Answers
The formula for Octane is given by :
\(C_8H_{18}\)STEP 1 : Write down the structural formula of Octane• The condensed formula can be determined by grouping :
• CH3 -CH2 -CH2-CH2-CH2-CH2 -CH2 -CH3
This can be expressed as :CH3-(CH2)6-CH3 .... condensed formula of Octane (C8H18)

Related Questions
What will happen if you weighed out a quantity of beans
Answers
Answer:
When we weigh beans in this mass ratio, we must obtain the same number of beans.Explanation:
Nothing will happen
2. Assume
60.0 mL
of a
2.5M
potassium chromate solution is mixed with
40.0 mL
of a
3.2M
solution of iron (III) chloride. a) Will a reaction occur and if so, what reaction will occur? b) How much precipitate will be produced in grams? c) What is the concentration of each spectator ion in the final solution? What is the concentration of left-over ions in the solution? (Calculate the final concentration of each ion).
Previous qu
Answers
The displacement reaction will occur. The concentration of each spectator ion in the final solution is 3/2 moles of Fe2(CrO4)3 will be formed and Concentration of CrO4^2- will be 0.033 M
Step 1:
The balanced chemical equation for the reaction is given below:
K2CrO4 + FeCl3 -> Fe2(CrO4)3 + 2KCl
Hence, the reaction occurs between potassium chromate and iron (III) chloride.
Step 2:
We need to find out how much precipitate will be produced in grams.
Let's calculate the moles of reactants and then use mole ratio to find out the limiting reagent:
\(\[\text{Moles of potassium chromate} = \text{Molarity} \times \text{Volume} \div 1000\][Molarity of K2CrO4 = 2.5 M; Volume of K2CrO4 = 60.0 mL]\)
Moles of K2CrO4 = (2.5 x 60.0) / 1000 = 0.150 mol
\(\[\text{Moles of iron (III) chloride} = \text{Molarity} \times \text{Volume} \div 1000\][Molarity of FeCl3 = 3.2 M\) = 3.2 M;
Volume of FeCl3 = 40.0 mL]Moles of FeCl3 = (3.2 x 40.0) / 1000 = 0.128 mol
As we see, K2CrO4 is the limiting reagent. So, FeCl3 is in excess.
Therefore, amount of Fe2(CrO4)3 precipitated is given by moles of K2CrO4 and mole ratio:
\(\[\text{Moles of Fe2(CrO4)3} = \text{Moles of K2CrO4} = 0.150 mol\]\)
Now, we will find the molecular weight of Fe2(CrO4)3 as 479.87 g/mol.
\(\[\text{Mass of Fe2(CrO4)3} = \text{Moles of Fe2(CrO4)3} \times \text{Molecular weight}\]\)
\(\[\text{Mass of Fe2(CrO4)3} = 0.150 \times 479.87 = 71.98\]\)
Therefore, the amount of precipitate produced is 71.98 g.c
We need to find out the concentration of each spectator ion in the final solution.
Firstly, we can write down the ionic equation for the reaction:
\(2 K+ + CrO4^2- + 3 Fe^3+ + 3 Cl^- - > 2 K+ + 3 Cl^- + Fe2(CrO4)3\)
Now, we will check which ions remain in the final solution. We see that potassium and chloride ions are spectator ions. Hence, we don't need to calculate their concentration. The concentration of remaining ions can be calculated as follows:Fe3+ ions: In the given reaction, 3 moles of FeCl3 reacts with 2 moles of K2CrO4.
Hence, 3/2 moles of Fe2(CrO4)3 will be formed.
Therefore,
= \(\frac{3/2 \times 3.2 \times 40.0 \div 1000}{60.0 + 40.0}\)
= 0.034 M\]CrO42- ions:
In the given reaction, 2 moles of K2CrO4 reacts with 3 moles of FeCl3.
Hence, 2/3 moles of Fe2(CrO4)3 will be formed.
Therefore,
Concentration{ of CrO4^2-}
= \(\frac{2/3 \times 2.5 \times 60.0 \div 1000}{60.0 + 40.0}\)
= 0.033 M\]
For more such questions on displacement reaction , Visit:
https://brainly.com/question/20690229
#SPJ11
If a car has an EPA mileage rating of 30 miles per gallon, what is this rating in
kilometers per liter? (1 L = 1.06 qt)
Answers
The rating in kilometers per liter is 12.75 kilometer per liter
How to convert 30 miles per gallon to kilometer per gallon1 mile per gallon = 1.60934 Kilometer per gallon
Therefore,
30 miles per gallon = 30 × 1.60934
30 miles per gallon = 48.2802 kilometer per gallon
How to convert 48.2802 kilometer per gallon to kilometer per liter1 kilometer per gallon = 0.264172 kilometer per liter
Therefore,
48.2802 kilometer per gallon = 48.2802 × 0.264172
48.2802 kilometer per gallon = 12.75 kilometer per liter
Thus, the rating in kilometer per liter is 12.75 kilometer per liter
Learn more about conversion:
https://brainly.com/question/1624880
#SPJ1
coenzyme q carries electrons between which stages of the electron-transport chain? check all that apply.
Answers
Coenzyme q carries electrons from complex I to complex III and complex II to complex III in the electron-transport chain.
Coenzyme q (CoQ), also known as ubiquinone, is the electron carrier in the electron transport system (ETS) present on the inner membrane of mitochondria.
Ubiquinone is a ubiquitous quinone, which accepts electrons from complex II ( succinate dehydrogenase) and reduces to ubiquinol ( reduced form)
The purpose of the ETS is to generate an H+ ion concentration, by carrying electrons obtained from NADH AND \(FADH_{2}\) produced by the Krebs cycle and glycolysis in the mitochondrial matrix. This H+ ion potential will be used by ATP synthase to generate ATP.
To know more about ETS :
https://brainly.com/question/876880
https://brainly.com/question/18686654
The inner mitochondrial membrane contains CoQ, a key part of the mitochondrial electron transport chain (ETC), which moves electrons from complexes I and II to complex III to provide energy for proton translocation to the intermembrane gap.
What is mitochondria ?An organelle called a mitochondrion can be found in the cells of the majority of Eukaryotes, including mammals, plants, and fungi. Adenosine triphosphate, which is produced by aerobic respiration in mitochondria with their double membrane structure, is used as a source of chemical energy throughout the entire cell.
Coenzyme Q10 takes electrons from reducing equivalents produced during the metabolism of fatty acids and glucose and then transports them to electron acceptors as part of the mitochondrial electron transport chain.
Ubiquinone, also known as coenzyme Q, is a lipophilic molecule that is found in all tissues and cells and is mostly found in the inner mitochondrial membrane. It is generally known that Coenzyme Q is an essential part of the oxidative phosphorylation process in mitochondria.
Thus, The inner mitochondrial membrane contains CoQ, a key part of the mitochondrial electron transport chain (ETC).
To learn more about mitochondria, follow the link;
https://brainly.com/question/10688306
#SPJ12
Which of the following statements are true concerning the results of the Human Genome Project? Check all that apply.
Researchers now have maps of every human’s genome.
The Human Genome Project has raised many complicated ethical issues.
All medical conditions can be attributed to a specific gene.
Researchers now have a map of an “average” human genome.
Answers
The following statement is true concerning the results of the Human Genome Project:
The Human Genome Project has raised many complicated ethical issues.
What is Human Genome?
The human genome refers to the complete set of genetic information (DNA) present in human cells. It includes both the protein-coding and non-coding regions of DNA. The human genome is made up of about 3 billion base pairs and is organized into 23 pairs of chromosomes. The study of the human genome is an important field of genetics and has many implications for understanding human biology, evolution, and disease.
The Human Genome Project was a scientific research project that aimed to identify and map all the genes in the human genome, which contains all the genetic information needed for human development and function. The project was completed in 2003 and provided a wealth of information about the structure and function of the human genome, including the location and sequence of all human genes.
Learn more about Human Genome from the given https://brainly.com/question/29479722
#SPJ1
In each term equation below a blank line shows one term missing the terms may be an elemental symbol a coefficient or a subscript decide which term would balance the equation write the missing term in the second column the. Determine the reactants third column and products forth column of the reaction some reactions may have only one reactant or product and other reactions may have only one reactant or product and other reactions may have multiple reactants or products write your answers in the spaces in the table the first reaction involves sodium chloride not sodium carbon and iodine

Answers
Answer:
1) Cl2 2 is missing
2) H H is missing
Explanation:
List the 2 pKa's for H2SO4
Answers
calculate the number of moles for the quanity 8.06 x 1021 atoms of Pt
Answers
The number of moles for the quanity 8.06 x\(10_{21\) atoms of Pt is approximately 2.61 grams.
To calculate the number of moles for a given quantity of atoms, we can use Avogadro's number and the molar mass of the element. Avogadro's number is 6.022 x 10²³ atoms/mol.
In this case, you have 8.06 x 10²¹ atoms of Pt. To find the number of moles, divide this quantity by Avogadro's number:
8.06 x 10²¹ atoms Pt / 6.022 x 10²³ atoms/mol = 0.0134 mol Pt
So, there are approximately 0.0134 moles of Pt in 8.06 x 10²¹ atoms of Pt.
The molar mass of Pt (platinum) is 195.08 g/mol. To convert the number of moles to grams, multiply the number of moles by the molar mass:
0.0134 mol Pt x 195.08 g/mol = 2.61 g Pt
Therefore, there are approximately 2.61 grams of Pt in 8.06 x10²¹ atoms of Pt.
In summary, the number of moles for the quantity 8.06 x 10²¹ atoms of Pt is approximately 0.0134 moles. This is equivalent to approximately 2.61 grams of Pt. Remember to use Avogadro's number and the molar mass to perform these calculations accurately.
Know more about moles here:
https://brainly.com/question/29367909
#SPJ8
what properties of a natural resource make it useful for humans as a materials or energy source?
Answers
The properties of a natural resource that make it useful for humans as a material or energy source is the ability to convert mass into energy and vice versa.
What are natural resources?The expression natural resources make reference to all types of matter and energy extracted from nature that can be used to produce goods and services.
Some examples of natural resources include for example irreversible resources such as fossil fuels (i.e., oil, or coal, gas, minerals such as metals, rocks, etc) as well as those based on the use of reversible energy such as eolic air energy, solar radiation or sunlight, soil and hydric resources or water.
Therefore, with this data, we can see that natural resources can be defined as any material and or energy obtained from nature that may be irreversible or reversibly used to produce goods and services.
Learn more about natural resources here:
https://brainly.com/question/24514288
#SPJ1
Magnesium hydroxide reacts with chlorine to form magnesium chloride,
magnesium chlorate and water. How many grams of magnesium hydroxide is
needed to yield 8.00 moles of magnesium chlorate?
77.8 g Mg(OH)2
9178.1 g Mg(OH)2
2799.6 g Mg(OH)2
.823 g Mg(OH)2
How many grams of sodium sulfato pro
Answers
The grams of magnesium hydroxide needed to yield 8.00 moles of magnesium chlorate is approximately 466.64 g. None of the options provided match the calculated value of 466.64 g.
To determine the grams of magnesium hydroxide (Mg(OH)2) needed to yield 8.00 moles of magnesium chlorate (Mg(ClO3)2), we need to consider the balanced chemical equation for the reaction between magnesium hydroxide and chlorine.
The balanced equation is as follows:
2 Mg(OH)2 + 6 Cl2 → 2 Mg(ClO3)2 + 2 H2O
From the balanced equation, we can see that 2 moles of Mg(OH)2 react with 6 moles of Cl2 to produce 2 moles of Mg(ClO3)2.
Therefore, the stoichiometric ratio is 2 moles of Mg(OH)2 : 2 moles of Mg(ClO3)2.
To calculate the grams of Mg(OH)2 needed, we can use the stoichiometric ratio and the given moles of Mg(ClO3)2.
Given:
Moles of Mg(ClO3)2 = 8.00 moles
Using the stoichiometric ratio, we have:
8.00 moles Mg(ClO3)2 × (2 moles Mg(OH)2 / 2 moles Mg(ClO3)2) = 8.00 moles Mg(OH)2
To convert moles to grams, we need to multiply by the molar mass of Mg(OH)2.
The molar mass of Mg(OH)2 = (24.31 g/mol) + (2 * 16.00 g/mol) = 58.33 g/mol
Grams of Mg(OH)2 = 8.00 moles Mg(OH)2 × 58.33 g/mol = 466.64 g
Therefore, the grams of magnesium hydroxide needed to yield 8.00 moles of magnesium chlorate is approximately 466.64 g.
For more such questions on magnesium chlorate
https://brainly.com/question/12358640
#SPJ11
HELP ASAP PLS Is an oxygen ion and fluorine ion bigger and why?
Answers
Answer:
From top to bottom of the periodic table ions will increase in radii. However, now left to right the radius is more of a function of the number of electrons. ... Similarly, O2- will be larger than F- as both have 10 electrons but Z=8 for oxygen and Z=9 for fluorine.
The oxygen ion is bigger than the fluorine ion , the reason is explained below
What is an Atomic Structure ?
An atom is composed of electrons , protons and neutrons .
Electrons have negative charge and are grouped in different shells around the nucleus
Protons and neutrons are present in positively charged nucleus
The nucleus has the most mass .The atomic number is the number of protons which is equal to the number of electrons.
Both oxygen atom and fluorine atoms are isoelectronic , they have 10 electrons in the shell , but oxygen has 8 protons while fluorine have 9 protons in the nucleus.
It is believed that as the atomic number increases so the attraction forces of the nuclei increases , making the nucleus smaller and the overall atom smaller
while the oxygen atom on reduction forms oxide anion , which is a dianion and a fluorine atom forms fluoride ion which has a single negative charge and the ion which has more electrons have more electron electron repulsion and the size of the ion is bigger.
Hence the oxygen ion is bigger than the fluorine ion.
To know more about Atomic Structure
https://brainly.com/question/14156701
#SPJ2
MSG (monosodium glutamate has the following composition by mass: 51% C; 4.77 % H, 37.8 % 0; 8.29 % N and 13.60 % Na. What is the molecular formula if its molar mass is 169 g
Answers
Following the composition by mass, the molecular formula of monosodium glutamate would be \(C_7H_8O_4NNa\)
The empirical formula can first be derived such that:
C = 51/12 = 4.25
H = 4.778/1 = 4.77
O = 37.8/16 = 2.36
N = 8.29/14 = 0.59
Na = 13.60/23 = 0.59
Dividing by the smallest (0.59):
C = 7
H = 8
O = 4
N = 1
Na = 1
Hence, the empirical formula would be \(C_7H_8O_4NNa\)
Molar mass of empirical formula = 12x7 + 8x1 + 16x4 + 14 + 23
= 195
Recall that: , (\(C_7H_8O_4NNa\))n = molecular formula
Where n = molar mass/empirical formular mass
Molar mass/empirical formular mass = 169/195
= 0.87
≈ 1
Thus, (\(C_7H_8O_4NNa\)) x 1 = \(C_7H_8O_4NNa\)
More on molecular formula calculation can be found here: https://brainly.com/question/21629131
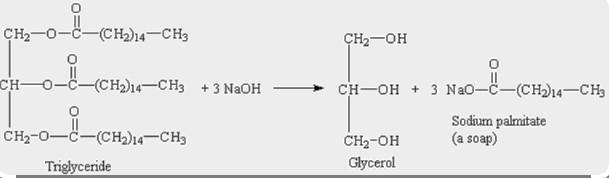
1) write a balanced equation to show the hydrolysis of glycerol tristearate (tristearin, a triple ester of glycerol) using water and sodium hydroxide to make soap. in addition to the molar ratios, give the mass ratios, i.e., the ratios of masses that react according to the reaction stoichiometry) based on 20 g of tristearin.
Answers
Mass ratio of Tristearin that react according to the reaction : \(NaOH\) is
20 : 27
The main fat in beef is tristearin. A molecule of glycerine that has interacted with three (3) molecules of the fatty acid stearic acid is known as a triglyceride.
1 mole of Tristearin requires 3 moles of \(NaOH\) to react to give 1 mole of glycerol & 3 moles of sodium sterate.
Molar ratio of reactant Tristearin : \(NaOH\) = 1 : 3
Molar weight of Tristearin = 891.5 g/mol
=> 20 g Tristearin = \(\frac{20}{891.5} moles\)
=> \(NaOH\) required to react with \(\frac{20}{891.5}\) moles of Tristearin = \(3\times\frac{20}{891.5} moles\) = 0.0673 moles
=> Molecular weight of \(NaOH\) = 40 g/mol
=> \(NaOH\) mass required to react with 20 g Tristearin = 0.0673 × 40g = 2.7g
∴ Mass ratio of Tristearin: \(NaOH\) is 20 : 27.
Learn more about Tristearin:
brainly.com/question/7175814
#SPJ4
A mixture can be separated into simpler parts based on the components of the mixture's _____ properties.
volatile
reaction
physical
chemical
Answers
Answer:
Chemical is best answer
Explanation:
Mark me as brilliant and thank you
Please
Answer:
chemical is the answer
Explanation:
A 1.85-mole sample of H₂O2 weighs
(A) 33.3 amu
(B) 35.9 g
C) 62.9 g
(D) 1.85 g
E 33.3 g
Answers
Considering the definition of molar mass, the correct answer is option c): the mass of 1.85 moles H₂O₂ is 62.9 grams.
Definition of molar massThe molar mass of substance is a property defined as the amount of mass that a substance contains in one mole.
The molar mass of a compound is the sum of the molar mass of the elements that form it (whose value is found in the periodic table) multiplied by the number of times they appear in the compound.
Molar mass of H₂O₂In this case, you know the molar mass of the elements is:
O= 16 g/moleH= 1 g/moleSo, the molar mass of the compound H₂O₂ is calculated as:
H₂O₂= 2× 1 g/mole + 2× 16 g/mole
Solving:
H₂O₂= 34 g/mole
Mass of 1.85 moles H₂O₂You can apply the following rule of three: If by definition of molar mass 1 mole of the compound contains 34 grams, 1.85 moles of the compound contains how much mass?
mass= (1.85 moles× 34 grams)÷ 1 mole
mass= 62.9 grams
Finally, the mass of 1.85 moles H₂O₂ is 62.9 grams.
Learn more about molar mass:
brainly.com/question/5216907
#SPJ1
How many moles of C2H2 are required to produce 0.60 mol of H2O?
Express your answer to two significant figures and include the appropriate units.
Answers
Answer:
0.60 moles
Explanation:
From the reaction:
\(2C_2H_2 + 5O_2 \to 4CO_2+2H_2O\)
From above, Only 2 moles of CH2CH2 are required to produce 2 moles of water (H_2O).
As such, 0.6 moles of H2O will require:
x (CH2CH2) × 2 moles of (H_2O) = 0.6 moles (CH2CH2) × 2 mole of (H_2O)
x mole of (CH2CH2) = 0.60 moles
∴
0.6 moles of H2O will require 0.60 moles of CH2CH2
If you burn 9.1 L of octane gas (C8H18), how many liters of steam will you produce?
Answers
From the stoichiometry of the reaction, the volume of the steam produced is 82.9 L
What is combustion?The term combustion is the term that is used to describe the kind of reaction that occurs when a substance is being burnt in oxygen. It is an oxidation reaction and the octane is burnt to produce carbon dioxide and steam.
We can try to write down the equation of the reaction as follows;
2C8H18(g) + 25O2(g) ------> 16CO2(g) + 18H2O(g)
Now 1 mole of octane gas occupies 22.4 L
x moles of octane gas occupies 9.1 L
x = 9.1 L * 1 mole /22.4 L
x = 0.41 moles
Now;
2 mole of octane gas produces 18 moles of steam
0.41 moles of octane gas produces 0.41 moles * 18 moles/2 mole
= 3.7 moles
1 mole of steam occupies 22.4 L
3.7 moles of steam occupies 3.7 moles * 22.4 L/1 mole
= 82.9 L
Learn more about stoichiometry:https://brainly.com/question/9743981
#SPJ1
The data in the table below represents the pressure of the gas as the temperature changes. Plot a graph of this data, using the blank graph provided below. Draw a trend line and calculate its slope. How are the variables related? What will the pressure of the gas be at 0°C?
Answers
Answer: A
Explanation:
Which option is formed by the process of fusion in stars?
O compounds
Oelectrons
O quarks
Oelements
Answers
D. The option that is formed by the process of fusion in stars is elements.
How fusion in stars form elements
When the new star reaches a certain size, a process called nuclear fusion ignites, generating the star's vast energy.
The fusion process forces hydrogen atoms together, transforming them into heavier elements such as;
helium, carbon and oxygen.Thus, the option that is formed by the process of fusion in stars is elements.
Learn more about nuclear fusion here: https://brainly.com/question/17870368
#SPJ1
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
A sample of Neon gas occupies 25 mL at 3.2 atm. What would be the volume at
202.6kPa?What law are you using?
O
Charles' Law: V1T2 = V2T1
Gay-Lussac's Law: P1T2 = P2T1
OBoyle's Law: V1P1 = V2P2
Answers
Answer:
Using OBoyle's Law, the volume at 202.6kPa would be 16.25 mL.
how does the position of an electron relates to its energy
Answers
The position and energy of an electron are intricately linked in the quantum realm.
The position of an electron is related to its energy through the principles of quantum mechanics. According to the Bohr model, electrons occupy specific energy levels or orbitals around the nucleus of an atom. These energy levels are quantized, meaning they can only have specific values. Electrons with lower energy are found closer to the nucleus in lower energy levels, while those with higher energy occupy outer energy levels. The energy of an electron is directly proportional to its distance from the nucleus. As the electron transitions between energy levels, it either absorbs or emits energy in discrete quantities known as photons. The energy of an electron can also be influenced by external factors such as electric and magnetic fields.
For more such questions on electron
https://brainly.com/question/26084288
#SPJ11
Which of the following molecules would you expect to have the highest boiling point?
1
O Molecule 3
O Molecule 1
O Molecule 4
O Molecule 2
2
3
OH
O
4

Answers
The highest boiling point based on the data is option 4
What is the highest boiling point?Compared to alcohols of comparable molecular weight, carboxylic acids often have higher boiling temperatures. Between the hydrogen atoms of adjacent molecules and the oxygen in the carboxyl group of carboxylic acids, strong intermolecular hydrogen bonds can develop. Because it takes more energy to break the intermolecular interactions and change the substance from a liquid to a gas during boiling, these hydrogen bonds help materials have higher boiling temperatures.
Although carboxylic acids and alcohols are both capable of forming hydrogen bonds, carboxylic acids have higher boiling temperatures due to the extra carboxyl group that they contain.
Learn more about boiling point:https://brainly.com/question/1514229
#SPJ1
The skeletal structure in line-angle (line-bond) mode of 2,3,3,5-tetramethylhexane is shown. Identify the number of hydrogen atoms bound to each carbon atom in the structure.

Answers
Answer:
There are 22 hydrogen in 2,3,3,5-tetramethylhexane (C10H22)
From the left to the right of the skeletal structure:
1st Main Carbon = 3 hydrogen
2nd Main Carbon = 1 hydrogen
1st Branch Carbon = 3 hydrogen
3rd Main Carbon = 2 hydrogen
2nd Branch Carbon = 3 hydrogen
3rd Branch Carbon = 3 hydrogen
4th Main Carbon = 0 hydrogen
4th Branch Carbon = 3 hydrogen
5th Main Carbon = 1 hydrogen
6th Main Carbon = 3 hydrogen
Total = 22 hydrogen
Explanation:
This is the skeletal formula for 2,3,3,5-tetramethylhexane - the 2D chemical structure. It is an organic compound. The carbon atoms in the structure are known as the carbon backbone. The hydrogen atoms are linked to the carbon backbone. This provides each carbon atom with four bonds.
Write a balanced equation for the reaction between copper metal and nitric acid to produce copper II nitrate, nitrogen dioxide, and water.
Answers
Nitric acid basically reacts with copper as per to the reaction given 4 HNO3(l) + Cu(s) -----> Cu(NO3)2(aq) + 2 NO2(g) + 2 H2O(l).
What is a balanced reaction?A balanced reaction includes the same number of elements on both sides of the equation.
In a chemical reaction, the stoichiometric coefficient is the number written in front of atoms, ions, and molecules to balance the number of each element between the reactant and product sides of the equation.
The balanced reaction for reaction between copper metal and nitric acid to produce copper II nitrate, nitrogen dioxide, and water will be as follows:
\(4HNO_3(l)+Cu(s)----- > Cu(NO_3)_2(aq)+2NO_2+2H_2O\)
Thus, the above mentioned is the balanced reaction for the given scenario.
For more details regarding balanced reactions, visit:
https://brainly.com/question/12299665
#SPJ1
A balloon has a volume of 145 mL at room temperature (25°C = 298°K). Alyssa decides to place the balloon in the freezer to see what happens. After being in the freezer for an hour, the balloon has a new volume of 35mL. What is the temperature inside the freezer?
Answers
The temperature inside the freezer is approximately -164°C.
To solve this problem, we can use the combined gas law equation:
\((P1V1)/T1 = (P2V2)/T2\)
where P is the pressure, V is the volume, and T is the temperature of the gas.
We know that the initial volume of the balloon is 145 mL and the final volume is 35 mL. We also know that the initial temperature is 25°C or 298 K, and we need to find the final temperature.
Assuming the pressure of the gas remains constant, we can rearrange the combined gas law equation to solve for the final temperature:
\(T2 = (P2V2/T1)(P1/V1)\)
Plugging in the values we know, we get:
\(T2 = (1 atm * 35 mL/298 K)(1 atm/145 mL) = 0.0808 atm/mL\)
Multiplying both sides by 298 K and dividing by 0.0808 atm/mL, we get:
T2 = 109.15 K or approximately -164°C
Therefore, the temperature inside the freezer is approximately -164°C.
Learn more about combined gas law, here:
https://brainly.com/question/30458409
#SPJ1
Ten non metals formula
Answers
Answer:
plz mark me as a brainelist
Explanation:
Names and symbols/formulas of 10 non-metals are as follows.
1). Boron (B)
2). Carbon (C)
3). Silicon (Si)
4). Nitrogen (N)
5). Phosphorus (P)
6). Arsenic (Ar)
7). Oxygen (O)
8). Sulfur (S)
9). Selenium (Se)
10). Bromine (Br)
Problems 307 How many milliliters of a 2.5 M NaCl solution would be needed to prepare each solution? a. 1.5L of a 0.75m solution
Answers
Answer:
450 mL
Explanation:
First we calculate how many NaCl moles would there be in 1.5 L of a 0.75 M solution, using the definition of molarity:
Molarity = moles / LitersMoles = Molarity * LitersMoles = 1.5 L * 0.75 M =1.125 molOnce again using the definition of molarity, we now calculate how many liters of a 2.5 M solution would have 1.125 moles:
Liters = 1.125 mol / 2.5 M = 0.45 LFinally we convert 0.45 liters to mL:
0.45 L * 1000 = 450 mLThe sugars we have been discussing are made directly through a series of reactions in the____ , which uses co2 as a starting input. a. light reaction a. recall, that under thermodynamic laws, building complex molecules requires the input of energy in the form of sunlight. that energy in photosynthesis comes from the . b. calvin cycle a. through a series of reactions in the , light energy is transformed into energy that is in the form of atp and high-energy electrons carried by nadph. b. in the , h2o is used and outputs as o2. a. in the , atp and nadph enter and output as adp and nadp.
Answers
The sugars we have been discussing are made directly through a series of reactions in the Calvin Cycle, which uses CO2 as a starting input.
Recall that under thermodynamic laws, building complex molecules requires the input of energy in the form of sunlight. That energy in photosynthesis comes from the Light Reaction. Through a series of reactions in the Light Reaction, light energy is transformed into energy that is in the form of ATP and high-energy electrons carried by NADPH. In the Light Reaction, H2O is used and outputs as O2. In the Calvin Cycle, ATP and NADPH enter and output as ADP and NADP. These processes work together to produce glucose and other sugars that are essential for life. The sugars we have been discussing are made directly through a series of reactions in the Calvin Cycle, which uses CO2 as a starting input.
Learn more about Calvin Cycle :
https://brainly.com/question/17600594
#SPJ4
Explain what is a chemical reaction.
Answers
Answer:
Chemical reaction, a process in which one or more substances, the reactants, are converted to one or more different substances, the products. Substances are either chemical elements or compounds. A chemical reaction rearranges the constituent atoms of the reactants to create different substances as products.
Explanation:
Answer:
a process in which one or more substances, the reactants, are converted to one or more different substances, the products. Substances are either chemical elements or compounds. A chemical reaction rearranges the constituent atoms of the reactants to create different substances as products.
Explanation:
Here's an example. When you burn wood, It releases Carbon dioxide, water vapor, and ashes. It just depends what chemicals you use ^^