can occupy the interlayer sites, with hydroxyl (OH) being more common. Fluorine can substitute for hydroxyl in some micas.So in summary, micas have a layered aluminosilicate structure with interlayer cations that can vary, but they are generally characterized by an approximate 3:1 ratio of silicon to aluminum within the silica tetrahedral sheets. The chemical formula given is the generalized structural formula for micas, but the actual compositions can vary based on the specific mica.
Will mark brainliest
Calculate the specific heat of a substance given that 512 joules of heat is required to raise the temperature of 255.0 g of the substance by 15.0 ºC.
Answers
Answer:
\(c=0.133\ J/g^{\circ}C\)
Explanation:
Given that,
Heat required, Q = 512 J
Mass of the substance, m = 255 g
The change in temperature, \(\Delta T=15^{\circ} C\)
Let c be the specific heat of the substance. We know that the heat required to raise the temperature is given by :
\(Q=mc\Delta T\)
Where
c is the specific heat of a substance
So,
\(c=\dfrac{Q}{m\Delta T}\\\\=\dfrac{512}{255\times 15}\\\\c=0.133\ J/g^{\circ}C\)
So, the specific heat of the substance is equal to \(0.133\ J/g^{\circ}C\).
Explain why crude oil is a fossil fuel
Answers
Answer:
fossil fuels are exhaustible, available in limited quantity, takes a long time to replenish & is found under the earth.
Since crude oil satisfies all these conditions, it is a fossil fuel.
Draw the structure of phosphatidylserine and discuss its components
Answers
Phosphatidylserine is a type of phospholipid that is mainly found in cell membranes. Its structure is made up of two fatty acid chains, a phosphate group, a serine molecule, and a glycerol molecule.
The fatty acid chains are hydrophobic, meaning they repel water, while the phosphate group and serine molecule are hydrophilic, meaning they attract water.
The glycerol molecule acts as a bridge that connects the two fatty acid chains to the phosphate group and serine molecule.
The structure of phosphatidylserine is important for its function in the cell membrane.
Because of the hydrophobic and hydrophilic components of its structure, phosphatidylserine is able to form a lipid bilayer, which is a barrier that separates the inside of the cell from the outside environment.
The hydrophilic heads of the phosphatidylserine molecules face outward and interact with water, while the hydrophobic tails face inward and repel water.
Phosphatidylserine also plays a role in cell signaling and apoptosis, which is programmed cell death.
It acts as a signaling molecule by binding to proteins that are involved in cellular pathways.
In addition, phosphatidylserine is translocated to the outer leaflet of the cell membrane during apoptosis, which signals to immune cells that the cell is ready to be removed.
In conclusion, the structure of phosphatidylserine is made up of two fatty acid chains, a phosphate group, a serine molecule, and a glycerol molecule. Its hydrophobic and hydrophilic components allow it to form a lipid bilayer in cell membranes, and it also plays a role in cell signaling and apoptosis.
For more such questions on Phosphatidylserine
https://brainly.com/question/16179573
#SPJ8
10 grams of CaO (Mr = 56.08) were needed for the reaction of a 2,5 L solution containing hydrochloric acid (Mr = 36.46). How many grams of CaCl2 (Mr = 110.98) can be prepared? What was the concentration of the hydrochloric acid?
CaO + HCl = CaCl2 + H¬2O
Answers
10 grams of CaO were needed for the reaction of 2.5 L solution containing hydrochloric acid. grams of CaCl₂ can be prepared is 19.75 g The concentration of HCl is 0.142 M.
The balanced equation is given as :
CaO + 2HCl ------> CaCl₂ + H₂O
no. of moles of CaO = mass / molar mass
= 10 g / 56.08 g/mol
= 0.178 moles
1 mole of CaO produce 1 mole of CaCl₂
0.178 moles produced 0.178 moles of CaCl₂
mass of CaCl₂ = number of moles × molar mass
= 0.178 mol × 110.98 g/mol
= 19.75 g
1 mole of CaO react with 2 moles of HCL
0.178 moles react with 2 × 0.178 = 0.356 moles of HCl
Molarity = number of moles / volume in L
= 0.356 moles / 2.5 L
= 0.142 M
Thus, 10 grams of CaO were needed for the reaction of 2.5 L solution containing hydrochloric acid. grams of CaCl₂ can be prepared is 19.75 g The concentration of HCl is 0.142 M.
To learn more about concentration here
https://brainly.com/question/16112491
#SPJ1
What is the pH of a solution of 0.300 mole of acetic acid (CH3CO₂H) (Ka = 1.8 × 10-5) and 1.50
moles of sodium acetate (NaCH3CO₂) dissolved in 1.00 L of water?
Answers
The pH of the solution is 2.44.
we first need to write the chemical equation for the dissociation of acetic acid in water:
\(CH_3CO_2H + H_2O - > CH_3CO_2^- + H_3O^+\)
We can use the acid dissociation constant (Ka) for acetic acid to determine the concentration of hydronium ions (H3O+) in the solution, and then use the pH equation to calculate the pH:
Ka = [CH₃CO₂⁻][H₃O⁺] / [CH₃CO₂H]
We know the concentrations of acetic acid and sodium acetate, so we can calculate the concentration of acetate ions ([CH₃CO₂⁻]) using the stoichiometry of the reaction:
[CH₃CO₂⁻] = [NaCH₃CO₂] = 1.50 moles / 1.00 L = 1.50 M
We can assume that all of the acetic acids dissociate into acetate ions and hydronium ions, so the initial concentration of acetic acid is equal to the change in concentration of acetate ions:
[CH₃CO₂H] = 0.300 moles / 1.00 L = 0.300 M
[CH₃CO₂⁻] = [H3O+] = x
Substituting these values into the Ka expression, we get:
1.8 x 10⁻⁵ = (1.50 M)(x) / (0.300 M)
Solving for x, we get:
x = 0.0036 M
Now, we can use the pH equation to calculate the pH:
pH = -log[H₃O+]
pH = -log(0.0036)
pH = 2.44
Therefore, the pH of the solution is 2.44.
learn more about pH here
https://brainly.com/question/172153
#SPJ9
Exercise is an effective stress reliever.True or False
Answers
Answer:
yes it can
Explanation:
What do the symbols tell you about theconditions of the reaction shown to the right
Answers
Answer:
the arrow
Explanation:
this show reaction give product which always appear on right side
determine the rate law and the value of k for the following reaction using the data provided. 2NO(g) + O2(g) -----> 2NO2
Answers
The rate law and the value of k for the given reaction is 1.7×103 M⁻²s⁻¹. Therefore, option D is correct.
What is rate law ?The word "rate law" refers to an expression that expresses reaction rate as the product of the stoichiometric coefficient of the reacting species in a balanced chemical equation multiplied by the molar concentration of the reactants, with each term raised to a power.
\(\rm Rate = k \times [NO]^{n} x [O_{2}]^ {m}\)
Thus, m must = 1
\(\rm Rate\ 1 = k \times [NO 1]^ {m} x [O_{2} 1]^ {n}\\Rate\ 2 = k \time [NO 2]^ {m} x [O_2 2]^ {n}\)
Rearranging this equation
\(Rate1 / [O_2 1]^{n} = k x [NO1]^ {m}Rate2 / [O_2 2]^{n} = k x [NO2]\)
but [NO 1] = [NO 2]
Hence,
\(Rate1 / [O_2 1]^ {n}= Rate2 / [O_2 2]^{n}\)
Rearranging and substituting in the values
\(([O_2 2] / [O_2 1])^{n} = Rate2 / Rate 1\)
\(2^{n} = 2\)
So, n = 1
Same from run 1 to 3
[NO] doubled
[O₂] stayed constant
Rate quadrupled
\((2)^{n} = 4\)
n = 2
we know that rate = k x [NO]² x [O₂]
Substitute in any value for [NO], [O₂] and rate and calculate K
k = rate / [NO]² x [O₂]
= (8.55x10⁻³ M / sec) / ((0.030M)² x (0.0055M))
= 1.7×103 M⁻²s⁻¹
Thus, option D is correct.
To learn more about the rate law follow the link;
https://brainly.com/question/30379408
#SPJ9
Your question is incomplete, most probably complete question is
Determine the rate law and the value of k for the following reaction using the data provided.
2 NO(g) + O2(g) 2 NO2(g)
[NO]i (M) [O2]i (M) Initial Rate (M-1s-1)
0.030 0.0055 8.55 x 10-3
0.030 0.0110 1.71 x 10-2
0.060 0.0055 3.42 x 10-2
A. Rate = 57 M-1s-1[NO][O2]
B. Rate = 3.8 M-1/2s-1[NO][O2]1/2
C. Rate = 3.1×105 M-3s-1[NO]2[O2]2
D. Rate = 1.7×103 M-2s–1[NO]2[O2]
How many grams of Aluminum Sulfate are produced when 4 g of Aluminum Nitrate react with 3 g of Sodium Sulfate?
Al(NO3)3 + Na2SO4 ---------> Al2(SO4)3 + NaNO3
Answers
3.21 grams of Aluminum Sulfate are got when 4 g of Aluminum Nitrate reacts chemcially with 3 g of Sodium Sulfate.
WHat is the balanced equation for this reaction? How many grams of Aluminum Sulfate are produced?The equation given is not balanced. Thus, when balanced the equation becomes:
2 Al(NO₃)₃ + 3 Na₂SO₄ → Al₂(SO₄)₃ + 6 NaNO₃
The molar mass of Al(NO₃)₃ is:
Al(NO₃)₃ = 1(Al) + 3(N) + 9(O) = 213 g/mol
The molar mass of Na₂SO₄ is:
Na₂SO₄ = 2(Na) + 1(S) + 4(O) = 142 g/mol
From the balanced equation, we can see that 2 moles of Al(NO₃)₃ react with 3 moles of Na2SO4 to produce 1 mole of Al₂(SO₄)₃. Therefore, we can calculate the number of moles of Al(NO₃)₃ and Na₂SO₄ that react:
Number of moles of Al(NO₃)₃ = 4 g / 213 g/mol = 0.0188 mol
Number of moles of Na₂SO₄ = 3 g / 142 g/mol = 0.0211 mol
From the balanced equation, we can see that 2 moles of Al(NO₃)₃ produce 1 mole of Al₂(SO₄)₃. Therefore, the number of moles of Al₂(SO₄)₃ produced is:
Number of moles of Al₂(SO₄)₃ = 0.0188 mol / 2 * 1 = 0.0094 mol
The molar mass of Aluminum Sulfate (Al₂(SO₄)₃) is:
Al₂(SO₄)₃ = 2(Al) + 3(S) + 12(O) = 342 g/mol
Therefore, the mass of Aluminum Sulfate produced is:
Mass of Al₂(SO₄)₃ = Number of moles of Al₂(SO₄)₃ * Molar mass of Al₂(SO₄)₃
= 0.0094 mol * 342 g/mol
= 3.21 g
Hence, 3.21 grams of Aluminum Sulfate are liberated when 4 g of Aluminum Nitrate change state with 3 g of Sodium Sulfate.
Learn more about balanced chemical equation here:
https://brainly.com/question/28294176
#SPJ1
Cells are the basic unit of structure and function in living
things. True or false
Answers
Answer:
This statement is true
1) Consider the dissolution of CaCO3 compound in aqueous medium.
a) Write down the equation of the chemical reaction that represents this dissolution.
b) Write the expression of the equilibrium constant for this reaction.
c) Explain how the addition of a certain amount of sodium carbonate to
water would affect this balance
2) The equilibrium constant for the reaction 2 SO2(g) + O2(g) 2 SO3(g) has a Kc value = 2.5x10^10 to 500 K. Find the kc value for each of the following reactions at the same temperature
(a) SO2(g) +1/2 O2 SO3(g).
(b) SO3(g) SO2(g)+1/2 O2(g)
(c) 3SO3(g)+ 3/2 O2(g) 3SO3(g)
3) A reaction mixture consisting of 0.400 mol H 2 and 1.60 mol I 2 was prepared in a 3.00 L flask and heated. In balance, 60.0% of the hydrogen gas reacted. What is the equilibrium constant for the reaction H 2(g) + I 2(g) 2 HI(g) at this temperature?
Answers
Explanation:
1a) CaCO₃(s) → Ca²⁺(aq) + CO₃²⁻(aq)
1b) Remember, solids are not included in the equilibrium equation.
K = [Ca²⁺] [CO₃²⁻]
1c) Adding CO₃²⁻ ions will shift the reaction to the left, producing CaCO₃(s) until equilibrium is restored.
2) 2 SO₂(g) + O₂(g) → 2 SO₃(g)
Kc = 2.5×10¹⁰ = [SO₃]² / ([SO₂]² [O₂])
2a) SO₂(g) + ½ O₂(g) → SO₃(g)
Kc = [SO₃] / ([SO₂] [O₂]^½)
Kc² = [SO₃]² / ([SO₂]² [O₂])
Kc² = 2.5×10¹⁰
Kc ≈ 1.58×10⁵
2b) SO₃(g) → SO₂(g) + ½ O₂(g)
Kc = [SO₂] [O₂]^½ / [SO₃]
Kc = 1 / (1.58×10⁵)
Kc ≈ 6.33×10⁻⁶
2c) 3 SO₂(g) + ³/₂ O₂(g) → 3 SO₃(g)
Kc = [SO₃]³ / ([SO₂]³ [O₂]^³/₂)
Kc = ([SO₃] / ([SO₂] [O₂]^½))³
Kc = (1.58×10⁵)³
Kc ≈ 3.95×10¹⁵
3) H₂(g) + I₂(g) → 2 HI(g)
K = [HI]² / ([H₂] [I₂])
Make an ICE table.
\(\left[\begin{array}{cccc}&Initial&Change&Equilibrium\\H_{2}&0.400&-0.240&0.160\\I_{2}&1.60&-0.240&1.360\\HI&0&+0.480&0.480\end{array}\right]\)
K = (0.480)² / (0.160 × 1.360)
K = 1.06
A gas has a volume of 100 mL and a pressure of 6 atm. What volume will the gas occupy when the pressure is reduced to 1 atm (total)?
Answers
We are given:
V1 = 100 mL P1 = 6 atm
V2 = x mL P2 = 1 atm
Solving for 'x' :
According to the Boyle's law:
P ∝ 1/V (pressure and volume are inversely proportional)
PV = k (where k is a constant)
since the constant k will be the same:
P1V1 = P2V2
replacing the variables
6 * 100 = 1 * x
x = 600 mL OR 0.6L
Therefore, the gas will have a volume of 600mL or 0.6L
Hydrolysis of the compound B5H9 forms boric acid, H3BO3. Fusion of boric acid with sodium oxide forms a borate salt, Na2B4O7. Part A Without writing complete equations, find the mass (in grams) of B5H9 required to form 148 g of the borate salt by this reaction sequence.
Answers
Answer:
37.09 g of B₅H₉ is required to form 148 g of borate salt( Na₂B₄O₇ )
Explanation:
Given the data in the question;
B₅H₉ → H₃BO₃ → Na₂B₄O₇
find the mass (in grams) of B5H9 required to form 148 g of the borate salt by this reaction sequence.
S M.W
B₅H₉ 63
H₃BO₃ 62
Na₂B₄O₇ 201
So, moles of Na₂B₄O₇ = 148 / 201 = 0.736
Since Boron will be conserved,
Moles of Boron atoms in Na₂B₄O₇ will be;
⇒ 4 × 0.736 = 2.944
Now, Moles of Boron in Na₂B₄O₇ = Moles of Boron in H₃BO₃ = Moles of Boron in B₅H₉
Hence,
Moles of Boron in B₅H₉ = 2.944
Moles of B₅H₉ = 2.944 / 5 = 0.5888
Mass of B₅H₉ = 0.5888 × 63 = 37.09 g
Therefore, 37.09 g of B₅H₉ is required to form 148 g of borate salt( Na₂B₄O₇ )
a 1 or 2 letter symbol that stands for the name of an element
Answers
Answer:
the answer is chemical element because when a symbol consists of two letters the first letter is always capitalized
What is the term used to describe the scientific study of how living things are classified?
Answers
Answer:
Taxonomy
Explanation:
The term used to describe the scientific study of how living things are classified is known as taxonomy.
Taxonomy describes includes the nomenclature and classification of organisms based on several relationships between organisms.
Carl Linnaeus introduced the basis of modern classification system and this has been refined through the years.
Different relationships between organisms are used to classify them.
