Why is John Dalton known as the originator of atomic theory?
Answers
Answer:
he thought of atoms first
Answer:
He was the first to be the one to propose that matter is made out of atoms. He also proved that the atoms are divisible in nature.
Related Questions
Do chemical formulas have charge even though they may be made from ions
Answers
Density is defined as
Responses
A volume/massvolume/mass
B length to mass ratiolength to mass ratio
C mass to length ratiomass to length ratio
D mass/volumemass
Answers
Answer:
D. Density is defined as mass/volume.
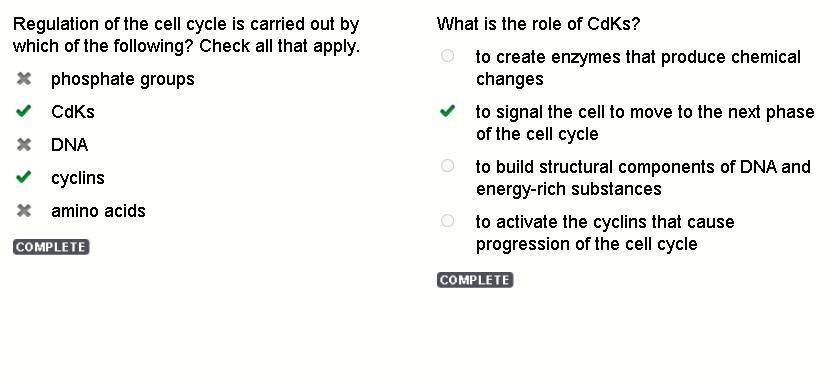
What is the role of CdKs? to create enzymes that produce chemical changes to signal the cell to move to the next phase of the cell cycle to build structural components of DNA and energy-rich substances to activate the cyclins that cause progression of the cell cycle
Answers
Answer:
It’s b
Explanation:
Answer:
Regulation of the cell cycle is carried out by which of the following? Check all that apply.
CdKs
cyclins
What is the role of CdKs?
to signal the cell to move to the next phase of the cell cycle
Explanation:
Water from jordan lake was analyzed for its fe3+ content. A 20. 0-ml sample of lake water was acidified with nitric acid and treated with excess kscn to form a red complex (kscn itself is colorless). The solution was then diluted to 50. 0-ml and put in a 1. 00 cm pathlength cell, where it yielded an absorbance of 0. 345. For comparison, a 5. 0-ml reference sample of 4. 80 x 10-4 m fe3+ was treated with hno3 and kscn and diluted to 50. 0 ml. The reference solution was also placed in a 1. 00-cm cell and gave an absorbance of 0. 512. What is the concentration of fe3+ in jordan lake?.
Answers
The concentration of Fe³⁺ in Jordan Lake is = 8.09 × 10⁻⁵ M
What is Lambert-Beer law?The Beer-Lambert Law, also known as Beer's Law, Lambert-Beer Law, or Beer-Lambert-Bouguer Law, relates the attenuation of light to the properties of the material through which the light passes.
According to Lambert-Beer's law, the absorbance of a sample is directly proportional to its concentration.
The reaction that produces a red complex
Fe³⁺ + KScN ⇒ Fe ( SCN )₃ ( red complex )
Concentration of Fe³⁺ in reference sample
= 4.80x10⁻⁴ × ( 5.0 / 50.0 ) = 4.80 × 10⁻⁵M
Reference sample was diluted from 5.0 mL to 50.0 mL
Concentration of 4.80 × 10⁻⁵M has an absorbance = 0.512
Given that Lake sample absorbance is 0.345
Concentration of lake sample :
= absorbance of lake sample × ( conc of reference sample / absorbance )
= 0.345 × (4.80* 10⁻⁵ / 0.512) = 3.23 × 10⁻⁵M.
Concentration of Fe³⁺ in Jordan lake
= 3.23 × 10⁻⁵ × ( 50.0mL / 20.0mL) = 8.09 × 10⁻⁵ M
Solution was diluted from 20.0 mL to 50.0 mL
Hence we can conclude that The concentration of Fe³⁺ in Jordan Lake is = 8.09 × 10⁻⁵ M.
To know more about Lambert-Beer law visit:
https://brainly.com/question/24183759
#SPJ4
a buffer is formed by adding 1.0 mol of methylamine, ch3nh2, and 1.0 mol of methylammonium, ch3nh3 , in a 1.0 l container. if a 10.0 ml sample of this buffer is diluted to 1000 ml with water, what is the ph of the diluted buffer? for methylamine, kb
Answers
If a 10.0 ml sample of this buffer is diluted to 1000 ml with water,the ph of the diluted buffer is 9.26.
pH of Buffer Solution can be calculated as :
pH = pKa - log ( [ Salt ] / [ Base ] )
Since Kb = 1.8 x 10-5
then , pKb = 4.74
Since , pKa + pKb = 14 \Rightarrow pKa = 9.26
Initial concentration of NH3 and (NH4)+ is 1 M.
Now 10 mL of the sample is taken and diluted to 1000 mL
Since , M1V1 = M2V2
Where M1 is the Molarity of the solution before dilution and V1 is the volume taken ( M1 = 1 M ; V1 = 10 mL)
M2 is the Molarity of the solution after dilution and V2 ( V2 =1000 mL ) is the Final volume of the solution.
M2 = M1V1 / V2 \Rightarrow M2 = ( 1 x 10 ) / 1000 = 0.01 M .
Final concentration of NH3 and (NH4)+ in the sample is 0.01 M.
then , [ Salt ] / [ Base ] = 1
Since , log( 1 ) =0
Then pH = pKa = 9.26
The pH of the Buffer solution is 9.26.
Learn more about Buffer here:
https://brainly.com/question/22821585
#SPJ4
Spacing between atoms in a crystal is on the same order as the de Broglie wavelength of accelerated electrons.
A) observation a
B) observation b
C) observation c
D) observation d
E) observation e
The answer is D but I dont know why and I don't understand the question clearly
Answers
The de Broglie connection between both the momentum p or the wavelength of an electron (=h/p, h is Hubble constant) is used to calculate the wavelength of the an electron for a given power.
What is the accelerated electron formula?The following equation gives the kinetic energy of the an electron accelerated thru a voltage differential of V volts: where e is indeed the electron charge and 1/2 mv2 = eV (1.6x10-19 C) If you want to respond to this question, you'll need to know the electron charge as well as the Planck constant.
What is electron accelerated motion?Yet, a speeding electron creates a shifting magnetic field, which in turn causes a changing electromagnetic current, which in turn causes a shifting magnetic field, etc. Or to put it another way, it produces an electromagnetic wave.
To know more about wavelength visit:
https://brainly.com/question/31143857
#SPJ1
Consider the half reactions below for a chemical reaction.
ZnZn2+ (aq) + 2e
Cu?" (aq) + 2e → Cu(s)
What is the overall equation for this chemical reaction?
Zn(s)+ Cu?* (aq) —>Zn2+ (aq) + Cu(s)
O Zn(s) + Cu2+ (aq) — Cu2+ (aq) + 2e-
O Zn2*(aq) + Cu(s) —> Cu2* (aq) + Zn(s)
O Zn2+ (aq) + 22 —> Cu2(aq) + 2e
Answers
Answer:
Option A:
Zn(s) + Cu^(2+) (aq) → Cu(s) + Zn^(2+)(aq)
Explanation:
The half reactions given are:
Zn(s) → Zn^(2+)(aq) + 2e^(-)
Cu^(2+) (aq) + 2e^(-) → Cu(s)
From the given half reactions, we can see that in the first one, Zn undergoes oxidation to produce Zn^(2+).
While in the second half reaction, Cu^(2+) is reduced to Cu.
Thus, for the overall reaction, we will add both half reactions to get;
Zn(s) + Cu^(2+) (aq) + 2e^(-) → Cu(s) + Zn^(2+)(aq) + 2e^(-)
2e^(-) will cancel out to give us;
Zn(s) + Cu^(2+) (aq) → Cu(s) + Zn^(2+)(aq)
uses activity-based costing. The company produces 001 and 002. Information relating to the two products is as follows: 001 002 Units produced 19,000 25,000 Machine-hours 7,500 8,500 Direct labor-hours 8,000 12,000 Materials handling (number of moves) 4,000 6,000 Setups 5,000 7,000 The following costs are reported: Materials handling $160,000 Labor-related overhead 280,000 Setups 240,000 Labor-related overhead costs assigned to product 001 are: Select one: A. $112,000 B. $224,000 C. $192,000 D. $232,000
Answers
The labor-related overhead costs assigned to product 001 are $224,000. To calculate the labor-related overhead costs assigned to product 001 using activity-based costing, we need to allocate the overhead costs based on the activity drivers for each product.
In this case, the activity drivers are machine-hours, direct labor-hours, materials handling moves, and setups. First, we need to determine the overhead cost per unit for each activity driver. We do this by dividing the total overhead cost for each activity driver by the total units produced for each product.For product 001: Overhead cost per unit for machine-hours = Labor-related overhead cost / Total machine-hours Overhead cost per unit for direct labor-hours = Labor-related overhead cost / Total direct labor-hours Overhead cost per unit for materials handling moves = Labor-related overhead cost / Total materials handling moves Overhead cost per unit for setups = Labor-related overhead cost / Total setups Next, we multiply the overhead cost per unit for each activity driver by the respective activity driver amount for product 001. Finally, we sum up the costs calculated for each activity driver to obtain the total labor-related overhead costs assigned to product 001. By performing these calculations, the labor-related overhead costs assigned to product 001 amount to $224,000.
Learn more about labor-related here;
https://brainly.com/question/15279902
#SPJ11
5. A certain radio wave has a wavelength of 7 Find the frequency of this radio wave.
Answers
In the electromagnetic spectrum, radio waves are a form of electromagnetic radiation having wavelength longer than infrared light.
Describe a spectrum?
The characteristic frequencies of electromagnetic waves (or a subset of it) that are released or absorbed by a material, atom, or molecule are known as a spectrum.The range of colours that appear on a display when white light goes thru a prism and separates into its individual colours is known as the spectrum. Vi (V), indigo (1), blues (B), greenish (E), yellow (X), oranges (O), and red are the colours of the rainbow (R).
Describe a prism ?
A prism is a transparent glass piece or another material that has been cut with exact angles on plane faces for use in reflecting and analysing light in optics a typical triangle
To know more about spectrum visits :
brainly.com/question/6836691
#SPJ1
A metal ball has a mass of 6 kg and a volume of 42 cubic meters. What is its density
Answers
Answer:
0.143kg/m^3
Explanation:
density= mass / volume
Answer:
1/7 kg/cubic metres
Explanation:
Here, Density=mass /volume
=6/42
=1/7 kg/cubic metres.
On Greg's last visit to his doctor, he complained of feeling tired. Sandra, the registered nurse, withdrew 6.5 mL of blood, which was sent to the lab and tested for iron. The normal range for serum iron in men is 80 to 160 mcg/dL. Greg's iron test showed a blood serum iron level of 39 mcg/dL, How many micrograms of iron were in the 6.5-mL sample of Greg's blood?
Answers
Answer:
2.5 mcg of iron
Explanation:
Density is given as;
D= m/v
Where;
m= mass
v= volume
mass= ?
volume= 6.5 ml or 0.065 dl
d= 39 mcg/dL
m= density × volume
m= 39 mcg/dL × 0.065 dl
m= 2.5 mcg of iron
would a mix of ki and na2so3 perform the same reduction and precipitation of copper(ii) as a mix of nai and na2so3 ?
Answers
No, a mixture of KI and Na2SO3 would not perform the same reduction and precipitation of copper(II) as a mixture of NaI and Na2SO3. This is because the reducing power of the two mixtures is different due to the different nature of the cations (K+ vs Na+).
What is Reduction?
Reduction is a chemical process in which a substance gains electrons and undergoes a decrease in oxidation state. It is a key aspect of many chemical reactions, including combustion, corrosion, and the synthesis of organic compounds.
In the reduction of copper(II) to copper(I), iodide ion (I-) acts as the reducing agent, which gets oxidized to iodine (I2). In the presence of sodium sulfite (Na2SO3), the iodine reacts with sulfite ion (SO32-) to form iodide ion and sulfate ion (SO42-), while copper(I) ions get precipitated as copper(I) sulfite.
Learn more about Reduction
https://brainly.com/question/13892498
#SPJ1
when copper metal is heated it reacts with a gas in the air . what iz the name of the product formed when copper reacts with a gas in the air ?
Answers
Answer:
Heated copper metal reacts with oxygen to form the black copper oxide. The copper oxide can then react with the hydrogen gas to form the copper metal and water.
Answer:
answer-COMPOUND COPPER OXIDE
what relative masses of dimethyl amine and dimethyl ammonium chloride do you need to prepare a buffer solution of ph = 10.54?
Answers
To prepare a buffer solution of pH = 10.54, the relative masses of dimethyl amine and dimethyl ammonium chloride needed are 0.079 g and 0.067 g respectively.
A buffer solution is a solution that has the ability to resist changes in pH upon the addition of small amounts of acid or base. A buffer solution contains a weak acid and its conjugate base or a weak base and its conjugate acid. It can be prepared by mixing equal volumes of a weak acid and its conjugate base or a weak base and its conjugate acid.
Dimethyl amine is an organic compound with the formula (CH3)2NH. It is a weak base and can act as a proton acceptor. Dimethyl ammonium chloride is an organic compound with the formula (CH3)2NH2Cl. It is a salt of a weak base and a strong acid and can act as a proton donor.
Calculation of relative masses:
The pKa of dimethyl amine is 10.73.
To prepare a buffer solution of pH = 10.54,
the ratio of [A-]/[HA] should be 1/9.
Using the Henderson-Hasselbalch equation;
pH = pKa + log([A-]/[HA])10.54 = 10.73 + log([A-]/[HA])
log([A-]/[HA]) = -0.19[A-]/[HA] = 0.67/1.00
The sum of the masses of dimethyl amine and dimethyl ammonium chloride is 0.146 g. The ratio of their masses is 0.67:1.00.
So, the relative masses of dimethyl amine and dimethyl ammonium chloride needed are 0.079 g and 0.067 g respectively.
To know more about Buffer solution refer here :
https://brainly.com/question/13076037
#SPJ11
14 What is the greenhouse gas among the following list? a. Carbon Dioxide b. Nitrogen c. Oxygen d. Argon
Answers
The greenhouse gas among the following list of gases is a. Carbon Dioxide. Carbon dioxide (CO2) is a greenhouse gas. It is produced by burning fossil fuels (coal, oil, and natural gas) for energy. I
It is also produced by the decomposition of organic matter and other chemical reactions. Carbon dioxide, methane, and nitrous oxide are the three primary greenhouse gases. Carbon dioxide is the most important greenhouse gas among them. Other greenhouse gases include water vapor, ozone, and some industrial gases.The term "greenhouse gas" refers to any gas that traps heat in the atmosphere. These gases contribute to global warming by allowing sunlight to enter the atmosphere but preventing the heat that is generated from leaving it. As a result, the Earth's temperature rises, resulting in climate change.
Know more about greenhouse gas here: https://brainly.com/question/30674591
#SPJ11
What is the approximate concentration of reaction product in a solution that has an absorbance of 0.7 at ph 6.0?
Answers
The approximate concentration of reaction product in a solution that has an absorbance of 0.7 at ph 6.0 is based on the equation A = εbc given in the passage, the concentration c is the absorbance A divided by the absorptivity ε in a 1 cm path length cell. 0.7 divided by approximately 1400 gives 500 μM.
A = εbc
0.7 = 1400 x c
7/14000 = 1/2000 = 0.0005 = 5x10^-4 = 500 μM
1 μM = 1 x10 ^-6
The concentration of a substance is the amount of solute present in a given volume of solution. Concentration is usually expressed in molarity, defined as the number of solutes in 1 L of solution. Solution concentration is a measure of the amount of solute dissolved in a given amount of solvent or solution.
Concentrated solutions are solutions that contain relatively large amounts of solutes. Dilute solutions are solutions that contain relatively small amounts of solutes. The definition of concentration means the amount of an ingredient or part relative to other ingredients or parts. An example of concentration is the amount of salt to water in a brine solution.
Learn more about concentration here
https://brainly.com/question/23437000
#SPJ4
Where are hormones produced in animals, and how are they transported throughout the body?
A. Animal hormones are produced in endocrine glands and travel through the blood.
B. Animal hormones are produced in lymph nodes and travel through the lymphatic system.
C. Animal hormones are produced inside the bones and travel through the nervous system.
D. Animal hormones are produced in sweat glands and travel through the skin.
Answers
Answer:
Vertebrate Endocrine Glands and Hormones
Unlike plant hormones, animal hormones are often (though not always) produced in specialized hormone-synthesizing glands (shown below). The hormones are then secreted from the glands into the blood stream, where they are transported throughout the body.
What is the purpose of adding base in the aldol condensation reaction?.
Answers
The purpose of adding base in the aldol condensation reaction is to generate the enolate ion, which is a strong nucleophile and is critical for the reaction to take place. The enolate ion attacks the carbonyl group of another molecule, resulting in the formation of a carbon-carbon bond.
In the aldol condensation reaction, the base serves two primary functions. Firstly, it generates the enolate ion, which is an essential intermediate for the reaction. Secondly, the base helps to remove the alpha hydrogen atom from the carbonyl compound, allowing it to become acidic and therefore more susceptible to the removal of the proton. When the base is added to the carbonyl compound, it accepts a proton to form an anion. This anion then attacks the carbonyl group of another molecule, resulting in the formation of a carbon-carbon bond. As a result, the aldol product is formed. The product can either be an α,β-unsaturated carbonyl compound or a β-hydroxy carbonyl compound depending on the reaction conditions.
In summary, the addition of base in the aldol condensation reaction serves to generate the enolate ion, which is a strong nucleophile and is essential for the reaction to occur. It also helps to remove the alpha hydrogen atom from the carbonyl compound, allowing it to become acidic and more reactive.
To know more about aldol condensation , click here
https://brainly.com/question/31558115
#SPJ11
why is lightning categorized as plasma? A. It consists of tightly packed particles B. it has a definite volume. C. it has no definite shape D. it has extremely high levels of energy
Answers
Lightning is categorized as plasma because it has extremely high levels of energy.
When the electrons are freed from their host atoms for a short time, due to high temperatures, it is plasma. An electrical discharge consisting of moving electrons and ions, is lightning. Lightning strikes create plasma via a very strong jolt of electricity.
By the passage of electricity through a gas, the plasma was created. An electrical discharge through air and it ionizes the atoms when this happens. The electrons from the atoms strips and leaves positively charged ions in the gas, is lightning.
Lightning as an example of plasma present at Earth's surface. Plasma temperatures approaches 30000 K and electron densities may exceed 10²⁴ m−³.
To learn more about plasma and lightening,
brainly.com/question/18207038
#SPJ1
The element lithium (Li) has 3 protons and 3 electrons. The element fluorine (F) has 9 protons and 9 electrons. An atom of the element lithium (Li) transfers an electron to an atom of the element fluorine (F). Which type of bond results between the atoms, and what happens to the charges in each of the atoms?
Answers
When an atom of the element lithium (Li) transfers an electron to an atom of the element fluorine (F), then the bond results between the atoms is ionic bond.
what is chemical bond?A chemical bond is defined as the bond which holds atoms together in molecules.
Bonds arise due to the electrostatic forces present between positively charged atomic nuclei and negatively charged electrons.
Types of chemical bondIonic bondCovalent bondCoordinate bondWhat is Ionic bond ?Ionic bond is defined as the transfer of electron from one atom to another atom.
Since, electron transfer from lithium to fluroine. Thus lithium get positive charge and fluorine occupy negative charge.
Thus, the bond form between lithium atom and fluorine atom is ionic bond.
learn more about ionic bond:
https://brainly.com/question/977324
#SPJ1
what is the molarity of a solution made by dissolving 7.50 g of magnesium nitrate in enough water to make 25.0 ml of solution?
Answers
The molarity of a solution made by dissolving 7.50 g of magnesium nitrate in enough water is 2.04 M
To solve this problem, the formulas and the procedures that we have to use are:
M = n(solute)/v(solution) Ln = m / MWMW= ∑ AWTWhere:
M= molarityn = molesm = massv = volumeMW = molecular weightAWT = atomic weightInformation about the problem:
m = 7.50 gv = 25.0 mlM = ?MW Mg(NO₃)₂ = ?AWT(Mg) = 24 g / molAWT(N) = 14 g / molAWT(O) = 16 g / molConverting the volume units from (ml) to (L) we have:
v(solution) = 25.0 ml * (1 L/1000 ml)
v(solution) = 0.025 L
We calculate the moles of the Mg(NO₃)₂ from the MW:
MW = ∑ AWT
MW Mg(NO₃)₂= AWT (Mg) + AWT (N)*2 + AWT (O)*6
MW Mg(NO₃)₂= 24 g/mol + 14 g/mol*(2) + 16 g/mol*(6)
MW Mg(NO₃)₂= 24 g/mol + 28 g/mol + 96 g/mol
MW Mg(NO₃)₂= 148 g/mol
Having the MW we calculate the moles of Mg(NO₃)₂:
n = m / MW
n Mg(NO₃)₂ = 7.50 g / 148 g/mol
n Mg(NO₃)₂ = 0.051 mol
Applying the molarity formula, we get:
M = n(solute)/v(solution) L
M =0.051 mol / 0.025 L
M = 2.04 M
What is a solution?In chemistry a solution is known as a homogeneous mixture of two or more components called:
SolventSoluteLearn more about chemical solution at: brainly.com/question/13182946 and brainly.com/question/25326161
#SPJ4

if you mistakenly extract the solution first with naoh (aq), and then with nahco3(aq), what results you will observe and why?
Answers
The NaOH extraction step would remove some acidic components, while the NaHCO3 extraction step may have limited effect if the significant acidic components have already been neutralized.
If you mistakenly extract a solution first with NaOH (aq) and then with NaHCO3 (aq), you would observe the following results:
NaOH Extraction:
When NaOH (aq) is added to the solution, it will react with acidic components present in the solution, such as carboxylic acids, phenols, or acidic functional groups. This reaction results in the formation of water-soluble salts or compounds, which will dissolve in the aqueous NaOH solution. As a result, the acidic components will be removed from the solution.
NaHCO3 Extraction:
When NaHCO3 (aq) is added to the remaining solution from the previous step, it will react with acidic components that were not neutralized by NaOH. NaHCO3 is a weaker base compared to NaOH and is primarily used to extract acidic compounds such as phenols and carboxylic acids. These acidic components will react with NaHCO3 to form water-soluble salts, which will dissolve in the aqueous NaHCO3 solution.
However, if NaOH is mistakenly used first, it is possible that some acidic components in the solution may have already reacted and been removed in the previous step. Therefore, the NaHCO3 extraction step may not yield significant additional changes or observable results.The results of mistakenly extracting the solution first with NaOH (aq) and then with NaHCO3 (aq) would depend on the nature and concentration of the acidic components present in the solution.
Learn more about carboxylic acids visit:
brainly.com/question/4721247
#SPJ11
Explain why boiling a solution to remove the CO2 doesn’t affect the analysis for carbonate (co3 2- in the solution). The experiment is titration of soda ash.
The other answers on chegg all say that carbonate can't break down into carbon dioxide with heat, but when I look online, it says that all carbonates with heat will eventually decompose into carbon dioxide and other things. Another answer said that the carbon dioxide released will return to the solution when cooled, but I don't think that's right either. Our experiment doesn't mention anything to cover the flask or trap the gas, so if we were just relying on the CO2 cooling and returning, then a majority of the carbonate would not be in the solution and it would affect the analysis.
Answers
Boiling a solution to remove CO2 does not affect the analysis for carbonate (CO3 2-) in the soda ash titration because the carbon dioxide produced during the boiling process escapes from the solution, and the remaining carbonate ions do not undergo any significant decomposition.
When a carbonate solution is boiled, it causes the release of carbon dioxide gas (CO2) due to the thermal decomposition of the carbonate ions. However, in the experimental setup you described, where there is no mechanism to capture or trap the released CO2, the gas will escape from the solution into the surrounding atmosphere. Therefore, the concentration of carbonate ions in the solution remains unchanged.
The decomposition of carbonate ions into CO2 and other products is a slow process, and at the relatively short boiling time during the experiment, the amount of carbonate that decomposes into CO2 is negligible compared to the initial concentration of carbonate ions. Additionally, the CO2 gas released does not return to the solution upon cooling since there is no mechanism to capture it.
Boiling the solution to remove CO2 in the soda ash titration does not affect the analysis for carbonate because the carbonate ions remain in the solution. The boiling process only removes the gaseous CO2, while the remaining carbonate ions are not significantly affected. Therefore, the analysis for carbonate can proceed without concern for loss of carbonate ions during the boiling step.
To know more about thermal decomposition , visit;
https://brainly.com/question/14949019
#SPJ11
Which of the following is a subsurface event that takes place during the rock cycle?
Group of answer choices
Cementing
Erosion
Melting
Weathering
Answers
Answer:
Melting
Explanation:
Could not have been erosion because erosion happens on the surface.
Could not have been weathering because weathering happens on the surface.
So we only have Cementing and Melting left....
The answer is melting, I got 100% on my test.
Hope this helps!!!! :D
Please give brainliest!!!
Answer:
D
Explanation:
In the rock cycle, surface events takes place at the surface of earth. Thus,
weathering and erosion are surface events, while the subsurface events he take place in the deeper section of the earth . Examples of sub surface events are plate tectonics and mountain building or anything that takes place in the inner core of earth.
The age of a piece of wood from an archeological site is to be determined using the Carbon-14 method. The activity of the sample is measured to be 0.407 times the Carbon-14 activity of living plants. What is the age of the sample in years
Answers
The age of the piece of wood from the archeological site, using the Carbon-14 method, is approximately 8,263 years.
How to determine the age of wood using Carbon-14 method?The Carbon-14 method is a radiometric dating technique used to determine the age of organic materials, such as wood, charcoal, and bone, that are up to about 50,000 years old. The method is based on the fact that Carbon-14, a radioactive isotope of Carbon, is formed in the upper atmosphere by cosmic ray bombardment of Nitrogen-14, and it subsequently decays with a half-life of about 5,700 years.
To determine the age of the piece of wood using the Carbon-14 method, we need to use the following formula:
Age = (\(t_{1/2}\) / ln(2)) * ln(\(A_{o}\) / A)
Where:
- Age is the age of the sample in years
- \(t_{1/2}\) is the half-life of Carbon-14, which is 5,730 years
- ln is the natural logarithm function
- \(A_{o}\) is the initial activity of Carbon-14 in living plants (1.0)
- A is the activity of the sample (0.407)
Step 1: Substitute the given values into the formula:
Age = (5730 / ln(2)) * ln(1.0 / 0.407)
Step 2: Calculate the natural logarithms:
Age = (5730 / 0.6931) * ln(2.4549)
Step 3: Calculate the result:
Age ≈ 8263 years
To know more about Carbon-14 method:
https://brainly.com/question/29261279
#SPJ11
what is relative abundance isotopes
Answers
The relative abundance of isotopes is the number of atoms of a particular isotope divide by the total number of atoms of all isotopes of that element, multiplied 100 percent.
What is relative abundance isotopes?The relative abundance of an isotope is the percentage of atoms with a specific atomic mass found in a naturally occurring sample of an element.
Also relative abundances refers to the relative proportions of the stable isotopes of each element. They are most often quoted as atom percentages
To calculate the percent abundance of each isotope in a sample of an element, the number of atoms of a particular isotope is usually divide by the total number of atoms of all isotopes of that element and then multiply the result by 100 since it is expressed in percentage.
Mathematically, the formula for relative abundance is given as;
R.A = ( number of atoms of isotope / total number of atoms ) x 100%
Learn more about relative abundance here: https://brainly.com/question/6844925
#SPJ1
What happens to the number of neutrons as you move across the table?
left to right
Answers
Answer: The number of neutrons will increase as we move from left to right in a periodic table.
Explanation:
Atomic number is equal to the number of protons.
Mass number is the sum of number of neutrons and number of protons.
As we move from left to right, both the atomic number and mass number increases.
For example: As we move from Lithium to berrylium to boron to carbon to nitrogen to oxygen to fluorine to neon , the number of neutrons increase from 4 to 5 to 6 to 6 to 7 to 8 to 10 to 10.
Thus the number of neutrons will also increase as we move from left to right in a periodic table.
what are the structural characteristics of glycophorin that help it to maintain asymmetric orientation
Answers
The structural characteristics of glycophorin that help it to maintain asymmetric orientation is the center of the proteins contains hydrophobic amino acid residue.
The glycophorin A is the type I single membrane protein. the glycophorin plays an important tole in the process of the invasion of the red blood cells by the malaria. glycophorin is the membrane of the red blood cell. the membrane of the spanning protein. glycoprotein of plasma is situated with the sugar residue.
Thus , in the glycophorin a segment in the contains protein residue of 75 to 93. theses are hydrophobic residue of amino acid. it is a transmembrane segment.
To learn more about glycophorin here
https://brainly.com/question/29588507
#SPJ4
For a chemical reaction, given ∆H < 0 and ∆S > 0. Which of the following statement is/are true?I) The reaction is spontaneous at low temperature only.II) As temperature increases, the reaction becomes more spontaneous.III) The reaction is spontaneous at any temperature.
Answers
Statement III is false, as the reaction is spontaneous only for certain temperatures.
The spontaneity of a reaction is determined by the sign of the Gibbs free energy change (∆G). The ∆G = ∆H - T∆S, where ∆H is the enthalpy change and ∆S is the entropy change of the reaction. Thus, if we have ∆H < 0 and ∆S > 0, then ∆G will be negative, indicating a spontaneous reaction. In this case, Statement II is true, as an increase in temperature (T) will make the negative value of ∆G more negative, and thus the reaction will become more spontaneous. Statement I is also true, as a lower temperature would result in a larger positive value of T∆S, which would make the ∆G value more positive, thus rendering the reaction less spontaneous. However, Statement III is false, as the reaction is spontaneous only for certain temperatures.
Learn more about Gibbs free energy change here:
https://brainly.com/question/4002787
#SPJ4
Before considering the effects of radiative heat transfer, you are tasked with investigating the temperature profile and conductive/convective heat transfer aspects of a multi-layered silicon-PV (photovoltaic) solar panel. Currently, most PV solar panels utilize silicon cells to convert photons from the sun into electrons to generate electricity. In order to capture the photos in an energy-efficient manner, solar panels contain several key layers. The silicon cell layer (0.4 mm thick) is coated with ethylene vinyl acetate (0.4 mm) on both the front and back sides; the "top" side is then coated with a thick layer of glass (4 mm), while the "bottom" side is coated with a backing sheet (0.3 mm). To test the convective and conductive heat transfer properties of the panel prior to radiative testing (which will be the focus of Assignment 2), the panel is placed into a testing rig so that the top side is exposed to a range of varying conditions, while the bottom side atmospheric conditions are kept constant at T[infinity]=293 K and convective heat transfer coefficient, h, of 3 W/m2 K. In this assignment, you are tasked with investigating heat transfer through the multi-layered solar panel, investigating effects on the temperature profile of the system, and finally discussing what you expect to happen when the panel is moved into a solar farm. a) If the top side convective heat transfer coefficient (h) is 10 W/m^2 K, and the heat transfer flux in the system is 360 W/m^2, determine the steady-state temperature in the air above the panel. Provide assumptions and references for any data that you need to find for your calculations. b) Using the same conditions and assumptions as per (a) above, determine the interface temperatures throughout the solar panel, and plot the temperature profile as a function of distance through the panel.
Answers
The steady-state temperature in the air above the panel is 233 K.. The temperature of the air above the panel will increase as the heat transfer flux increases. This is because the heat transfer coefficient is constant.
The steady-state temperature in the air above the panel is calculated using the following equation:
T_s = T_∞ - h * q_s
where:
T_s is the steady-state temperature in the air above the panel (K)
T_∞ is the ambient temperature (K)
h is the convective heat transfer coefficient (W/m^2 K)
q_s is the heat transfer flux (W/m^2)
Substituting the given values, we get:
T_s = 293 K - 10 W/m^2 K * 360 W/m^2 = 233 K
When the panel is moved into a solar farm, the ambient temperature will be higher than the ambient temperature in the testing rig. This will cause the temperature of the air above the panel to increase, which will in turn increase the heat transfer rate. The increase in heat transfer rate will cause the temperature of the silicon cell layer to increase. This could potentially damage the silicon cell layer, so it is important to ensure that the panel is designed to operate at the expected ambient temperature.
In addition to the increase in heat transfer rate, the panel will also be exposed to more sunlight in a solar farm. This will increase the amount of heat that is generated in the silicon cell layer. The increase in heat generation will also cause the temperature of the silicon cell layer to increase.
It is important to consider both the increase in heat transfer rate and the increase in heat generation when designing a solar panel for a solar farm. The panel should be designed to operate at the expected ambient temperature and the expected amount of sunlight.
To know more about radiative heat transfer, click here:-
https://brainly.com/question/14221689
#SPJ11