Write a balanced equation for the complete oxidation reaction that occurs when ethane burns in air
Answers
Alkanes are saturated hydrocarbons that have single bonds in chains
General formula for alkanes :
\(\tt \large{\bold{C_nH_{2n+2}}\)
Hydrocarbon combustion reactions (specifically alkanes)
\(\large {\box {\bold{C_nH _ (_2_n _ + _ 2_) + \dfrac {3n + 1} {2} O_2 \Rightarrow nCO_2 + (n + 1) H_2O}}}\)
So that the burning of ethane with air (oxygen):
\(\tt C_2H_6+\dfrac{7}{2}O_2\rightarrow 2CO_2+3H_2O\)
2C₂H₆ (g) + 7O₂ (g) ⟶ 4CO₂ (g) + 6H₂O (ℓ)
or we can use mathematical equations to solve equilibrium chemical equations by giving the coefficients for each compound involved in the reaction
C₂H₆ (g) + aO₂ (g) ⟶ bCO₂ (g) + cH₂O (ℓ)
C : left 2, right b ⇒ b=2
H: left 6, right 2c⇒ 2c=6⇒ c= 3
O : left 2a, right 2b+c⇒ 2a=2b+c⇒2a=2.2+3⇒2a=7⇒a=7/2
Related Questions
how many moles are in 6.4grams of copper
Answers
Answer:
One mole of copper atoms has Avogadro number of copper atoms i.e. 6.022×10
23
atoms.
Given the mass of Copper is 6.4g
Number of Copper atoms in 6.4g =
molar mass
given mass
×Avogadro number
=
63.55
6.4
×6.022×10
23
copper atoms
=6.064×10
22
copper atoms
Explanation:
chloride per milliliter (MW of CaCl2 = 147) [Round to the nearest whole number 5. What weight of magnesium chloride (MgCl2, formula weight = 95.3) is required to prepare 200 ml solution that is 5.0 mi
Answers
The weight of magnesium chloride required to prepare the 200 ml solution that is 5.0 M is approximately 48 grams.
To calculate the weight of magnesium chloride (\(MgCl_{2}\)) required to prepare a 200 ml solution that is 5.0 M, we need to use the formula: Weight (in grams) = Volume (in liters) × Concentration (in moles/liter) × Molecular Weight (in grams/mole)
First, we convert the volume from milliliters to liters by dividing it by 1000: Volume = 200 ml ÷ 1000 = 0.2 L. Next, we multiply the volume, concentration, and molecular weight: Weight = 0.2 L × 5.0 mol/L × 95.3 g/mol = 47.65 grams
Rounding to the nearest whole number, the weight of magnesium chloride required to prepare the 200 ml solution that is 5.0 M is approximately 48 grams.
This calculation ensures that the desired concentration is achieved by accurately measuring the appropriate amount of magnesium chloride, taking into account its molecular weight and the desired volume of the solution.
To know more about magnesium chloride, refer here:
https://brainly.com/question/30671024#
#SPJ11
the general term that describes all organisms that obtain their nutrition by breaking down pre-made organic compounds is
Answers
The general term that describes all organisms that obtain their nutrition by breaking down pre-made organic compounds is heterotrophs.
A heterotroph is an organism that eats other plants or animals for energy and nutrients. The term stems from the Greek words hetero for “other” and trophe for “nourishment.”
Organisms are characterized into two broad categories based upon how they obtain their energy and nutrients: autotrophs and heterotrophs. Autotrophs are known as producers because they are able to make their own food from raw materials and energy. Examples include plants, algae, and some types of bacteria. Heterotrophs are known as consumers because they consume producers or other consumers. Dogs, birds, fish, and humans are all examples of heterotrophs.
To know more about heterotrophs, click here:-
https://brainly.com/question/10618463
#SPJ11
A spectrophotometric method for the analysis of iron has a linear calibration curve for standards of 0. 00, 5. 00, 10. 00, 15. 00, and 20. 00 ppm. An iron ore sample with an expected iron content of 40–60% w/w is to be analyzed by this method. An approximately 0. 5 g sample is taken, dissolved in a minimum of concentrated HCl, and diluted to 1 L in a volumetric flask using distilled water. A 5. 00-mL aliquot is removed with a pipet. To what volume (10, 25, 50, 100, 250, 500, or 1000 mL) should it be diluted to minimize the uncertainty in the analysis? Explain
Answers
To calculate the concentration of the iron sample by using a spectrophotometric method, it is necessary to dilute the sample. The volume to which the sample should be diluted is a crucial question in achieving the most accurate result.
The process involves diluting the sample, and the concentration must be calculated to determine the precise result of the dilution. This question can be answered by calculating the uncertainty and identifying the value of the uncertainty. The value with the lowest uncertainty will be the best value to choose. The volume with the lowest uncertainty will be the ideal volume to dilute the 5 ml aliquot of the iron sample to achieve a result with the minimum level of uncertainty.
To determine the optimal volume for dilution, the uncertainty should be calculated.
This can be done by using the equation for propagation of uncertainty, which states that the uncertainty of the result is equal to the square root of the sum of the squares of the uncertainties of the individual components. When calculating the uncertainty of the diluted sample, the uncertainty of the initial sample and the uncertainty of the diluent must be considered. The uncertainty of the initial sample can be calculated using the calibration curve. As the expected iron content is 40-60%, the concentration of the sample is expected to be 8-12 ppm. The uncertainty of the calibration curve is given by the standard deviation of the calibration standards.
The diluent has a negligible uncertainty. The uncertainty of the diluted sample will be lower if a larger volume is used for dilution because the relative contribution of the uncertainty of the initial sample will decrease. However, the uncertainty of the measurement will increase if the sample is diluted too much because the concentration of the analyte will be too low to be detected accurately. A 100 mL volume is a good choice because it balances the need for sufficient dilution to reduce the uncertainty of the initial sample with the need for sufficient concentration to allow for accurate detection of the analyte.
The volume of the sample that should be diluted is 5 ml. The minimum level of uncertainty is obtained at a dilution of 100 ml. When the volume of the diluent is greater than 100 ml, the uncertainty of the measurement increases, and when the volume of the diluent is less than 100 ml, the uncertainty of the measurement also increases. Thus, a 100 ml volume of diluent is the ideal volume to minimize the uncertainty in the analysis of iron.
to know more about spectrophotometric visit:
brainly.com/question/31632843
#SPJ11
which electron is, on average, further from the nucleus: an electron in a 3p orbital or an electron in a 4p orbital?
Answers
An electron in a 4p orbital is, on average, further from the nucleus than an electron in a 3p orbital. This is because the 4p orbital is larger than the 3p orbital, and so the electron is more likely to be found further away from the nucleus on average.
It is generally accepted that, on average, an electron in a 3p orbital is further from the nucleus than an electron in a 4p orbital. This is due to the fact that the 3p orbitals are larger in size than the 4p orbitals. Additionally, the 3p orbital is closer to the 2p orbital than the 4p orbital, and thus the electron experiences less Coulombic attraction from the nucleus in the 3p orbital.
Hence the electron in a 3p orbital is, on average, further from the nucleus than the electron in a 4p orbital. This is because the 3p orbital is larger than the 4p orbital, and the electron is more likely to be found further away from the nucleus in a larger orbital.
Electrons are one of the three fundamental subatomic particles. Electrons are negatively charged, and are found in atoms. Electrons orbit the nucleus of an atom, and are responsible for the chemical properties of atoms. Electrons are also responsible for the electrical and magnetic properties of atoms and are responsible for electrical dipole present in it .
Learn more about nucleus at : https://brainly.com/question/23366064
#SPJ4
If you create 2.3 L of a 1.5 M solution of hydrochloric acid and react it with excess magnesium hydroxide, how many grams of magnesium chloride can you produce
Answers
You can produce approximately 328.497 grams of magnesium chloride by reacting 2.3 liters of 1.5 M hydrochloric acid with excess magnesium hydroxide.
To determine the number of grams of magnesium chloride that can be produced, we need to first calculate the amount of hydrochloric acid (HCl) in moles, then determine the stoichiometry of the reaction between HCl and magnesium hydroxide (Mg(OH)2), and finally calculate the mass of magnesium chloride (MgCl2) produced.
Given:
Volume of HCl solution = 2.3 L
Molarity of HCl solution = 1.5 M
Step 1: Calculate the moles of HCl
Moles of HCl = Volume (L) × Molarity
Moles of HCl = 2.3 L × 1.5 mol/L
Moles of HCl = 3.45 mol
Step 2: Determine the stoichiometry of the reaction
The balanced chemical equation between HCl and Mg(OH)2 is:
2 HCl + Mg(OH)2 → MgCl2 + 2 H2O
According to the stoichiometry, 2 moles of HCl react with 1 mole of Mg(OH)2 to produce 1 mole of MgCl2.
Step 3: Calculate the mass of MgCl2 produced
Molar mass of MgCl2 = 95.211 g/mol (24.305 g/mol for Mg + 2 × 35.453 g/mol for Cl)
Mass of MgCl2 = Moles of MgCl2 × Molar mass
Mass of MgCl2 = 3.45 mol × 95.211 g/mol
Mass of MgCl2 = 328.497 g
Therefore, you can produce approximately 328.497 grams of magnesium chloride by reacting 2.3 liters of 1.5 M hydrochloric acid with excess magnesium hydroxide.
To know more about Reaction of acids, visit:
https://brainly.com/question/20162424
#SPJ11
How can the location of an electron in an atom be described?
Answers
Answer:
electron are on the outside of the nucleus and form rings
Explanation:
What is the empirical formula for limonene?
Answers
The empirical formula for limonene is C5H8, which represents the simplest whole-number ratio of carbon to hydrogen atoms in the compound. This information can be useful in determining the properties and behavior of limonene in various chemical reactions and applications.
Limonene is a hydrocarbon compound found in the essential oils of citrus fruits. It is a common ingredient in many household and personal care products, and is often used as a flavoring agent in food and beverages. The empirical formula for limonene is a representation of its molecular composition in terms of its simplest whole-number ratio of atoms.
To determine the empirical formula for limonene, we first need to know its molecular formula. The molecular formula for limonene is C10H16, meaning it contains 10 carbon atoms and 16 hydrogen atoms.
The next step is to find the simplest whole-number ratio of carbon to hydrogen atoms in the compound. This can be done by dividing both the carbon and hydrogen atoms by their greatest common factor, which is 2 in this case. Dividing by 2 gives us a ratio of C5H8, which is the empirical formula for limonene.
The empirical formula for limonene, C5H8, tells us the simplest whole-number ratio of carbon to hydrogen atoms in the compound, but it does not provide any information about the arrangement of atoms in the molecule. To understand the molecular structure of limonene, we would need to determine its molecular geometry and bonding arrangement through more advanced chemical analysis techniques.
Here you can learn more about empirical formula
https://brainly.com/question/14044066#
#SPJ11
Can someone please help me ☺️

Answers
Answer:
a)calcium nitrate + hydrogen
b) sodium sulfate+ water.
c)barium chloride +carbon dioxide+hydrogen
d) magnesium phosphate +water
e) chlorine+aluminum = aluminum chloride + water
f) potassium bicarbonate +sulphuric acid=potassium sulfate +water +carbon dioxide

What’s the balanced equation for the reaction between zinc oxide and dilute hydrochloric acid ?
Answers
Answer:
ZnO + 2HCl → ZnCl₂ + H₂O
Explanation:
The unbalanced reaction between zinc oxide (ZnO) and dilute hydrochloric acid (HCl) is:
ZnO + HCl → ZnCl₂ + H₂ONow we'll procede to balance the equation:
There is 1 Cl atom on the left side and 2 on the right, so we change that:ZnO + 2HCl → ZnCl₂ + H₂OThe number of atoms for each element is the same on both sides, so the equation is now balanced.
What components must be present in the atmosphere to create photochemical smog in addition to volatile organic compounds VOCs?
Answers
To create photochemical smog, in addition to volatile organic compounds (VOCs), the presence of nitrogen oxides (NOx) is required. The combination of these two groups of pollutants can lead to the formation of ground-level ozone and other harmful secondary pollutants.
In addition to volatile organic compounds (VOCs), the presence of nitrogen oxides (NOx) is required to create photochemical smog. NOx is a term used to describe the family of nitrogen oxides, which include nitrogen monoxide (NO) and nitrogen dioxide (NO2), both of which are produced mainly from vehicle emissions, industrial processes, and combustion of fossil fuels.
When VOCs and NOx are emitted into the atmosphere and are exposed to sunlight, a series of complex photochemical reactions occur. This can result in the formation of ground-level ozone, a key component of photochemical smog, as well as other harmful secondary pollutants such as peroxyacetyl nitrate (PAN) and aldehydes.
Therefore, to create photochemical smog, the presence of both VOCs and NOx is necessary.
Learn more about photochemical smog here: brainly.com/question/15728274
#SPJ4
describe the three states of matter that are present when snow melts
Answers
Answer:
solid liquid and gas
Explanation:
ice is a solid then it melts to liquid and evaporates into gas
Answer:
Snow is basically water;
ice/snow is solid state
water is liquid state
vapour is gaseous state
Explanation:
Write and solve an equation for the following problem: How many liters of pure acid should be added to 2 liters of 30% concentrate to raise the concentration to 50% ?
Answers
To raise the concentration of a 2-liter solution of 30% acid to 50%, 0.8 liters of pure acid should be added. The amount of acid before and after mixing is equated to find the necessary quantity.
Let us assume that x liters of pure acid should be added. The given 2 liters of 30% concentrate is mixed with x liters of pure acid to raise the concentration to 50%.
According to the question, the amount of acid before mixing is:
2 × 0.3 = 0.6 liters of acid
The amount of acid after mixing is (2 + x) × 0.5
The quantity of acid before mixing is equal to the quantity of acid after mixing, hence
0.6 = (2 + x) × 0.50.6/0.5 = 2 + xx = 1.2 - 2x = -0.8
Therefore, x should be equal to 0.8 liters.
Hence, 0.8 liters of pure acid should be added to 2 liters of 30% concentrate to raise the concentration to 50%.
To know more about concentration, refer to the link below:
https://brainly.com/question/31597130#
#SPJ11
i need help on science lol.

Answers
Answer:
yes your right its is 4
Explanation:
what factors are involved in determining the solubility of an ionic salt
Answers
The solubility of an ionic salt depends on factors such as the nature of the solvent, ion size, charge, temperature, and the presence of common ions, which collectively determine its ability to dissolve in a particular medium.
Several factors play a role in determining the solubility of an ionic salt. These factors include:
1. Nature of the solvent: Different solvents have different polarities and interactions with ions. The solubility of an ionic salt depends on the compatibility between the ions in the salt and the solvent molecules. For example, polar solvents like water tend to dissolve salts that contain ions with strong ionic interactions, while nonpolar solvents may dissolve salts with weaker ionic interactions.
2. Ion size: The size of the ions in the salt affects their ability to interact with the solvent molecules. Smaller ions can more easily fit between solvent molecules and form favorable interactions, increasing solubility. Larger ions may have difficulty fitting into the solvent structure and exhibit lower solubility.
3. Charge of the ions: The charge of the ions influences their attraction or repulsion to the polar solvent molecules. Ions with higher charges can have stronger interactions with the solvent, promoting solubility. Conversely, ions with like charges may repel each other and exhibit lower solubility.
4. Temperature: In general, increasing the temperature can increase the solubility of an ionic salt. Higher temperatures provide more energy for solvent molecules to overcome the attractive forces between ions and dissolve the salt. However, this relationship may vary depending on the specific salt and solvent involved.
5. Presence of common ions: The presence of other ions in the solvent, either from the same salt or other sources, can affect solubility through the principle of common ion effect. When a common ion is present in the solvent, it can reduce the solubility of the salt by shifting the equilibrium in the direction of reduced ionization.
These factors collectively determine the solubility of an ionic salt and influence the extent to which it dissolves in a given solvent.
To know more about the ionic salt refer here :
https://brainly.com/question/30420333#
#SPJ11
If each fission reaction in a nuclear reactor emits three neutrons, how many neutrons are produced from three fission reactions?
Answers
answer:
I think nine
explanation:
Nuclear fission : It is defined as the splitting of bigger nuclei into two smaller nuclei. During this process neutrons and some energy also released.
1. Describe What happens to a hydrogen
atom in an acid when the acid is dissolved
in water?
Answers
Answer:
The H+ atoms will become neutralized by the OH- ions in the water. This is due to the fact that when acids are dissovled in water, they give H+ ions (Hydrogen ions). When the H+ ions from the acid join with the OH- ions in the base (water), they'll become neutralized and form H₂O (Water molecule).
What is the name of the functional group that is attached to this hydrocarbon?
alkyl halide
alcohol
carbonyl
ketone
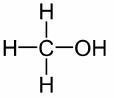
Answers
Answer:
alcohol
Explanation:
Functional group of an organic compound is that part of its structure that contains atoms, which determines its characteristic chemical properties. The functional group is what makes an organic molecule UNIQUE and different from others.
In the structure displayed in the attachment, the functional group is identified as -OH. R-OH is the functional group for ALCOHOLS, which compounds like ethanol, butanol etc fall into. Specifically, the structure in the attachment is METHANOL.
how many protons and neutrons does a silicon atom have
Answers
Silicon (Si) has a total of 14 protons and 14 neutrons in its atomic nucleus. A neutral silicon atom has 14 electrons that balance the 14 positively charged protons in its nucleus.
Silicon is a chemical element with the symbol Si and the atomic number 14. It is a hard and brittle crystalline solid with a blue-grey metallic lustre, and it is classified as a metalloid, which means it has characteristics of both metals and nonmetals. It is a fundamental element of the Earth's crust and is the second most abundant element in it, after oxygen. Silicon is essential for the manufacture of electronic components, such as semiconductors and solar cells. The element's ability to pass electricity makes it useful in the production of electronic circuits. Furthermore, silicon is used to create other materials like silicone and glass, which are used in construction and other applications.
To know more about element visit-
https://brainly.com/question/31950312
#SPJ11
In the Bohr model of the atom, ________both electrons travel in circular paths called orbitals and electron energies are quantized
Answers
In the Bohr model of the atom, electrons travel in circular paths called orbitals, and electron energies are quantized.
The Bohr model of the atom was proposed by Niels Bohr in 1913. According to this model, electrons move in circular paths, also known as orbitals, around the nucleus of an atom. These orbitals have discrete energy levels, and the electrons can only occupy these levels, which are quantized.
The energy of an electron is proportional to the distance between the electron and the nucleus, and electrons can move between energy levels by absorbing or emitting energy in the form of photons. The Bohr model was significant in helping to explain the spectra of atoms and provided a basis for further understanding of atomic structure.
However, it has since been replaced by more complex models, such as the quantum mechanical model, which provide a more accurate description of atomic behavior.
To know more about Bohr model, refer here:
https://brainly.com/question/3964366#
#SPJ11
Which of these changes to a modern lifestyle would promote the
development of more-sustainable practices in the world food system?
O A. Switching from chicken to beef
B. Eating fewer processed foods
O C. Buying tropical fruits
D. Eating fresh vegetables year-round

Answers
Eating fewer processed foods would promote the development of more-sustainable practices in the world food system.
What are Processed food?
This is the food which has undergone some degree of alteration during preparation such addition of preservatives etc.
Eating less of it will ensure that more sustainable practices in the world food system are promoted.
Read more about Processed food here https://brainly.com/question/21201084
#SPJ2
A teacher sprinkles some iron shavings on a clear tray . She places two magnets in the shavings with the north ends facing each other . Based on the cause-and-effect relationship between the magnets, which phenomenon is the teacher modeling for her students ?
Answers
Answer:
Field of repulsion.
Explanation:
An experiment can be defined as an investigation which typically involves the process of manipulating an independent variable (the cause) in order to be able to determine or measure the dependent variable (the effect).
This ultimately implies that, an experiment can be used by scientists to show or demonstrate how a condition causes or gives rise to another i.e cause and effect, influence, behavior, etc in a sample.
Cause and effect can be defined as the relationship between two things or events in which an occurrence one (cause) leads to the occurrence of another (effect).
In this scenario, a teacher sprinkles some iron shavings on a clear tray. She places two magnets in the shavings with the north ends facing each other. Based on the cause-and-effect relationship between the magnets, the phenomenon the teacher is modeling for her students is field of repulsion.
Generally, the two north ends of a magnet have the same polarities and as such are considered to be like charges.
Hence, the field of repulsion is a phenomenon that is based on the fact that like charges repel while unlike charges attract. Thus, the north end of a magnet would repel the north end of another magnet placed beside it.
Find the mass, in grams, of 11.2 L H2 at STP
Answers
11.2L
22.4L/mol
=48g/mol
Note: At s.t.p., 1 mole of any gas occupies a volume of 22.4 L.
Ascorbic Acid is a organic compound with formula C6H8O6, originally called Hexuronic Acid. It's a white solid, but impure samples can be yellowish. It dissolves well in water to give mildly acidic solutions. It is a mild reducing agent.
What is the other name of Ascorbic Acid?
Answers
Explanation:
ascorbic acid is lemon
lemon contain ascorbic acid....
it is organic acid.....
Na2O is it ionic or covalent
Answers
Answer:
Sodium oxide (Na2O) is an ionic compound
Explanation: can i have brain plz!
which of the following require oxygen to grow? group of answer choices facultative anaerobes aerobes anaerobes all of the above
Answers
Aerobes are organisms that require oxygen to grow and survive. The correct answer is: aerobes.
They have metabolic pathways that depend on the presence of oxygen for efficient energy production through aerobic respiration. Without oxygen, aerobes cannot carry out their metabolic processes effectively.
Facultative anaerobes, on the other hand, can grow and survive in the presence or absence of oxygen. They have the ability to switch between aerobic and anaerobic metabolic pathways depending on the availability of oxygen. In the presence of oxygen, facultative anaerobes can utilize aerobic respiration, and in the absence of oxygen, they can switch to anaerobic fermentation.
Anaerobes are organisms that do not require oxygen for growth and can even be inhibited or killed by its presence. They have metabolic pathways that allow them to carry out fermentation or other anaerobic processes for energy production.
Therefore, the correct answer is aerobes, as they specifically require oxygen to grow.
Learn more about Aerobes from the link given below.
https://brainly.com/question/29615844
#SPJ4
32. A toothpick found in a pizza is an example of what type of contamination? a) biological b) chemical c) physical d) cross
Answers
The toothpick found in a pizza is an example of Physical contamination.
Physical contamination is any physical object, such as hair, wood, glass, plastic, or other foreign objects, that contaminates a food item. These contaminants may be brought in by the individuals preparing the food, by machinery, by packaging, or by the food itself. Physical contamination refers to the presence of unwanted or harmful substances or objects in a material or environment. It can occur in various contexts, including food and beverages, manufacturing processes, laboratory settings, and everyday objects. Physical contaminants can pose health risks, compromise product quality, or affect the safety of a particular environment.
Examples of physical contaminants include Hair, Fingernails, Bandages, Jewelry or jewelry parts (such as beads), Broken glass, staples, etc.
Learn more about Physical contamination:
https://brainly.com/question/29550013
#SPJ11
All of the food that we eat, liquids that we drink and medications that we take are
O chemicals.
O vitamins.
O proteins.
O nucleic acids.
O carbohydrates.
HURRY PLEASE!!!!
Answers
A) Chemicals
(I'm pretty sure)
Balance equations H2O-> OH
Answers
Answer: Reaction is already balanced
Explanation: The balanced chemical equation for the reaction H2O -> OH- can be written as:
H2O → OH-
However, this equation is already balanced as there is only one atom of oxygen on each side of the arrow and two atoms of hydrogen on each side of the arrow. There is no need to adjust the coefficients of the reactants or products to balance the equation.
This reaction represents a dissociation reaction, where a molecule of water (H2O) dissociates into a hydroxide ion (OH-) and a hydrogen ion (H+).
brainly.com/question/31373946
You poured out a little (or a lot) too much of a chemical solution. it is ok to pour it back into the bottle.
a) true
b) false
Answers
(b) false
It is not ok to pour it back in the bottle
Once a chemical is taken outside its bottle or container , it reacts with various gases present in the natural environment and also with those chemical which are being released in that laboratory.This will become a source of possible contamination for the entire contents of the stock bottle.The disposal of entire chemicals should be done as per instructed on the reagent bottle to prevent any dangerYou should never put the used spatula inside the reagent bottle.Do not put the excess chemicals inside the sink also , dispose it as it was instructed on the reagent bottle.To know more about chemical safety please refer:
https://brainly.com/question/4430099
#SPJ4