Why might it be important to test one variable at a time when doing an experiment?
Answers
Answer:
If you change everything at once, you may not know what caused the end result.
Explanation:
If you have Variable A (Salt), Variable B (Potassium), and the Control of Water, you might mix everything together, so you mix salt, potassium and water together. It is known that Potassium reacts very aggressively in water, but since you mixed everything together, you might think that salt+potassium+water goes boom, while it is actually potassium+water=boom.
Related Questions
How many moles are on a 7.0 cm x 10.0 cm sheet of 1.0 mm thick aluminum foil? The density of the material is 2.702 g/mL.
Answers
The number of mole present in the aluminum foil, given that the foil has a thickness of 1.0 mm is 0.7 mole
How do I determine the number of mole?We'll begin by obtaining the mass of the aluminum foil. Details below:
Density of aluminum = 2.702 g/mLDimension = 7 cm × 10 cm × 1 mm = 7 cm × 10 cm × 0.1 cmVolume of aluminum = 7 cm × 10 cm × 0.1 cm = 7 cm³ = 7 mLMass of aluminum =?Density = mass / volume
Cross multiply
Mass = Density × Volume
Mass of aluminum = 2.702 × 7
Mass of aluminum = 18.914 g
Finally, we shall determine the number of mole present. Details below:
Mass of aluminum = 18.914 gMolar mass of aluminum = 27 g/mol Number of mole of aluminum =?Mole = mass / molar mass
Number of mole of aluminum = 18.914 / 27
Number of mole of aluminum = 0.7 mole
Thus, the number of mole is 0.7 mole
Learn more about mole:
https://brainly.com/question/13314627
#SPJ1
please answer fast!! very easy! correct answer will get 30 points!

Answers
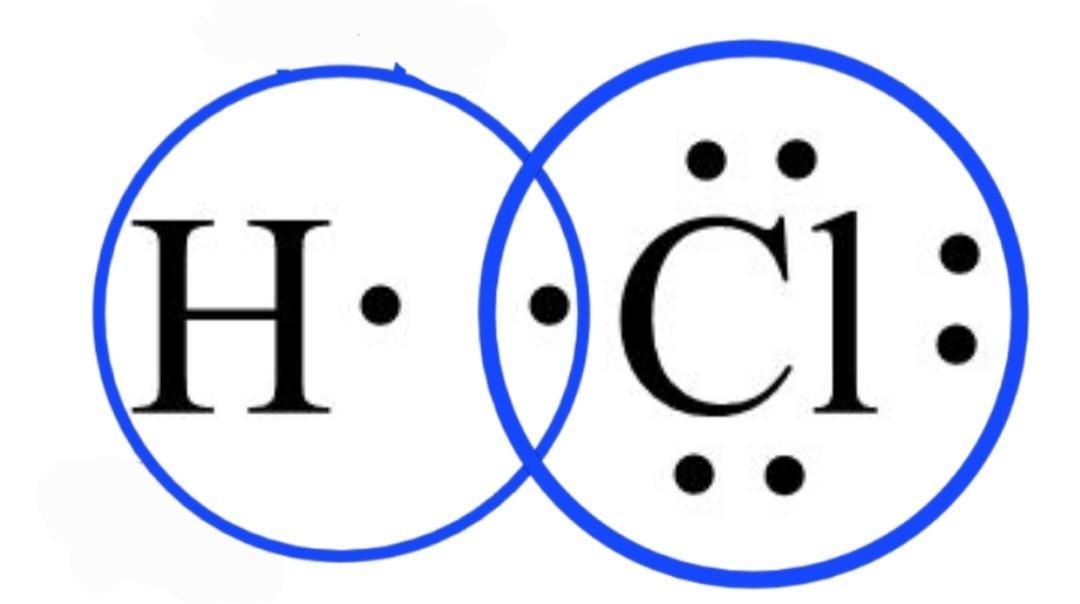
The electron-dot formula of the hydrogen chloride HCl is shown in the attached diagram below.
What is the lewis electron dot diagram?A lewis electron dot structure can be used to express the number of bonds, the bonding atoms, and the lone pairs remaining in the atoms in the molecule.
Lines are represented between atoms that are bonded with each other and excess electrons or lone pairs are shown as dot pairs and are placed next to the atoms.
As the valence electrons in the chlorine atom are equal to 7 from the electronic configuration of the chlorine atom. First, the total number of valence electrons in the HCl molecule is 7 + 1 = 8.
A chlorine atom requires only one electron to complete its octet configuration. As the octet completes, the rest of the electrons of Cl are assigned as the lone pairs of the Cl atom. Therefore, the chlorine atom has 3 lone pairs of electrons on it and the hydrogen atom completes its duplet.
Learn more about the lewis dot diagram, here:
brainly.com/question/14091821
#SPJ1
a sample of carbon contains 54.3 g. to three significant figures, this sample contains:
Answers
A sample of carbon contains 54.3 g. to three significant figures, this sample contains moles of carbon. Option a is correct.
To determine the number of moles in the given sample, we can use the formula:
moles = mass / molar mass
The molar mass of carbon (C) is 12.01 g/mol.
Student question: A sample of carbon contains 54.3 g. To three significant figures, this sample contains:
Write down the given mass of carbon and its molar mass.
mass = 54.3 g
molar mass = 12.01 g/mol
Calculate the number of moles using the formula:
moles = mass / molar mass
Substitute the values and solve:
moles = 54.3 g / 12.01 g/mol = 4.52 moles (to three significant figures)
So, the sample contains 4.52 moles of carbon.
For more such questions on carbon, click on:
https://brainly.com/question/5296530
#SPJ11
Probable question would be
A sample of carbon contains 54.3 g. to three significant figures, this sample contains mol of ?
a. carbon
b. oxygen
c. molar
d. atom
What two types of matter are closest in
density? How do you know?
ings.
Answers
Answer:
Density: The molecules of a liquid are packed relatively close together. Consequently, liquids are much denser than gases. The density of a liquid is typically about the same as the density of the solid state of the substance.
In a gas, the distance between molecules, whether monatomic or polyatomic, is very large compared with the size of the molecules; thus gases have a low density and are highly compressible. In contrast, the molecules in liquids are very close together, with essentially no empty space between them
I hope it helps you
Which describes the amplitude of a wave when it carries more energy?
It is higher.
It is lower.
It is darker.
It is lighter.
Answers
Explaining
I think that’s the answer I had a quiz in it
Answer:
it’s higher
Explanation:
i had already had mine up but someone erased it somehow
In ICD-10-CM, what type of burn is considered corrosion? a) Sunburn b) Burn from a fire c) Burn from a hot appliance d) Burn from a chemical.
Answers
The type of burn considered corrosion in ICD-10-CM is burn from a chemical. The correct option is d).
ICD-10-CM is a medical classification system used to code and classify diagnoses and procedures in the United States. The codes are used by healthcare providers and insurance companies to accurately identify and bill for medical services. In the context of burn injuries, the system classifies burns by their type and severity.
Corrosion burns are caused by exposure to chemicals that damage the skin and underlying tissues. These types of burns are classified as thermal burns in ICD-10-CM because they cause similar types of tissue damage as burns from fire or hot appliances.
However, they are distinguished from other types of thermal burns by the fact that they are caused by chemical exposure rather than heat.
Sunburn is a type of radiation burn, which is another category of burn injury. In ICD-10-CM, burns are further classified by their depth and extent, which can affect the severity of the injury and the recommended course of treatment. Therefore, option d) is a correct answer.
To know more about Sunburn refer here:
https://brainly.com/question/26609703#
#SPJ11
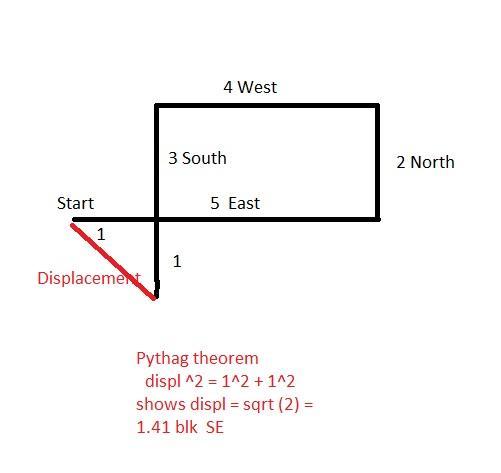
What is the total displacement of a student who walks 5 blocks East,2 blocks north,4 blocks west, and then 3 blocks south?
Answers
Explanation:
See image
what is hard water and soft water
Answers
Answer:
Hard water is water that has high mineral content, Soft water is free from dissolved salts of such metals as calcium, iron, or magnesium
Explanation:
A chemical change by definition involves a reaction that rearranges the atoms of one or more substances, resulting in a change in their chemical properties or “make-up,” forming at least one NEW substance. From your own experience, what is one thing that you observed that underwent a change chemically? Explain why it would be classified under chemical change.
Answers
Answer:
Explanation:
Rust used to be a very common reaction. Iron was used in autos and they formed oxides of iron or rust as the auto or truck aged. You can still see this in older vehicles, especially those used in the country.
To make wine from grapes, the grapes need to ferment which is a process where the grape just changes to alcohol or sometimes vinegar, depending on how the grapes ferment. You can certainly tell the difference between grape juice, wine an vinegar when you taste them. A chemical reaction has taken place.
B2H6+3O2 -> 2HBO2+2H2O If you start with 10.0 of each reactant, what mass of water will you produce?
Answers
From the equation, we can see that for every 1 mole of B2H6 reacted, 2 moles of H2O are produced. We can use this relationship to determine the amount of water produced from the given amount of B2H6.
What is the mass of water will you produce?The balanced chemical equation for the reaction is:
\(B_2H_6 + 3O_2 \rightarrow 2HBO_2 + 2H_2O\)
First, we need to calculate the number of moles of \(B_2H_6\) Present in \(10.0\)Grams. The molar mass of \(B_2H_6\) is:
2 × atomic mass of B + 6 × atomic mass of H
\(= 2 × 10.81 + 6 × 1.01\)
\(= 27.64 g/mol\)
So, \(10.0 g\) of \(B_2H_6\) is equivalent to:
\(n(B_2H_6) = m/M\)
\(= 10.0 g / 27.64 g/mol\)
\(= 0.362 mol\)
According to the balanced chemical equation, 1 mole of \(B_2H_6\) reacts to produce 2 moles of \(H_2O\) . So, the number of moles of water produced is:
\(n(H_2O) = 2 × n(B_2H_6)\)
\(= 2 × 0.362 mol\)
\(= 0.724 mol\)
Finally, we can calculate the mass of water produced using its molar mass:
\(m(H_2O) = n(H_2O) × M(H_2O)\)
\(= 0.724 mol × 18.02 g/mol\)
\(= 13.04 g\)
Therefore, if you start with \(10.0 g\) of \(B_2H_6\)and \(10.0 g\) of \(O_2\), you will produce \(13.04 g\) of water.
Learn more about water here:
https://brainly.com/question/26789700
#SPJ1
true or false: radiation can be detected because of its green glow, intense heat, crackling sound and ammonia smell.
Answers
False.
Radiation itself does not typically have a green glow, intense heat, crackling sound, or ammonia smell. These descriptions do not accurately represent the properties of radiation.
The emission of energy in the form of particles or electromagnetic waves is referred to as radiation. Our senses cannot immediately notice it. Radiation is measured and detected using specialized apparatus and detectors.
Alpha particles, beta particles, gamma rays, and X-rays are a few examples of different forms of radiation that have unique characteristics and may be identified with the right tools. For instance, ionizing radiation is typically detected using Geiger-Muller counters or scintillation detectors, whereas radiation exposure is measured using dosimeters.
For precise radiation risk identification and protection, it's crucial to rely on the right detection tools and follow safety procedures.
To know more about radiation:
https://brainly.com/question/31106159
#SPJ4
An object has 15 grams of atoms and displaces 2.5 mL of water when dropped into a cylinder. What is the objects density... HELP IM IN CLASS RN AND I NEED ANSWERS ASAP, grades are due in couple hours
Answers
Answer:
6g/mL
Explanation:
The density, which is the mass per unit volume of an object, can be calculated using the formula;
D= m/v
Where; D = density (g/mL)
M = mass of substance (g)
V = volume (mL)
In this question, the object's mass = 15g, volume of water= 2.5 mL, d =?
Therefore,
Density = 15/2.5
Density = 6 g/mL
The object's density is 6g/mL
the base protonation constant of 1-h-imidazole () is . calculate the ph of a solution of 1-h-imidazole at . round your answer to decimal place.
Answers
To calculate the pH of a solution of 1-H-imidazole (C3H4N2) at a given concentration, we need to consider the protonation equilibrium of the imidazole molecule. The protonation constant (Ka) of 1-H-imidazole is given as 1.0 × 10^-7.
The protonation equilibrium can be represented as follows:
C3H4N2 + H2O ⇌ C3H4N2H+ + OH-
At equilibrium, the concentrations of the species can be related using the equilibrium constant expression:
Ka = [C3H4N2H+][OH-] / [C3H4N2]
Since we are given the value of Ka and want to find the pH, we can assume that [OH-] is negligible compared to [H+] and simplify the equilibrium expression:
Ka ≈ [C3H4N2H+][H+] / [C3H4N2]
Since the concentration of [OH-] is negligible, [H+] is approximately equal to [C3H4N2H+].
Now, we can rearrange the equation and solve for [H+]:
Ka = [H+]^2 / [C3H4N2]
[H+]^2 = Ka × [C3H4N2]
[H+] = sqrt(Ka × [C3H4N2])
Substituting the given values:
[H+] = sqrt(1.0 × 10^-7 × [C3H4N2])
At a pH of 7, the concentration of [H+] is 1.0 × 10^-7 M. Therefore, we can set up the following equation:
1.0 × 10^-7 = sqrt(1.0 × 10^-7 × [C3H4N2])
1.0 × 10^-7 = 1.0 × 10^-7 × sqrt([C3H4N2])
Solving for [C3H4N2]:sqrt([C3H4N2]) = 1
[C3H4N2] = 1
Therefore, the concentration of 1-H-imidazole is 1.0 M.The pH of a 1-H-imidazole solution with a concentration of 1.0 M at 25°C is approximately 7.0 (neutral pH).
To know more about equilibrium, click here https://brainly.com/question/30624359
#SPJ11
SiS is what type of mineral?
Answers
why was thiosulfate, s2o32−, added to the reaction mixture in the kinetic lab?
Answers
Adding thiosulfate, S2O32−, to the reaction mixture in the kinetic lab was to stop the reaction and preserve the concentration of the reactants at a specific time.
In the kinetic lab, the reaction mixture contained an iodine solution and a thiosulfate solution. The addition of thiosulfate solution stops the reaction by reducing the concentration of iodine to a negligible level, thereby preserving the concentration of the reactants at that specific moment. This is important for determining the rate of reaction, which can be calculated from the changes in reactant concentrations over time. Without stopping the reaction, it would be difficult to determine the exact concentration of the reactants at a given time, and the rate of reaction would be inaccurate.
In the context of molecular biology, DNA transcription is the process by which the genetic information encoded in DNA is transcribed into RNA. This process involves the enzyme RNA polymerase, which synthesizes an RNA molecule that is complementary to the DNA template strand. The RNA molecule produced during transcription is known as messenger RNA (mRNA), which carries the genetic information from the DNA in the nucleus to the ribosomes in the cytoplasm where it is translated into protein.
DNA transcription is an essential process for gene expression, which refers to the process by which the information contained within a gene is used to direct the synthesis of a functional gene product. The process of transcription is highly regulated in cells to ensure that genes are expressed at the appropriate times and in the appropriate cells, and defects in transcriptional regulation can lead to a variety of diseases, including cancer. Understanding the molecular mechanisms underlying transcription is therefore critical for understanding both normal and pathological cellular processes.
To know more about DNA transcription.
https://brainly.com/question/22181919
#SPJ11
11. An alloy contains 62 % by mass of aluminum and 38% by mass of unknown element .If 10.0
grams of this alloy has a volume 4.20 cm³ ,use the table of density below to identify the
unknown element in the alloy.
Element
Density g/cm³
(A) Beryllium
Copper
8.96
Aluminum
2.70
(B) Copper
Beryllium
1.85
(C) Iron
Iron
7.87
(D) Silver
Silver
10.49
Answers
Based on the given information and the densities provided in the table, the unknown element in the alloy is most likely Beryllium. option(a)
To identify the unknown element in the alloy, we need to compare the density of the alloy with the densities of the elements listed in the table.
The density of the alloy can be calculated using the given information. We know that 10.0 grams of the alloy has a volume of 4.20 cm³. Density is defined as mass divided by volume, so we can calculate the density of the alloy as:
Density = Mass / Volume = 10.0 g / 4.20 cm³ ≈ 2.38 g/cm³
Now, we compare the calculated density of the alloy (2.38 g/cm³) with the densities listed in the table. From the given options, the closest density is that of aluminum, which is 2.70 g/cm³. The alloy's density is lower than the density of aluminum, which means it must contain an element with a lower density than aluminum.
The unknown element in the alloy is most likely Beryllium (option A) with a density of 1.85 g/cm³. The combination of 62% aluminum and 38% beryllium in the alloy would result in a density close to the calculated value of 2.38 g/cm³. option(a)
For such more questions on densities
https://brainly.com/question/1749900
#SPJ8
the salt obtained from the combination of the weak acid hydrogen peroxide, h2o2, and the weak base ammonia, nh3, is used to make an aqueous solution. is the solution acidic, basic, or neutral?
Answers
The solution obtained from the salt of hydrogen peroxide and ammonia is slightly basic.
The salt obtained from the combination of the weak acid hydrogen peroxide (H2O2) and the weak base ammonia (NH3) is ammonium peroxide (NH4)2O2. To determine the acidity or basicity of the resulting solution, we need to consider the nature of the ions present in the salt.
Ammonium (NH4+) is a weak acid, as it can donate a proton (H+) in water. Peroxide (O2^2-) is a strong base, as it can accept a proton (H+) from water. When ammonium peroxide dissolves in water, it dissociates into ammonium ions (NH4+) and peroxide ions (O2^2-).
The ammonium ion (NH4+) can slightly acidify the solution by donating protons (H+) to water, resulting in the formation of hydronium ions (H3O+). On the other hand, the peroxide ion (O2^2-) can slightly increase the pH by accepting protons (H+) from water, leading to the formation of hydroxide ions (OH^-).
Overall, the presence of ammonium peroxide in water will make the solution slightly basic. However, the exact pH of the solution would depend on the concentrations of the salt and other factors like temperature.
Learn more about solution from the given link:
https://brainly.com/question/25326161
#SPJ11
Which of the following combinations of peaks appears in the 1H NMR spectrum of diethyl ether, CH3CH2OCH2CH3? a. a triplet and a doublet b. a quartet and a sextet c. two singlets d. a triplet and a quartet Which of the following combinations of peaks appears in the 1H NMR spectrum of 1,2-dimethyoxyethane, CH3OCH2CH2OCH3? a. two singlets b. a singlet and a triplet c. a singlet and two triplets d. a doublet and a triplet Please explain!?
Answers
the 1H NMR spectrum of diethyl ether would display a triplet and a quartet, while the spectrum of 1,2-dimethoxyethane would exhibit a singlet and two triplets (option C).
Diethyl ether contains three types of hydrogen atoms: two methyl groups (CH3), one ethyl group (CH2CH3), and one methylene group (CH2) in the ether linkage.
The methyl group (CH3) adjacent to the ether oxygen (O) will appear as a triplet due to coupling with two equivalent neighboring hydrogens. The coupling constant (J) for this triplet will typically be around 7 Hz.
The methylene group (CH2) adjacent to the ether oxygen (O) will appear as a quartet due to coupling with three equivalent neighboring hydrogens. The coupling constant (J) for this quartet will typically be around 7 Hz as well.
The ethyl group (CH2CH3) in the diethyl ether molecule is not adjacent to the ether oxygen (O) and is therefore not coupled to it. Hence, it will appear as a singlet.
Thus, the correct answer is (d) a triplet and a quartet.
In the case of 1,2-dimethoxyethane (CH3OCH2CH2OCH3), the 1H NMR spectrum would display a combination of peaks consisting of a singlet and two triplets. Here's the explanation:
The methoxy group (-OCH3) on one end of the molecule will appear as a singlet since it is not coupled to any other hydrogens in the molecule.
The methylene group (-CH2-) adjacent to the ether oxygen (O) will appear as a triplet due to coupling with two equivalent neighboring hydrogens. The coupling constant (J) for this triplet will typically be around 7 Hz.
The methylene group (-CH2-) on the other end of the molecule, also adjacent to the ether oxygen (O), will also appear as a triplet due to coupling with two equivalent neighboring hydrogens. Again, the coupling constant (J) for this triplet will typically be around 7 Hz.
Therefore, the correct answer is (c) a singlet and two triplets.
In summary, the 1H NMR spectrum of diethyl ether would display a triplet and a quartet, while the spectrum of 1,2-dimethoxyethane would exhibit a singlet and two triplets.
To learn more about spectrum, visit
https://brainly.com/question/30138070
#SPJ11
what is the stoichiometry for the cobalt (iil) glycinate complex? explain the thinking behind having the conoentration of glycinate be more than 4 times greater than the concentration of cobalt ion
Answers
Glycinate donates an electron pair so it is a bidentate ligand.
The molecular formula is C₂H₄NO₂⁻. The octahedral complex is formed between glycinate molecules and cobalt(III) and the stoichiometry of the complex is [Co(gly)₃]. The reaction is as follows;
Co₃⁺(aq) + 3C₂H₄NO₂⁻ ⇒ [Co(C₂H₄NO₂⁻](aq)
A cobalt complex is formed when 3 glycinate ions equivalents react with one Co₃⁺ ion equivalent so, it is necessary to keep the glycinate ions concentration greater than the cobalt(III) ions at least three times more.
So, taking the concentration 4 times greater can facilitate the reaction.
For a complex whose concentration is 0.015M, 0.06M glycinate ions are required to obtain the desired cobalt(III) glycinate complex.
You can learn more about glycinate complex from the following answer;
https://brainly.com/question/28285145
#SPJ4
If someone could help that’d be nice, if not that’s okay
Answers
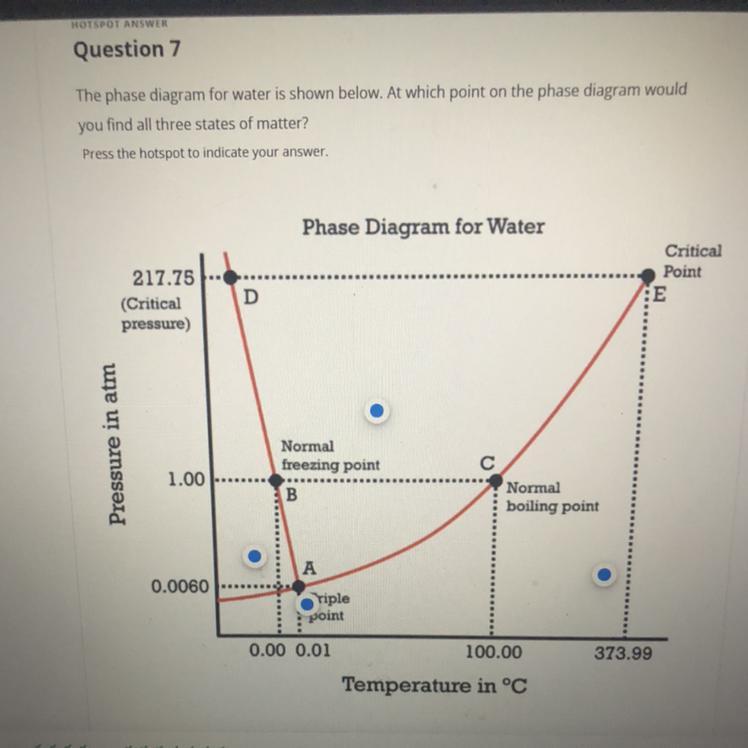
The point on phase diagram of water where all three states of matter can be found is point A, that is Triple point.
At what point on phase diagram of water would you find all three states of matter?Triple point is found on the phase diagram where all the three lines of equilibrium between the states of matter converge. Triple point is a temperature and pressure combination and at this point, all the three states of solid, liquid and gas exist simultaneously.
Triple point of water is the only temperature at which water can exist in all the three states of matter; solid (ice), liquid (water), and gas (water vapor) and this temperature is 0.01°C.
To know more about Triple point, refer
https://brainly.com/question/2402164
#SPJ1
name two bases which react with dillute sulfuric acid to give zinc sulfate
Answers
Answer:
Zinc Carbonate
Zinc Nitrate
Explanation:
On a job application how should you list the schools you attended
Answers
Answer:
Usually, you should lay down your educational background by listing the most recent or advanced degree first, working in reverse chronological order. But there are exceptions. Say you earned a degree in geography, but are now working in the field of online marketing. If you more recently completed coursework specific to social media or digital marketing, list that first to grab the reviewer’s attention.
Explanation:
Answer:
Start with the most recent or advanced degree first, working in reverse chronological order.
Explanation:
I need help with this one please it’s science

Answers
Answer: A change in appearance. (Please say thanks!)
Explanation:
The biggest sign of a chemical change is change in appearance.
Answer:
d
Explanation:
Which of the following is NOT a benefit of renewable energy? *
O generates power on smaller scale
O can decrease pollution
O combats climate change
Answers
Answer:
O generates power on a smaller scale
Explanation:
The other two options are benefits of renewable energy. You're looking for a con instead of a pro.
If the normal physiological concentration of hco3− is 24 mm , what is the ph of blood if pco2 drops to 35.0 mmhg ?
Answers
The pH of blood if pco2 drops to 35.0 mmhg is 7.459
The pH of the blood can be calculated using the Henderson- Hasselbalch equation, it explains the relation between acid dissociation constant pKa and pH in biological and chemical systems.
pH = pK + log ( HCO3- / ( 0.03 * PCO2 ) )
pK is 6.1 for bicarbonate buffer system.
HCO3- = 24mm
PCO2 = 35.0 mmhg
pH = 6.1 + log ( 24 / 0.03 * 35.0 )
= 6.1 + log ( 24 / 1.05 )
= 6.1 + log 22.8571
= 6.1 + 1.3590
= 7.459
Hence, the pH of blood is 7.459
Learn more about pH of blood on
https://brainly.com/question/3919636
#SPJ1
Give one paragraph of what the word Xenocryst mean?
Answers
Answer:
Explanation:
Xenocryst is a crystal in an igneous rock that is not derived from the original magma. This means that its origin is from a rock so itʻll be something thats on a rock that has a crystal base.
i hope this helps!
Choose the product of the aldol condensation reaction that occurs when the following compound is heated with sodium hydroxide. To NaOH, heat Н. of H H OH A) H OH B) H I D) H ОА В Ос D
Answers
The correct answer is option (A) H OH, which represents the product obtained after the loss of a water molecule from the intermediate formed during the aldol condensation reaction.
The given compound is a beta-hydroxy aldehyde. When heated with a base like sodium hydroxide, an aldol condensation reaction can occur where the aldehyde group can react with the hydroxyl group to form a new carbon-carbon bond and eliminate a water molecule.
The product of this reaction depends on which carbon of the aldehyde group reacts with which carbon of the hydroxyl group. In this case, the aldehyde group and hydroxyl group are on adjacent carbon atoms, so intramolecular aldol condensation is possible.
The reaction mechanism involves the formation of an enolate ion intermediate, followed by nucleophilic attack of the enolate on the carbonyl carbon of the aldehyde. The resulting intermediate then undergoes elimination of a water molecule to form a new carbon-carbon bond.
To know more about "Aldehyde" refer here:
https://brainly.com/question/30722723#
#SPJ11
geologists can estimate the age of rocks by their uranium-238 content. the uranium is incorporated in the rock as it hardens and then decays with first-order kinetics and a half-life of 4.5 billion years. a rock is found to contain 82.8�.8% of the amount of uranium-238 that it contained when it was formed. (the amount that the rock contained when it was formed can be deduced from the presence of the decay products of u-238u-238.)
Answers
The age of rocks can be estimated by measuring the uranium-238 content. Uranium-238 is incorporated into rocks as they form, and it decays over time with first-order kinetics. The half-life of uranium-238 is 4.5 billion years., geologists can estimate the age of rocks based on their uranium-238 content.
To estimate the age of a rock, geologists analyze the amount of uranium-238 present in the rock and compare it to the amount that the rock contained when it was formed. This initial amount can be deduced from the presence of the decay products of uranium-23Let's use an example to illustrate how this estimation works:
Suppose a rock is found to contain 82.8% of the amount of uranium-238 that it contained when it was formed - Since uranium-238 has a half-life of 4.5 billion years, we can use the formula:
To know more about uranium-238 visit :-
https://brainly.com/question/24285205
#SPJ11
Which set correctly orders the atoms from HIGHEST to LOWEST ionization energy?

Answers
Answer:
Option D
Explanation:
Ionization energy increases left to right in a period and decreases top to bottom in a groups.
Ar is in Group 13
S is in Group 15
P is in Group 16
Al is in Group 18
They are all in the same period so decide by the group numbers if left is the highest (group 18) and right (group 13) is the lowest.
The order: Ar, S, P, Al
Hope this is clear. Good luck with chemistry! :)
What is the median of the following set: 2, 4, 6, 8, and 10?
Answers
Answer:9
Explanation:
(3+8+10+15)/4 = 36/4 = 9