Answers
Answer:
11420.120569
Explanation:
i think idek of its wrong report my answe
Related Questions
129.13 mL of a 112.9 mM solution of NH4l is added to a 105.31 mL solution of 0.87 M Mgl2. What
is the final concentration of I ions in the resulting solution? Express your answer in units of
molarity using at least three significant figures.
Answers
The final concentration of I ions in the resulting solution is approximately 0.0311 M, expressed with three significant figures.
To determine the final concentration of I ions in the resulting solution, we need to consider the stoichiometry and volumes of the solutions being mixed.Given:
Volume of NH4l solution = 129.13 mL
Concentration of NH4l solution = 112.9 mM = 0.1129 M (converting from millimolar to molar)
Volume of Mgl2 solution = 105.31 mL
Concentration of Mgl2 solution = 0.87 M
First, we need to determine the moles of NH4l and Mgl2 in their respective solutions:
Moles of NH4l = Volume of NH4l solution * Concentration of NH4l solution
Moles of NH4l = 0.12913 L * 0.1129 M = 0.01459 moles NH4l
Moles of Mgl2 = Volume of Mgl2 solution * Concentration of Mgl2 solution
Moles of Mgl2 = 0.10531 L * 0.87 M = 0.09157 moles Mgl2
Next, we determine the limiting reagent, which is the reactant that is completely consumed and determines the maximum amount of product formed. In this case, the limiting reagent is NH4l because it has fewer moles than Mgl2.
The balanced chemical equation for the reaction between NH4l and Mgl2 is:
2 NH4l + Mgl2 → 2 NH4+ + MgI2
From the balanced equation, we can see that for every 2 moles of NH4l, we get 1 mole of MgI2.
Since the moles of NH4l is the limiting reagent, it will be completely consumed, and the moles of MgI2 formed will be half of the moles of NH4l.
Moles of MgI2 = 0.01459 moles NH4l * (1 mole MgI2 / 2 moles NH4l) = 0.007295 moles MgI2
Finally, we calculate the final concentration of I ions in the resulting solution:
Volume of resulting solution = Volume of NH4l solution + Volume of Mgl2 solution
Volume of resulting solution = 0.12913 L + 0.10531 L = 0.23444 L
Final concentration of I ions = Moles of MgI2 / Volume of resulting solution
Final concentration of I ions = 0.007295 moles / 0.23444 L = 0.0311 M
Therefore, the final concentration of I ions in the resulting solution is approximately 0.0311 M, expressed with three significant figures.
For more such questions on concentration
https://brainly.com/question/17251833
#SPJ8
Emperical formula of carbon dioxide
Answers
Empirical formula of Carbon dioxide :
\( \mathrm{CO_2}\)A container holds 40.0 mL of nitrogen at 30° C and at a constant pressure.
Find its volume if the temperature increases to 80° C?
Answers
Answer:
The correct answer is - 46.60 mL.
Explanation:
To find the volume of the gas at its new increased temperature we need to use Charl Law that shows the direct relationship between Volume and Temperature while Pressure remains constant.
V1 = 40 ml
T1 = 30 degree C + 273 = 303 K
V2 = ?
T2 = 80 degree C + 273 = 353 K
Charl Equation is:
V 1/T 1 = V 2/ T 2
(V1) * (T2)/ T1= V2
placing value:
40*353/303 = V2
= 14120/303
Vf = 46.60 mL
List and explain the five main characteristics of a mineral
Answers
Answer:
Most minerals can be characterized and classified by their unique physical properties: hardness, luster, color, streak, specific gravity, cleavage, fracture, and tenacity. Minerals Are Natural. You must find minerals in nature; substances concocted in laboratories don't qualify. ...
Minerals Are Inorganic. ...
Minerals Are Solids. ...
Definite Chemical Composition. ...
Crystalline Structure.
Which two actions are examples of science influencing technology?A. Using the internet to publish and share new scientific discoveriesB. Using research about kingfishers to design a more efficient high-speed trainC. Studying how infections spread through the body in order tobetter understand bacteriaD. Using observations about plant seed structures to develop hook-and-loop fasteners
Answers
The two actions that are examples of science influencing technology are:
A) Using the internet to publish and share new scientific discoveries.
D) Using observations about plant seed structures to develop hook-and-loop fasteners. Option A and D
These examples illustrate how scientific advancements and knowledge have influenced the development and application of technology.
Using the internet to publish and share new scientific discoveries is a prime example of science influencing technology. The internet has revolutionized the way scientific information is disseminated, allowing researchers to publish their findings rapidly and share them globally.
This has facilitated collaboration, accelerated the spread of scientific knowledge, and enhanced the efficiency of scientific communication. The internet has also given rise to platforms for open-access journals and online scientific communities, enabling greater accessibility and the democratization of scientific information.
Using observations about plant seed structures to develop hook-and-loop fasteners is another instance of science influencing technology. Scientists studying the natural world have observed the mechanisms by which certain plants disperse their seeds.
One such example is the observation of burrs or burdock plants that use tiny hooks to attach to animal fur or clothing. Inspired by these observations, scientists developed hook-and-loop fasteners, commonly known as Velcro, which mimics the natural hooking mechanism of the plant seeds.
This technology has found widespread applications in various fields, including clothing, aerospace, and medicine, offering a versatile and efficient fastening solution.
These examples highlight the reciprocal relationship between science and technology, where scientific discoveries and knowledge contribute to technological advancements, while technological developments, in turn, provide new tools and capabilities for scientific research and exploration.
For more such question on scientific discoveries visit:
https://brainly.com/question/28207278
#SPJ8
30 Points! Plz help
If you know that a ∝ b and a ∝ c, then you can also say that a ∝ bc, or the product of b and c. Take the above three proportionalities (including V ∝ n) and combine them into a single proportionality in the form: V ∝ ? Show your work below.

Answers
Answer:
V «T, 1/P, n (Product of
Temperature, Half of pressure, and Moles)
Explanation: If V o n, V « 1/P, and V T
then, V« T, 1/P, n
What is a spontaneous change?
A. One that occurs when one specific event happens.
B. One that occurs on its own.
C. One that has a random arrangement of particles.
D. One that has an ordered arrangement of particles.
Answers
Answer:B. One that occurs on its own
Explanation:
the gravitational energy of a golf ball at differnt heights is shown in the table below which graph best represent the relationship between the ball's gravitational energy and its height above the ground. I need helplease



Answers
Answer:
A because it's uniform
Answer:
a
Explanation:
salt in soap make the soap stronger
Answers
Answer:
yes correct salt hardens soap
Club soda is an aqueous solution of carbon dioxide. A sample of club soda is titrated with 0.04202M NaOH(aq) according to the reaction equation below:
CO2(aq)+2NaOH(aq)→Na2CO3(aq)
If it takes 32.14 mL of 0.04202M NaOH(aq) to react with a 25.00 mL sample of club soda, what is the concentration of CO2 in club soda (in g/L )?

Answers
The concentration of CO2 in club soda is approximately 1.1964 g/L.
To find the concentration of CO2 in club soda, we need to use the stoichiometry of the reaction and the volume and concentration of the NaOH solution used.
The balanced equation for the reaction is:
CO2(aq) + 2NaOH(aq) → Na2CO3(aq)
From the stoichiometry of the equation, we can see that 1 mole of CO2 reacts with 2 moles of NaOH. Therefore, the moles of CO2 can be calculated using the volume and concentration of NaOH solution used.
Given that 32.14 mL of 0.04202 M NaOH solution was used, we can calculate the moles of NaOH:
moles of NaOH = volume (L) × concentration (M)
moles of NaOH = 32.14 mL × 0.04202 mol/L
moles of NaOH = 0.001351 mol
According to the stoichiometry of the equation, 1 mole of CO2 reacts with 2 moles of NaOH. Therefore, the moles of CO2 can be calculated as:
moles of CO2 = (moles of NaOH) / 2
moles of CO2 = 0.001351 mol / 2
moles of CO2 = 0.0006755 mol
Now, we need to convert the moles of CO2 to grams. The molar mass of CO2 is approximately 44.01 g/mol.
mass of CO2 = moles of CO2 × molar mass of CO2
mass of CO2 = 0.0006755 mol × 44.01 g/mol
mass of CO2 = 0.02979 g
Finally, we need to express the concentration of CO2 in club soda in g/L. We are given that the sample of club soda used is 25.00 mL.
concentration of CO2 = (mass of CO2) / (volume of club soda in L)
concentration of CO2 = 0.02979 g / (25.00 mL × 0.001 L/mL)
concentration of CO2 = 1.1964 g/L
For more such questions on concentration visit:
https://brainly.com/question/28564792
#SPJ8
The total volume required to reach the endpoint of a titration required more than the 50 mL total volume of the buret. An initial volume of 49.17±0.04 mL was delivered, the buret was refilled, and an additional 1.56±0.04 mL was delivered before the endpoint was reached. The titration of a blank solution without the analyte required 0.60±0.04 mL . Calculate the endpoint volume corrected for the blank and its absolute uncertainty. Note: Significant figures are graded for this problem. To avoid rounding errors, do not round your answers until the very end of your calculations. volume: mL ± mL
Answers
Answer:
The answer is "\(\bold{50.42 \pm 0.08}\)".
Explanation:
Overall delivered volume \(= [(49.06 \pm 0.05) + (1.77 \pm 0.05)]\ mL\)
Its blank solution without any of the required analysis \(= (0.41 \pm 0.04)\ mL\)
Compute the volume of the endpoint as follows:
Formula:
\(\text{End point volume = Total Volume delivered - volume required}\)
\(= (49.06 \pm 0.05) + (1.77 \pm 0.05) - (0.41 \pm 0.04) \\\\= (49.06 + 1.77 - 0.41) \pm \ \ (absolute \ \ uncertainty)\)
therefore,
absolute uncertainty \(=\sqrt{(0.05)^2 + (0.05)^2 + (0.04)^2}\)
\(=\sqrt{0.0025 +0.0025 +0.0016} \\ \\=\sqrt{0.0066}\\\\=0.08124\\\)
The Endpoint volume \(= (49.06+1.77-0.41)\pm (0.08124)\)
\(= 50.42 \pm 0.08\)
Therefore, the volume of the endpoint adjusted for the blank is:
\(\bold { = 50.42 \pm 0.08}\)
Gаvе аn example of how energy from the sun gets into your cells
Answers
Answer:
production of vitamin D
A 39.2 g sample of copper took
up the 4.4 cm3 of space.
What is the density of the
copper piece in g/cm3?
[?] g/cm³
![A 39.2 g sample of copper tookup the 4.4 cm3 of space.What is the density of thecopper piece in g/cm3?[?]](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/ZeS4RdGYOgFbIFIgHndFGXhcUwRdTyry.png)
Answers
A 39.2 g sample of copper took up the 4.4 cm³ of space , so the Density of the copper sample is 8.9 g/cm³.
What is density?Density is the ratio of the mass of a substance and the volume occupied by that substance. Density measures how tightly-packed the particles in a substance are the more tightly packed the particles are, the denser the substance will be.
The formula for calculating the density of a substance is given below as:
Density = mass/volume
Given :
The mass of the copper sample = 39.2 g
The volume of the copper sample = 4.4 cm³
Hence, the density of the copper sample will be:
The density of copper sample = 39.2 g/4.4 cm³
The density of copper sample =8.9 g/cm³
Therefore, the density of the copper sample is 8.9 g/cm³
To learn more about density, refer to below link:
https://brainly.com/question/15164682
#SPJ1
Answer: 8.9
Explanation: sample of 39.2 grams sample of copper took up to 4.4 centimeters of space. And then you will get the density of the copper piece in g/cm3.
Intravenous lidocaine therapy is started for a patient. The doctor's order says to add 1.0 grams of lidocaine to 250 mL of I.V. solution and deliver it to the patient at 4.0 mg/min. In this particular I.V., 20. drops = 1.0 mL. What is the flow rate in drops per minute?
Answers
The flow rate of the IV solution in drops per minute is 80 drops/min.
To determine the flow rate in drops per minute, we need to consider the conversion factors and relationships between different units.
First, let's convert the lidocaine dose from grams to milligrams, as the flow rate is given in milligrams per minute:
1 gram = 1000 milligrams
So, 1.0 gram of lidocaine is equal to 1000 milligrams.
Next, we can calculate the total volume of the IV solution in milliliters:
250 mL
To find the flow rate in milligrams per minute, we divide the dose by the total time:
Flow rate = Dose / Time
The dose is 1000 milligrams (1.0 gram) and the time is 1 minute.
Flow rate = 1000 mg / 1 min = 1000 mg/min
Now, to determine the flow rate in drops per minute, we need to convert the IV solution volume from milliliters to drops. Given that 20 drops = 1.0 mL, we can set up a conversion factor:
20 drops / 1 mL
To find the flow rate in drops per minute, we multiply the flow rate in milligrams per minute by the conversion factor:
Flow rate (drops/min) = Flow rate (mg/min) * Conversion factor
Flow rate (drops/min) = 1000 mg/min * (20 drops / 1 mL)
Now we need to convert milliliters to drops:
Flow rate (drops/min) = 1000 mg/min * (20 drops / 250 mL)
Simplifying the expression:
Flow rate (drops/min) = 1000 mg/min * (4/50)
Flow rate (drops/min) = 80 drops/min
For more such question on flow rate visit;
https://brainly.com/question/1154328
#SPJ8
6. Observe the reaction below and choose the best answer which completes the
reaction.
C-C=C + HOH ===> ?
(will you be able to determine the answer?)
Answers
Answer:
The answer to this reaction would be C-C-OH + H2.
How many moles of He are in a
container with a volume
of 5.6 L at STP?

Answers
Mole measure the number of elementary entities of a given substance that are present in a given sample. Therefore, 0.25moles of He are in a container with a volume of 5.6 L at STP.
What is mole?The SI unit of amount of substance in chemistry is mole. The mole is used to measure the quantity or amount of substance. We know one mole of any element contains 6.022×10²³ atoms which is also called Avogadro number.
One mole of ideal gas has 22.4L volume at STP.
number of moles of Helium = Given volume ÷volume occupied by gas at STP
Given volume of helium = 5.6 L
Substituting all the given values, we get
number of moles of Helium = 5.6 L ÷22.4L
number of moles of Helium =0.25moles
Therefore, 0.25moles of He are in a container with a volume of 5.6 L at STP.
To know more about mole, here:
https://brainly.com/question/15209553
#SPJ1
Answer: 0.25 moles
Explanation:
Given volume of helium = 5.6 L
Substituting all the given values, we get
number of moles of Helium = 5.6 L ÷22.4L
number of moles of Helium =0.25moles
Therefore, 0.25moles of He are in a container with a volume of 5.6 L at STP.
Name the nine processes in the rock cycle.
Answers
The rock cycle involves changing the three types of rock (igneous, sedimentary and metamorphic) from one to another.
------------------------------------------------------------------------------------------------------------
1) WeatheringIt is the breaking of rocks into smaller fragments. This can occur in a few different ways.
Onion skin weatheringThis occurs when a rock's surface is warmed by the Sun during the day and cooled at night. As a result, the surface starts to expand and contract and eventually separates, much like an onion peel
Freeze-thaw weatheringWater expands as it freezes. This could expand the crack if it occurs in a rock crack. Several cycles of freezing and thawing cause pieces to separate.
------------------------------------------------------------------------------------------------------------
2) ErosionIt involves the wearing down of rocks. For example, by rain.
------------------------------------------------------------------------------------------------------------
3) TransportationIt is the process of spreading the eroded rock fragments across the globe, primarily using wind and water.
------------------------------------------------------------------------------------------------------------
4) DepositionIt is the laying down of sediment.
------------------------------------------------------------------------------------------------------------
5) Burial/Compression/CementationThe layers are squeezed and compressed, finally forming sedimentary rocks.
------------------------------------------------------------------------------------------------------------
6) Heat/PressureThe process that transforms rock into metamorphic rocks involves more crushing and heating
------------------------------------------------------------------------------------------------------------
7) MeltingThe rock partially melts and transforms into lava because of the intense heating.
------------------------------------------------------------------------------------------------------------
8) CoolingIt is the process by which melted rock solidifies to produce igneous rocks.
-----------------------------------------------------------------------------------------------------------
9) ExposureBack to erosion and weathering again. (Despite being weathered away, the amount of rock on the surface is always, about the same.)
-----------------------------------------------------------------------------------------------------------
Scientific notation.

Answers
Answer:
1.85 x 10^ -5
Explanation:
The vaporization of Br2 from the liquid to the gas state requires 7.4 kcal/mol. write a reaction showing heat as a product or reactant
Answers
Answer:
Br₂(l) + ΔV → Br₂(g)
Explanation:
When a chemical or phase change occurs during a chemical process some heat is absorbed or released. For the process of vaporization of a substance, the heat (Usually required) is ΔV (How many energy is required for the process occurs).
In the vaporization of Br2 there are required 7.4kcal/mol, Δv. The reaction is:
Br₂(l) + ΔV → Br₂(g)Draw the structure of the alkene with the molecular formula C6H10 that reacts with Br2 to give this compound.
Answers
Answer: Please, this question is not complete. I have attached the complete question.
The answer is in the attached picture below
Explanation:
The explanation is in the attached picture below


The structure of the alkene with the molecular formula \(C_6H_1_0\) that reacts with \(Br_2\)to give this compound is an alkene called 1-hexene
How do we explain?The alkene is called 1-hexene. It has a double bond between the first and second carbon atoms. When it reacts with Br2, the bromine atoms add to the double bond, resulting in the formation of 1,2-dibromohexane.
The reaction is a radical addition reaction. The first step is the formation of a radical by the homolytic cleavage of one of the bromine atoms in Br2. This radical then adds to the double bond in the alkene, forming a new radical. The second bromine atom then adds to the radical, forming 1,2-dibromohexane
Learn more about addition reaction at:
https://brainly.com/question/1433809
#SPJ6

Pure magnesium metal is often found as ribbons and can easily burn in the presence of oxygen. When 3.09 g of magnesium
ribbon burns with 8.75 g of oxygen, a bright, white light and a white, powdery product are formed.
Enter the balanced chemical equation for this reaction. Be sure to include all physical states.
equation:
What is the limiting reactant?
magnesium
oxygen
If the percent yield for the reaction is 90.3%, how many grams of product were
formed?
How many grams of the excess reactant remain?
Answers
Answer:
the answer is: Pure magnesium metal is often found as ribbons and can easily burn in the presence of oxygen. When 3.51 g of magnesium ribbon burns with 8.50 g of oxygen, a bright, white light and a white, powdery product are formed
In any food web, the organisms that are responsible for converting raw energy into usable chemical energy are collectively called ________, while organisms
that recycle the nutrients trapped in dead organisms are collectively called ________.
A) producers; consumers.
B) producers; decomposers.
C) heterotrophs; consumers.
D) heterotrophs; autotrophs.
Answers
Answer:
B. Producers and decomposers
Explanation:
Producers aka plants convert raw energy into chemical energy.
Decomposers are responsible for decomposing dead organisms.
Answer:
the answer is b
Explanation:
Because it provides for the restoration of the life cycle
Using Boyle's Law solve the following: An unknown gas has a volume of 200.0 mL and a pressure of 350.0 torr, pressure were increased to 700.0 torr, what is the resulting volume?
Answers
Answer:
400 mL
Explanation:
Boyle's Law: \(P_1*V_1 = P_2*V_2\)
Let x = the resulting volume
350 (200) = 700 (x)
x = 400 mL
40 g of ice at 0 °C and 80 g water at 40 oC are mixed thoroughly, the temperature of the mixture will be
Answers
The temperature of the mixture will be approximately 32°C.
To determine the final temperature of the mixture, we can use the principle of conservation of energy. The energy gained or lost by a substance can be calculated using the equation:
Q = m * c * ΔT
where:
Q = heat energy gained or lost
m = mass of the substance
c = specific heat capacity of the substance
ΔT = change in temperature
Let's calculate the heat energy gained or lost by each component separately and then equate them to find the final temperature.
For ice:
m_ice = 40 g
c_ice = 2.09 J/g°C (specific heat capacity of ice)
ΔT_ice = final temperature - 0°C (change in temperature)
Q_ice = m_ice * c_ice * ΔT_ice
For water:
m_water = 80 g
c_water = 4.18 J/g°C (specific heat capacity of water)
ΔT_water = final temperature - 40°C (change in temperature)
Q_water = m_water * c_water * ΔT_water
According to the principle of conservation of energy, the heat lost by the water will be equal to the heat gained by the ice:
Q_water = -Q_ice
Now let's substitute the respective values and solve for the final temperature:
m_water * c_water * ΔT_water = -m_ice * c_ice * ΔT_ice
80 g * 4.18 J/g°C * (final temperature - 40°C) = -40 g * 2.09 J/g°C * (final temperature - 0°C)
Simplifying the equation:
334.4 * (final temperature - 40) = -83.6 * final temperature
334.4 * final temperature - 13376 = -83.6 * final temperature
334.4 * final temperature + 83.6 * final temperature = 13376
418 * final temperature = 13376
final temperature = 13376 / 418
final temperature ≈ 32°C
Therefore, the temperature of the mixture will be approximately 32°C.
To learn more about conserconservation of energy from the given link
https://brainly.com/question/27422874
https://brainly.com/question/166559
Question 4 (4 points)
(01.03 MC)
An energy transformation flow diagram is shown.
X-
ELECTRICAL
ENERGY
What type of energy does X most likely represent? (4 points)
O a
X = gravitational energy
Oь
X = mechanical energy
Ос
= thermal energy
Od
X = radiant energy
Answers
Answer:
I think radiant I’m not sure
Explanation:
ntsttoncionsGiven the reaction:N2 (g) + 3 H2(g) -Check ALL that apply.2 NH3(g)Decomposition ReactionDouble Replacement ReactionNeutralization ReactionSynthesis/Combination ReactionPrecipitation ReactionSingle Replacement ReactionRedox Reaction
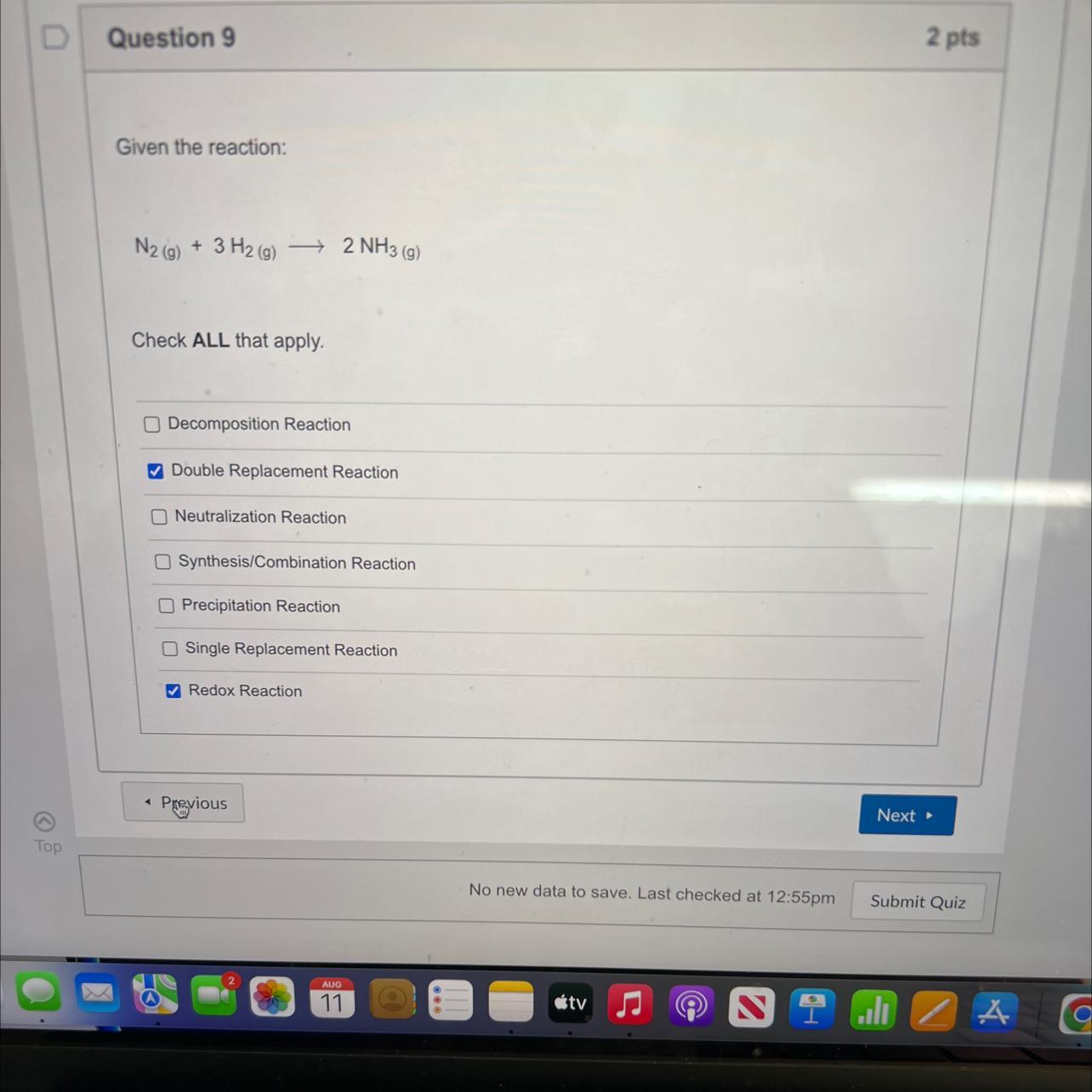
Answers
Explanation:
In the given reaction, a gas reacts with another gas to produce a gas. This indicates that this is a synthesis reaction. And the reaction exhibits redox reaction properties as nitrogen gains a hydrogen.
Answer:
Synthesis/combination reaction
Redox reaction
LAST TIME, I WILL AWARD BRAINLIEST TO WHOEVER ANSWERS ALL THE QUESTIONS RIGHT!!!!

Answers
Answer: Millard Reaction
Explanation:The yummy process, called the Maillard reaction, packs the cookie with riche taste
What did the Constitutional Convention decide to do about the slave trade?(1 point)
Responses
It expanded it.
It expanded it.
It restricted it to slave states.
It restricted it to slave states.
It banned it.
It banned it.
It delayed taking action.
Answers
2 C2H6 + 7O2 --> 4CO2 + 6H2O
What is the UNSIMPLIFIED molar ratio used to convert H2O into CO2?
Answers
Based on the equation of the reaction, the unsimplified molar ratio used to convert H₂O into CO₂ is:
moles of CO₂ = moles of H₂O * 4/6
What is the molar ratio of a reaction?The molar ratio of a reaction is the ratio in which the mole of the reactants combines to form products.
The molar ratio of a reaction can be expressed as the molar ratio of reactants and reactants, reactants and products, or products and products.
The molar ratio of a reaction is obtained from the balanced equation of the reaction.
Considering the given reaction;
equation of reaction: 2 C₂H₆ + 7 O₂ ----> 4 CO₂ + 6 H₂O
The molar ratio of the products carbon dioxide, CO₂ and water, H₂O is 4 : 6.
This means that in the given reaction of the combustion of ethane, for every 4 moles of carbon dioxide formed, 6 moles of water will be formed.
Hence the conversion factor to convert moles of H₂O to moles of CO₂ is:
moles of CO₂ = moles of H₂O * 4/6
Learn more about molar ratio at: https://brainly.com/question/19099163
#SPJ1
Will y’all find this answer real quick for me !!

Answers
The mass (in grams) of sodium carbonate, Na₂CO₃ required to produce 85 grams of barium carbonate, BaCO₃ is 45.7 grams
How do i determine the mass of sodium carbonate, Na₂CO₃ required?First, we shall observe the balanced equation to obtain useful information. This is shown below:
Na₂CO₃ + BaSO₄ -> BaCO₃ + Na₂SO₄
Molar mass of BaCO₃ = 197 g/molMass of BaCO₃ from the balanced equation = 1 × 197 = 197 g Molar mass of Na₂CO₃ = 106 g/molMass of Na₂CO₃ from the balanced equation = 1 × 106 = 106 gFrom the balanced equation above,
197 g of BaCO₃ were obtained from 106 g of Na₂CO₃
With the above information, we can obtain the mass of sodium carbonate, Na₂CO₃ required to produce 85 grams of barium carbonate, BaCO₃. This is illustrated below:
From the balanced equation above,
197 g of BaCO₃ were obtained from 106 g of Na₂CO₃
Therefore,
85 g of BaCO₃ will be obtain from = (85 × 106) / 197 = 45.7 g of Na₂CO₃
Thus, we can conclude from the above calculation that the mass of sodium carbonate, Na₂CO₃ required is 45.7 grams
Learn more about mass needed:
https://brainly.com/question/29263739
#SPJ1