Answers
Answer: \(Pb(NO_3)_2(aq)+2NaOH(aq)\rightarrow 2NaNO_3(aq)+Pb(OH)_2(s)\)
Explanation:
A double displacement reaction is one in which exchange of ions take place. The salts which are soluble in water are designated by symbol (aq) and those which are insoluble in water and remain in solid form are represented by (s) after their chemical formulas.
The balanced chemical equation for a precipitate forms when aqueous solutions of lead(II) nitrate and sodium hydroxide are combined is:
\(Pb(NO_3)_2(aq)+2NaOH(aq)\rightarrow 2NaNO_3(aq)+Pb(OH)_2(s)\)
Related Questions
How are a desert and a tundra similar?
They both have high levels of precipitation.
They have many trees and a lot of vegetation.
They have high average temperatures.
They have low humidity and low levels of precipitation.
Answers
Answer:
They have low humidity and low levels of precipitation.
Explanation:
Both the desert and the tundra are two extreames, one is really hot while the other is really cold. Deserts only receive less than 300 mm of rain each year. While the tundra receives 150 to 250 mm of precipitation each year, including melted snow. Which is less than the majority of the world's deserts.
Answer: They have low humidity and low levels of precipitation.
Explanation:
Differences: Tundra's are extremely cold, and doesn't get much rain.
Differences: The Dessert is Extremely hot and doesn't get rain.
Explanation: There is already our answer here, If we compare there differences the we will find our answer. This is what I found as the same "doesn't get rain." And we know that precipitation is rain so your answer would be precipitation. Your answer: They have low humidity and low levels of precipitation.
Today State the five steps or procedure that is involved in physical Examination of organic compound
Answers
The physical examination of an organic compound are appearance and physical state, melting point and boiling point, solubility, density, and spectroscopic analysis
What are the five steps required?Appearance and physical state: observe the appearance of the compound and note its physical state.
Melting point and boiling point: measure the melting point and boiling point of the compound to determine its purity and identity.
Solubility: Test the solubility of the compound in different solvents such as water, ethanol, acetone, etc.
Density: determine the density of the compound to calculate its molecular weight and to assess its purity.
Spectroscopic analysis: use spectroscopic techniques such as nuclear magnetic resonance (NMR), and mass spectrometry (MS) to determine the functional groups, molecular structure, and identity of the compound.
Learn more about organic compound here: https://brainly.com/question/5994723
#SPJ1
Which best describes the motion of pentane molecules in the liquid phase?
A. The pentane molecules move randomly in all directions to fill their
container.
O
B. The pentane molecules move past one another but are held close
together.
O C. The pentane molecules are locked in one place and do not move.
O D. The pentane molecules vibrate and are fixed in one position.
Answers
In the liquid state, the pentane molecules move past one another but are held close together.
What is the liquid phase?The liquid phase is a phase of matter that is made of molecules that in motion but do not posses as much kinetic energy as the molecules of gases. Thus implies that the molecules are able to translate.
Hence, the pentane molecules move past one another but are held close together.
Learn ore about liquid molecules:https://brainly.com/question/9437743
#SPJ1
how does the mass and size of an atom compare to the mass and size of the nucleus
Answers
Answer:
Explanation:
The nucleus of an atom is about 10-15 m in size; this means it is about 10-5 (or 1/100,000) of the size of the whole atom. ... (10-15 m is typical for the smaller nuclei; larger ones go up to about 10 times that.) Mass. Although it is very small, the nucleus is massive compared to the rest of the atom.
Answer:
The nucleus of an atom is about 10-15 m in size; this means it is about 10-5 (or 1/100,000) of the size of the whole atom. ... (10-15 m is typical for the smaller nuclei; larger ones go up to about 10 times that.) Mass. Although it is very small, the nucleus is massive compared to the rest of the atom.
Explanation:
Insert the term that correctly completes the paragraph.
Illustration of a rock showing that the layers are not stacked horizontally and look like a rainbow of layers.
Soledad studied rocks and how they help show the history of Earth. She knew that scientists use different ways to find out how old rock layers are and recognized one such example in the rock in the image. The image is an example of a/an
rock.
Answers
Gravity causes spacecraft such as stars, planets, moons, and other bodies to orbit one another. Revolution describes this style of movement.
What is Gravity ?Gravity causes spacecraft such as stars, planets, moons, and other bodies to orbit one another. Revolution describes this style of movement. Space is also home to several stationary things. Rotation describes this movement.We experience day and night because of how long it takes the Earth to complete one rotation. A year is the length of one Earth rotation around the sun. Due to their varying rates of rotation and revolving, other planets have days and years that differ from our own.In the same direction, every planet in our solar system orbits the sun. In addition, the majority of them rotate in the same direction (with the exception of Venus and Uranus). In the cosmos, approximately half of all galaxies rotate in a clockwise direction, and the other half in a counterclockwise direction. This may be due to the manner that the cosmos started, according to scientists.To Learn more About Gravity refer To:
https://brainly.com/question/557206
#SPJ1
which is the graph of the function g(x) = f(-x)
Answers
To graph the function g(x) = f(-x), you can start with the graph of f(x) and then reflect it about the y-axis.
What is a graph of the function g(x) = f(-x)?To find the graph of the function g(x) = f(-x), we can start with the graph of the function f(x) and then reflect it about the y-axis.
If the graph of f(x) is symmetric with respect to the y-axis, meaning it is unchanged when reflected, then g(x) = f(-x) will have the same graph as f(x).
However, if the graph of f(x) is not symmetric with respect to the y-axis, then g(x) = f(-x) will be a reflection of f(x) about the y-axis.
In either case, the resulting graph of g(x) = f(-x) will be symmetric with respect to the y-axis.
Learn more about the graph of functions at: https://brainly.com/question/17089414
#SPJ1

In your own words, explain why electronegativity increases from left to right across a period and increases from the bottom to the top of a group.
Answers
I have four questions hope you can help me


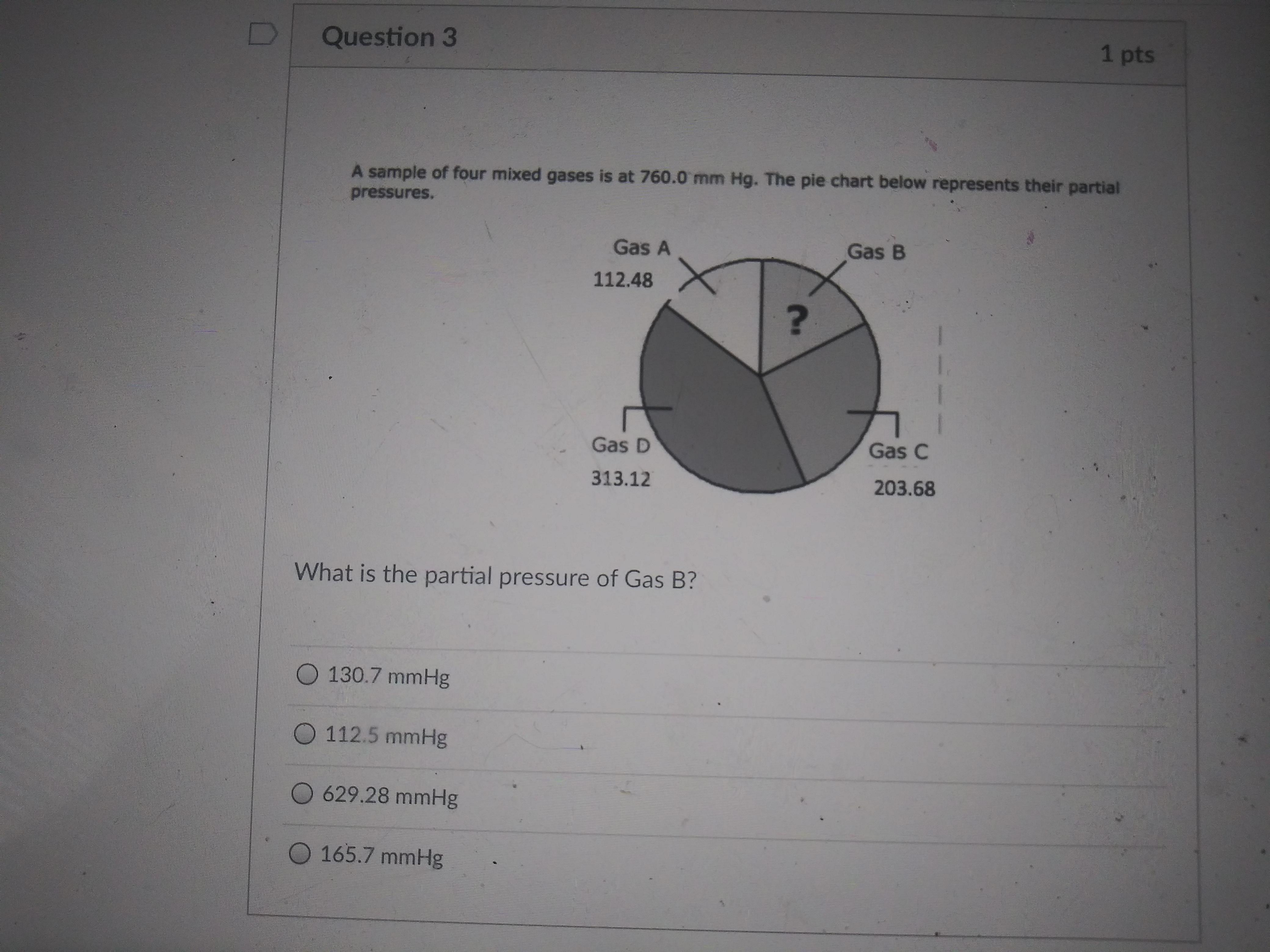

Answers
Answer:
2+2 kaszkqkq
Explanation:
If I wanted to convert from nanograms to centigrams which directions would I move my decimal?
Right or Left
Answers
Answer:
Left
Explanation:
To convert from Nanograms to centigrams, you have to move the decimal to the left hand side.
1 nanograms = 1 x 10⁻⁹g
1 centigrams = 1 x 10⁻² g
So to convert from nanograms to centigrams,
Multiply by 1 x 10⁻⁷ or move 7 places to the left
Please help me answer this question

Answers
How many moles of gas are in a 25 L container if the temperature is 289 K and the pressure is 125 atm?
Answers
Answer:
1.3 moles
Explanation:
pv = nrt
n = pv/rt
n = (125 * 25) / (8.3144598 * 289)
n = 1.3 moles
Planet A has a distance to the Sun of 10.0 AU, and a period of roughly 31.6 Earth
years, and Planet B’s period is 4.50 Earth years. Use the Kepler’s third law to
calculate Planet B’s distance to the Sun.
Answers
The distance of Planet B from the Sun is approximately 0.3149 AU.
Kepler's third law states that the square of the orbital period of a planet is proportional to the cube of its average distance from the Sun. Mathematically, it can be written as:
\(T^2 = (4\pi ^2/GM) * r^3\)
where T is the period of the planet, M is the mass of the Sun, G is the gravitational constant, and r is the distance of the planet from the Sun.
We can use this formula to calculate the distance of Planet B from the Sun, given that its period is 4.50 Earth years. We'll need to know the mass of the Sun, which is approximately \(1.99 * 10^{30\) kg.
First, we need to find the value of (T^2/r^3) for Planet A:
(T^2/r^3) for Planet A = (31.6 Earth years)^2 / (10.0 AU)^3
(T^2/r^3) for Planet A = 998.56 Earth years^2/AU^3
Now we can use this value to find the distance of Planet B from the Sun:
(T^2/r^3) for Planet B = (4.50 Earth years)^2 / r^3
Since the two planets are orbiting the same star, we can assume that the mass of the Sun is the same for both planets. Therefore, we can set the two expressions for (T^2/r^3) equal to each other:
(T^2/r^3) for Planet A = (T^2/r^3) for Planet B
998.56 Earth years^2/AU^3 = (4.50 Earth years)^2 / r^3
We can now solve for r:
r^3 = (4.50 Earth years)^2 / 998.56 Earth years^2/AU^3
r^3 = 0.020295 AU^3
r = 0.3149 AU
For more question on distance click on
https://brainly.com/question/14534420
#SPJ11
PLEASE HELP ILL GIVE BRAINLIESTTTT

Answers
Answer:
Look at it closer, its very easy to understand
Explanation:
read the questions thoroughly, then answer the questions.
How many moles of aluminum ions al3+ are present in 0.42 mol of al2so43
Answers
There are 0.84 moles of aluminum ions (Al3+) present in 0.42 mol of Al2(SO4)3.
To determine the number of moles of aluminum ions (Al3+) present in 0.42 mol of Al2(SO4)3, we need to consider the stoichiometry of the compound.
The formula of aluminum sulfate (Al2(SO4)3) indicates that for every 1 mole of the compound, there are 2 moles of aluminum ions (Al3+). This means that the mole ratio of Al3+ to Al2(SO4)3 is 2:1.
Given that we have 0.42 mol of Al2(SO4)3, we can calculate the moles of Al3+ as follows:
Moles of Al3+ = 0.42 mol Al2(SO4)3 x (2 mol Al3+ / 1 mol Al2(SO4)3)
Moles of Al3+ = 0.42 mol Al2(SO4)3 x 2
Moles of Al3+ = 0.84 mol Al3+
Therefore, there are 0.84 moles of aluminum ions (Al3+) present in 0.42 mol of Al2(SO4)3.
It's important to note that the stoichiometry of the compound determines the mole ratio between the different species involved in the chemical formula. In this case, the 2:1 ratio of Al3+ to Al2(SO4)3 allows us to determine the number of moles of Al3+ based on the given amount of Al2(SO4)3.
For more such question on aluminum visit:
https://brainly.com/question/30451292
#SPJ8
help me please :(
D & 1 is also a option

Answers
How many grams of iron are produced when 450 grams of iron (lll) oxide are reacted
Answers
To answer this question, we need to know the balanced chemical equation for the reaction between iron (III) oxide and another substance that would produce iron.
How many grams of iron will be produced when 450 grams of iron (lll) oxide are reacted?Assuming the reaction is:
Fe₂O₃ + 3CO → 2Fe + 3CO₂
This equation tells us that one mole of Fe₂O₃ reacts with three moles of CO to produce two moles of Fe. We can use this information to calculate the number of moles of Fe that can be produced from 450 grams of Fe₂O₃.
Convert the mass of Fe₂O₃ to moles in the first step:
Molar mass of Fe₂O₃ = 2 x atomic mass of Fe + 3 x atomic mass of O = 2 x 55.85 + 3 x 16.00 = 159.69 g/mol
Number of moles of Fe₂O₃ = 450 g / 159.69 g/mol = 2.82 mol
According to the balanced chemical equation, 1 mole of Fe₂O₃ produces 2 moles of Fe. Therefore, the number of moles of Fe that can be produced from 2.82 mol of Fe₂O₃ is:
Number of moles of Fe = 2.82 mol x (2 mol Fe / 1 mol Fe₂O₃) = 5.64 mol
Convert the number of moles of Fe to grams in the final step:
Molar mass of Fe = 55.85 g/mol
Mass of Fe produced = 5.64 mol x 55.85 g/mol = 315.89 g
Therefore, if 450 grams of iron (lll) oxide are reacted with carbon monoxide to produce iron, the amount of iron produced would be 315.89 grams.
Learn more about iron (lll) oxide here:
https://brainly.com/question/14401892
#SPJ1
Animals get energy from the food that they eat. However, when the molecules from the food enter your cells, how do the molecules turn in to energy?
Question 3 options:
The glucose molecules from the food are broken down by the mitochondria in the cell to produce ATP which the cells use as energy.
The ATP is broken down into glucose which the cells use for energy.
Upon hitting the stomach, the food molecules are changed to energy molecules which fuel the body.
The cells absorb ATP from food and use it for energy.
Answers
Answer:
The ATP is broken down into glucose which the cells use for energy.
how do you balance this equation
2h2s+3o2+so2
Answers
The balanced equation is: 4 \(H_2S\)+ 3 \(O_2\)→ 4 \(SO_2\)+ 8 \(H_2O\)
The given chemical equation is unbalanced. To balance it, we need to adjust the coefficients in front of each chemical species until the number of atoms on both sides of the equation is equal.
The unbalanced equation is:
2 \(H_2S\)+ 3 \(O_2\)→ \(SO_2\)
Let's start by balancing the sulfur (S) atoms. We have two sulfur atoms on the left side and one sulfur atom on the right side. To balance the sulfur, we can place a coefficient of 2 in front of the \(SO_2\):
2 \(H_2S\)+ 3 \(O_2\)→ 2 \(SO_2\)
Now, let's balance the hydrogen (H) atoms. We have four hydrogen atoms on the left side (2 from each \(H_2S\)) and none on the right side. To balance the hydrogen, we can place a coefficient of 4 in front of the water (H2O) on the right side:
2 \(H_2S\)+ 3 \(O_2\)→ 2 \(SO_2\)+ 4 \(H_2O\)
Finally, let's balance the oxygen (O) atoms. We have six oxygen atoms on the right side (3 from \(O_2\) and 3 from 2 \(SO_2\)) and three on the left side (2 from \(H_2S\)). To balance the oxygen, we can place a coefficient of 3/2 in front of the O2:
2 \(H_2S\)+ (3/2) \(O_2\)→ 2 \(SO_2\)+ 4 \(H_2O\)
To remove the fractional coefficient, we can multiply all coefficients by 2:
4 \(H_2S\) + 3 \(O_2\)→ 4 \(SO_2\)+ 8 \(H_2O\)
Now the equation is balanced, with an equal number of atoms on both sides. The balanced equation is:
4 \(H_2S\)+ 3 \(O_2\)→ 4 \(SO_2\)+ 8 \(H_2O\)
For more such questions on balanced equation visit:
https://brainly.com/question/23877810
#SPJ8
When two or more atoms chemically combine, they make a
1.mass
2.mono atom
3.molecule
4.synthesis
Answers
If there is sufficient fossil fuel , how will we cope ?
Answers
If there is sufficient fossil fuel, we will cope by utilizing it in a responsible and sustainable manner while actively transitioning towards alternative energy sources. Coping with the abundance of fossil fuels requires a multi-faceted approach that considers environmental, economic, and social aspects.
To cope effectively, we can:
1. Promote energy efficiency: Invest in technologies and practices that minimize energy waste and maximize efficiency in all sectors, including transportation, industries, and buildings.
2. Transition to renewable energy: Increase the adoption of renewable energy sources such as solar, wind, hydro, and geothermal power. This reduces our reliance on fossil fuels and mitigates environmental impacts.
3. Implement policy measures: Enact policies that incentivize the use of renewable energy, discourage excessive fossil fuel consumption, and promote sustainable practices.
4. Invest in research and development: Support and fund research efforts aimed at developing cleaner and more sustainable energy technologies, such as advanced battery storage, hydrogen fuel cells, and carbon capture and storage.
for more questions on fossil
https://brainly.com/question/14004257
#SPJ8
7. What is the volume of the
composite
solid?
4 in.
3 in.
3 in.

Answers
Answer:
The volume of Component 1 is 36 cubic inches.
Explanation:
To calculate the volume of a composite solid, we need to determine the individual volumes of the different components and then add them together.
In this case, the composite solid consists of multiple components with the following dimensions:
Component 1:
Length: 4 inches
Width: 3 inches
Height: 3 inches
To find the volume of Component 1, we multiply the length, width, and height together:
Volume of Component 1 = Length x Width x Height = 4 in x 3 in x 3 in = 36 cubic inches
Therefore, the volume of Component 1 is 36 cubic inches.
Please provide the dimensions of the remaining components of the composite solid, and I will calculate the total volume by summing up the individual volumes.
For the chemical reaction C2H6 + 137 kJ → C2H4 + H2, the chemical energy of the
a
reactant is greater than the chemical energy of the products.
b
products is greater than the chemical energy of the reactant.
c
reaction is conserved.
d
reactant and the chemical energy of the products are equal.
Answers
For a chemical reaction C₂H₆ + 137 kJ → C₂H₄ + H₂, the chemical energy of the reactant and the chemical energy of the products are equal. Option D is correct.
In an exothermic reaction, energy is released as the reactants are converted to products. The negative sign on the enthalpy change (ΔH) indicates that the reaction is exothermic, meaning that energy is released by the system to the surroundings. This energy comes from the chemical energy of the reactants, and in this case, 137 kJ of energy are released.
The chemical energy of a substance is the energy stored in the bonds between its atoms. In this reaction, C₂H₆ (ethane) is converted to C₂H₄ (ethylene) and H₂ (hydrogen gas), which means that some of the bonds in the reactant molecule are broken and new bonds are formed in the product molecules.
The total amount of energy stored in the bonds of the reactants is equal to the total amount of energy stored in the bonds of the products, since energy cannot be created or destroyed, only converted from one form to another. Therefore, the chemical energy of the reactant and the chemical energy of the products are equal.
Hence, D. is the correct option.
To know more about chemical energy here
https://brainly.com/question/30288262
#SPJ1
For the general gas phase reaction 2 A + 3 B ↔ 2 C the equalibrium constant is 5.00. Calculate the equilibrium quotient based on the following initial concentrations and predict whether the system with shift left or shift right.
Report your answer to 3 significant figures, not scientific notation, using the format "1.23 left" or "9.87 right".
A0 = 0.293 M
B0 = 2.1 M
C0 = 0.036 M
Answers
The equilibrium quotient based on the following initial concentrations and predict whether the system with shift left or shift right is 3.10 x 10⁻⁴ right
Gas Phase Reactions :There are two types of gas-phase reactions: intramolecular and intermolecular. Precursor molecules are broken down into activated species in intramolecular reactions, which are then used in the CVD process.
How can you calculate and determine the shift?Qc = [C] = equilibrium quotient ² / [A]² [B]³
Qc = (0.0383)² / (0.219)² (4.62) (4.62)³
Qc = 3.10 x 10⁻⁴
Kc = 5.00
Kc > Qc . so shifts to right
What is the formula for the gas-phase reaction?We use the ideal gas equation PV=nRT to account for these conditions, where P is the pressure in atmosphere (atm), V is the volume in liters (L), n is the number of moles, and R is the gas constant with a value of 08206 L atm mol-1 K-1, where T is the temperature in kelvin (K) and L is the atm.
Learn more gas phase reaction :
brainly.com/question/14789122?
#SPJ1
burning 12g of urea raise temp of water by 30C what is the enthalpy of combustion for 1kg urea
Answers
The enthalpy of combustion for 1kg of urea is -1223525.84 J/mol.
Urea is a compound that is used in fertilizers and in some plastics.The enthalpy of combustion for urea is the amount of energy that is released when urea is burned. In order to calculate the enthalpy of combustion for 1kg of urea, we need to use the information that is provided to us in the question. Let us start by writing down the balanced equation for the combustion of urea: CO(NH2)2 + 3/2 O2 → CO2 + 2H2O + N2
The balanced equation shows that 1 mole of urea reacts with 1.5 moles of oxygen gas to produce 1 mole of carbon dioxide, 2 moles of water, and 1 mole of nitrogen gas. The enthalpy change for this reaction is equal to the amount of energy that is released when 1 mole of urea is burned.
The heat of combustion (ΔHc) of urea is -632.6 kJ/mol. This means that 632.6 kJ of energy is released when 1 mole of urea is burned. We know that 12g of urea raised the temperature of water by 30°C. We can use this information to calculate the amount of energy that was released when 12g of urea was burned.
The specific heat capacity of water is 4.18 J/g°C. This means that it takes 4.18 J of energy to raise the temperature of 1 gram of water by 1°C. Therefore, it takes 4.18 x 1000 = 4180 J of energy to raise the temperature of 1 kg of water by 1°C.
We know that 12g of urea raised the temperature of water by 30°C. Therefore, the amount of energy that was released when 12g of urea was burned is:
Energy = mass x specific heat capacity x temperature change
Energy = 0.012 kg x 4180 J/kg°C x 30°C
Energy = 1497.6 J
We can now use this information to calculate the enthalpy of combustion for 1kg of urea:
Enthalpy of combustion = energy released / moles of urea burned
Enthalpy of combustion = 1497.6 J / (0.012 kg / 60.06 g/mol)
Enthalpy of combustion = - 1223525.84 J/mol
for such more questions on enthalpy
https://brainly.com/question/14047927
#SPJ8
What is the mass of 6.02 x 1024 molecules of the compound HCl?
Answers
Answer:
First, we need to determine the molar mass of HCl.
The molar mass of HCl = the mass of hydrogen (1.008 g/mol) + the mass of chlorine (35.45 g/mol) = 36.45 g/mol.
Next, we can use Avogadro's number (6.02 x 10^23 molecules/mol) to convert the number of molecules to moles:
6.02 x 10^24 molecules / 6.02 x 10^23 molecules/mol = 10 moles
Finally, we can use the molar mass to convert moles to grams:
10 moles x 36.45 g/mol = 364.5 grams
Therefore, the mass of 6.02 x 10^24 molecules of HCl is 364.5 grams.
:. It means 1 mole of Hcl
:. To find the mass of HcL
no of moles = mass/ molar mass
To get the molar mass of HCL {H=1 CL=35.5}
:. H+CL = 1+ 35.5 =36.5
So we have our molar mass and number of moles now
Then we input it in the eqn
1=x/36.5
X= 36.5g of HCl
According to Democritus, what is the composition of matter?
Answers
Answer:
he composition of matter is invisible particles called atoms.
Explanation:
I'm sure its correct
Answer:
The composition of matter is invisible particles called atoms. ... The root of the word "atom" is atomos, meaning uncuttable.
From Google
Hope I Helped
how do the biosphere and geosphere interact? give an example
Answers
Answer:
Plants
Explanation:
Plants, part of the biosphere, grow in the soil, which is part of the geosphere
hope this helped :)
What is the solar radius of a main sequence star?

Answers
Answer:I'm going to give some fictitious values just so that we can get some perspective on the matter.
Let's say that the surface temperature of our sun is 10, the surface temp of the bigger star- the red giant formed from leaving the main sequence, has a temp of 0.2. of that- 2.
We can also say that the radius of our sun is 10, and the radius of the red giant is 1000. (100 times more)
Using the equation:
L
=
σ
A
T
4
σ
= The Stefan-Boltzmann constant =
5.67
×
10
−
8
But we can ignore the constant, as we are only interested in a ratio of these values.
L
S
u
n
=
4
π
(
10
)
2
×
10
4
=
1.26
×
10
7
L
S
t
a
r
=
4
π
(
1000
)
2
×
2
4
≈
2.01
×
10
8
2.01
×
10
8
1.26
×
10
8
≈
16
So the newly formed, red giant star is almost 16 times more luminous than the sun. This is owing to the increased surface area of the star due to the massively increased radius.
A small sidenote:
There is an equation that might be useful for comparing the radii, temperature and luminosity of main sequence stars. As red giants are not on the main sequence it could not be used here, but if you stumble across a question where they ask you to find the radius, luminosity or temperature given the other two, you can relate it to the sun's characteristics:
r
s
t
a
r
r
s
u
n
=
√
L
s
t
a
r
L
s
u
n
×
(
T
s
u
n
T
s
t
a
r
)
2
(I know, it's not a beauty to look at- but it works)
Where
X
s
u
n
is the radius, temperature, and luminosity of the sun. These are not often given in numerical values, but this equation serves well when asked to find e.g the radius of a star, in solar radii given that a star is twice as luminous and has 5 times the temperature of that of the sun.
Hence:
T
s
t
a
r
=
5
T
s
u
n
L
s
t
a
r
=
2
L
s
u
n
r
s
t
a
r
r
s
u
n
=
√
2
L
s
u
n
L
s
u
n
×
(
T
s
u
n
5
T
s
u
n
)
2
(cancel the common terms)
r
s
t
a
r
r
s
u
n
=
√
2
×
(
1
5
)
2
r
s
t
a
r
≈
0.057
r
s
u
n
(divide both sides by 0.0057)
17.5
r
s
t
a
r
≈
r
s
u
n
So the star's radius is almost 17.5 times that of the sun.
Hopefully, you find this info useful!
Explanation:
The energy we get from the sun is called THERMAL ENERGY and the energy from the Earth is called SOLAR RADIATION.
True
or
False
Answers
The solar radiation that reaches the Earth's surface without being diffused is called direct beam solar radiation. The sum of the diffuse and direct solar radiation is called global solar radiation. Atmospheric conditions can reduce direct beam radiation by 10% on clear, dry days and by 100% during thick, cloudy days.
Brainbreak task:
Write a tweet as if you were Dmitri Ivanovich Mendeleev making his discovery
Answers
Mendeleev sketched out the table he had in mind. Mendeleev created the so-called Periodic Law while assembling these atomic data cards.
What is periodic law?Periodic law is defined as a rule that the elements fall into recurrent groupings when enumerated in order of their atomic numbers, causing elements with similar qualities to appear frequently. "The physical and chemical properties of the elements are periodic functions of their atomic numbers," is how the current Periodic law is best summarized.
Mendeleev found that when all the known chemical elements were arranged in order of increasing atomic weight, the resulting table revealed a periodicity, or repeated pattern, of characteristics within groupings of elements.
Thus, Mendeleev sketched out the table he had in mind. Mendeleev created the so-called Periodic Law while assembling these atomic data cards.
To learn more about periodic law, refer to the link below:
https://brainly.com/question/1258483
#SPJ1