Answers
Strong Acid: dissolves and dissociates 100% to produce protons (H+) 1. seven strong acids: HCl, HBr, HI, HNO3, H2SO4, HClO4, & HClO3 2. ... Weak Acid: dissolves but less than 100% dissociates to produce protons (H+) 1.
Related Questions
Giúp mình 2 câu này với ạ
1, Pha 500ml dung dịch HCl 10% (w/v) từ dung dịch 37% (w/w) d=1.19 g/ml
2, Cách pha như thế nào để nhận được 200g dung dịch HCl 25% (w/w) từ dung dịch HCL 37% (d=1.19 g/ml)
Answers
Answer:it is wrong answer
Explanation:estro man
Which reaction would most likely require the use of an inert electrode
Answers
The reaction that would most likely require the use of an inert electrode is a reaction involving highly reactive substances or species that can react with or be oxidized by the electrode material itself.
One such reaction that often requires the use of an inert electrode is the electrolysis of aqueous solutions containing halide ions (Cl-, Br-, I-). When halide ions are electrolyzed, they can undergo oxidation at the anode, resulting in the formation of halogen gas (Cl2, Br2, I2). However, these halogens are highly reactive and can react with many common electrode materials, such as metals, leading to unwanted side reactions.
To prevent these undesired reactions, an inert electrode, usually made of materials such as platinum, gold, or graphite, is employed. Inert electrodes do not react with the halogen gases formed during electrolysis, allowing the desired reaction to take place without interference from the electrode material itself.
For example, in the electrolysis of a sodium chloride (NaCl) solution, chloride ions (Cl-) can be oxidized at the anode to form chlorine gas (Cl2). To ensure that the chlorine gas is produced without any reactions involving the anode material, an inert electrode like platinum or graphite is used.
For more such questions on oxidized visit:
https://brainly.com/question/13182308
#SPJ8
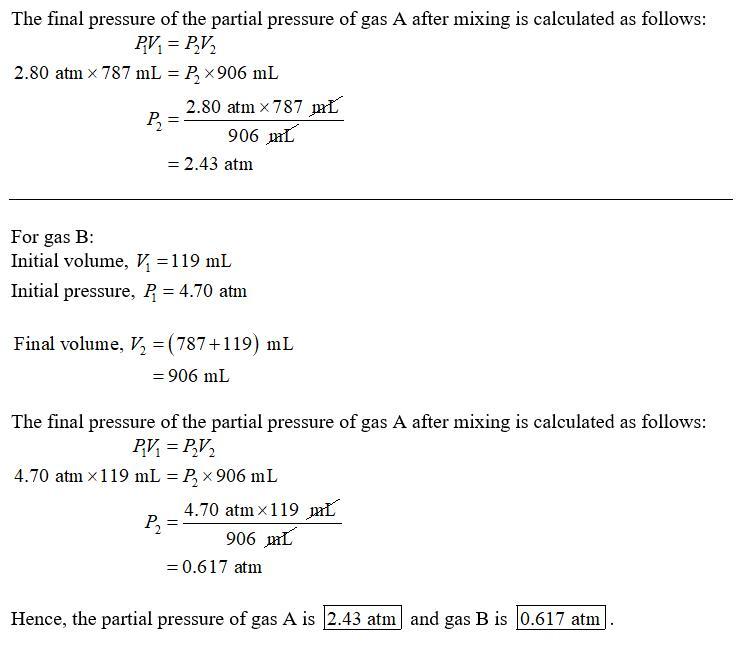
Container A holds 767 mL of an ideal gas at 2.80 atm. Container B holds 154 mL of a different ideal gas at 4.50 atm.
Container A and container B are glass spheres connnected by a tube with a stopcock. Container A is larger than container B.
If the gases are allowed to mix together, what is the resulting pressure?
Answers
Answer:
The answer is in the attachment below
Explanation:
1) How would the electron configuration of nitrogen change to make a stable configuration?
(1 point)
A) It would gain two electrons.
B) It would lose five electrons.
C) It would gain three electrons.
D) It would lose four electrons.
2. Which quantity determines how two atoms bond?(1 point)
A) their total number of valence electrons
B) the difference in their electronegativities
C) the sum of their electronegativities
D) the difference in the number of valence electrons
Answers
Answer:
C and B
Explanation:
1) In order for nitrogen to make a stable electronic configuration, it would have to gain three electrons.
Nitrogen is atomic number 7. Hence, the electronic configuration would be:
1s2, 2s2, 2p3
The p orbital is partially filled with 3 unpaired electrons. In order to be stable, the 3 electrons in the p orbital have to be paired. Hence, only by gaining 3 electrons can nitrogen obtain a stable electronic configuration.
2) The quantity the determines how two atoms would bond is the difference in the number of valence electrons in the atoms.
For example, an atom that is lacking 2 electrons will readily form a bond with another atom that has two free electrons.
More on bond formation can be found here; https://brainly.com/question/12937609
is the liter value for titration molarity calculation the difference between the final and inital values
Answers
NaOH has ais a liter value for titration molarity of 1 mole.
Molarity-
The amount of a substance in a specific volume of solution is known as its molarity (M). The number of moles of a solute per liter of a solution is known as molarity. The molar concentration of a solution is another term for molarity.
If you know how many grams of NaOH is dissolved in a known amount of solvent, you can use the formula below to calculate its molarity.
The molarity of NaOH is determined, for instance, when 10 gm of NaOH is dissolved in 250 ml of water.
Molarity is calculated as (Weight of NaOH taken*1000)/(250*NaOH's molecular weight).
Molarity equals (10*1000)/(250*40).
NaOH has a molarity of 1 mole.
Know more about Molarity
https://brainly.com/question/17138838
#SPJ4
The full question-
How do you calculate the molarity of NaOH?
3. How many grams of oxygen are required to completely react with 240g of C₂H6?
Answers
Approximately 766.08 grams of oxygen are required to completely react with 240g of C₂H₆.
To determine the amount of oxygen required to completely react with 240g of C₂H₆ (ethane), we need to set up a balanced chemical equation for the combustion of ethane.
The balanced equation for the combustion of ethane is as follows:
C₂H₆ + O₂ → CO₂ + H₂O
From the balanced equation, we can see that the stoichiometric ratio between C₂H₆ and O₂ is 1:3. This means that for every one mole of C₂H₆, three moles of O₂ are required for complete combustion.
To calculate the amount of oxygen required, we need to convert the given mass of C₂H₆ to moles using its molar mass, and then use the stoichiometric ratio to determine the moles of O₂ required. Finally, we can convert the moles of O₂ back to grams using the molar mass of oxygen.
The molar mass of C₂H₆ is calculated as follows:
(2 x atomic mass of carbon) + (6 x atomic mass of hydrogen)
(2 x 12.01 g/mol) + (6 x 1.01 g/mol) = 30.07 g/mol
Now, we can proceed with the calculation:
Calculate the moles of C₂H₆:
moles of C₂H₆ = mass of C₂H₆ / molar mass of C₂H₆
moles of C₂H₆ = 240 g / 30.07 g/mol ≈ 7.98 mol
Determine the moles of O₂ using the stoichiometric ratio:
moles of O₂ = moles of C₂H₆ x (3 moles of O₂ / 1 mole of C₂H₆)
moles of O₂ = 7.98 mol x 3 ≈ 23.94 mol
Convert moles of O₂ to grams:
mass of O₂ = moles of O₂ x molar mass of O₂
mass of O₂ = 23.94 mol x 32.00 g/mol = 766.08 g
For more such questions on oxygen visit:
https://brainly.com/question/28009615
#SPJ8
Difference between heavy chemicals and fine chemicals
Answers
Answer:
Fine chemicals are used as starting materials for specialty chemicals, particularly pharmaceuticals, biopharmaceuticals and agrochemicals. ... The term "fine chemicals" is used in distinction to "heavy chemicals", which are produced and handled in large lots and are often in a crude state.
Explanation:
How many grams of Ag are needed to react with 75.0 g of Ss in the following reaction?
16 Ag + S8 → 8 Ag2S
Answers
What is the condensed structural formula of C7H8N4O2
Answers
Answer:
C7H8N4O. The molecular formula C7H8N4O2 (molar mass: 180.16 g/mol) may refer to: Paraxanthine. Theobromine.
Hello How do you do?
What is logic bomb and time bomb?
Answers
Answer:
A logic bomb and a time bomb are both types of malicious software or code that are designed to cause harm to a computer system or network. Here's a brief explanation of each:
Logic Bomb:
A logic bomb is a piece of code or software that is intentionally inserted into a system to execute a malicious action when specific conditions are met. It remains dormant until triggered by a predefined event or circumstance, such as a specific date, time, or user action. Once triggered, the logic bomb may perform various harmful actions, such as deleting files, corrupting data, or disrupting system functionality. The purpose of a logic bomb is often to cause damage or to gain unauthorized access to a system.
Time Bomb:
A time bomb is similar to a logic bomb, but it is specifically designed to activate or execute its malicious payload at a certain date or time. It is usually programmed to remain undetected until the predetermined time arrives. The time bomb can be set to trigger after a specific time period or on a particular date, at which point it may carry out destructive actions. Time bombs can be used by attackers to target specific events or to coordinate an attack to occur simultaneously across multiple systems.
Both logic bombs and time bombs are considered forms of malicious code or malware and are used with malicious intent to disrupt, damage, or compromise computer systems or networks. They can be extremely harmful, and it is important to have strong security measures, such as antivirus software and regular system updates, to protect against such threats.
Explanation:
if .654g of oxygen dissolves in 1.5L of water 1.65atm at what pressure would 1.35g in 1.5L of water dissolve
Answers
the pressure required for 1.35 g of oxygen to dissolve in 1.5 L of water is 3.56 atm.
The first step in solving this problem is to identify the relevant equation.
Henry's law is the formula that relates the pressure of a gas above a liquid to the concentration of the gas that dissolves in the liquid.
In mathematical terms, Henry's law can be expressed as follows:P = kH * Cwhere P is the pressure of the gas, kH is Henry's law constant, and C is the concentration of the gas in the liquid.
To solve the problem, we need to first determine the value of kH using the given data.
kH can be calculated using the following formula:kH = P / CSubstituting the values given in the problem into this formula, we get:kH = 1.65 atm / (0.654 g / 1.5 L) = 3.97 atm/(g/L).
Now that we have the value of kH, we can use Henry's law to calculate the pressure required for 1.35 g of oxygen to dissolve in 1.5 L of water.
To do this, we simply rearrange the formula to solve for P:P = kH * CSubstituting the values of kH and C into this formula, we get:P = 3.97 atm/(g/L) * (1.35 g / 1.5 L) = 3.56 atm
Therefore, the pressure required for 1.35 g of oxygen to dissolve in 1.5 L of water is 3.56 atm.
for more questions on oxygen
https://brainly.com/question/15457775
#SPJ8
dicotyledonous plants
Answers
The dicotyledons, also known as dicots, are one of the two groups into which all the flowering plants or angiosperms were formerly divided. The name refers to one of the typical characteristics of the group, namely that the seed has two embryonic leaves or cotyledons.
6) Mendeleev put the elements on the periodic table in the order of how
many
it has. *
O electrons
O neutrons
O protons
Answers
Answer: Protons
Explanation:
5. The density of water at 4.00°C is 0.967 g/mL. How many molecules of water are present in a 499.8 mL bottle of water? Express your answer to the correct number of significant figures
Answers
There are approximately 1.62 x 10^25 water molecules in the 499.8 mL bottle of water.
To determine the number of water molecules in the given volume of water, we need to use the relationship between mass, volume, and molar mass of water.
First, we need to find the mass of water in the bottle:
Mass = Density * Volume
Mass = 0.967 g/mL * 499.8 mL = 483.9 g
Next, we need to convert the mass of water to moles using the molar mass of water. The molar mass of water (H2O) is approximately 18.015 g/mol.
Moles = Mass / Molar mass
Moles = 483.9 g / 18.015 g/mol = 26.88 mol
Finally, we can calculate the number of water molecules using Avogadro's number, which is approximately 6.022 x 10^23 molecules/mol.
Number of molecules = Moles * Avogadro's number
Number of molecules = 26.88 mol * (6.022 x 10^23 molecules/mol) = 1.62 x 10^25 molecules
Therefore, there are approximately 1.62 x 10^25 water molecules in the 499.8 mL bottle of water.
for more questions on molecules
https://brainly.com/question/24191825
#SPJ8
Consider the precipitation reaction: BaCl 2 + 2 AgNO 3 → 2 AgCl + Ba(NO 3) 2. How many grams of AgCl are generated when 85 g of BaCl 2 reacts?
Answers
The grams of AgCl are generated when 85 g of BaCl₂ reacts is 10.86 g.
The balanced equation is given as :
BaCl₂ + 2AgNO₃ -----> 2AgCl + Ba(NO₃)₂
the mass of the BaCl₂ = 85 g
molar mass of BaCl₂ = 208.23 g/mol
moles of BaCl₂ = mass / molar mass
= 85 / 208.23
= 0.408 mol
1 mole of BaCl₂ produce 2 moles of AgCl
0.408 moles of BaCl₂ = 2 × 0.408
= 0.816 mol of AgCl
moles of AgCl = 0.816 mol
mass of AgCl = moles × molar mass
= 0.816 g × 143.32 g/mol
= 10.86 g
Thus, The grams of AgCl are generated when 85 g of BaCl₂ reacts is 10.86 g.
To learn more about moles here
https://brainly.com/question/26416088
#SPJ9
as heat is added, the pressure in this gas . view available hint(s)for part a as heat is added, the pressure in this gas . increases decreases remains constant cannot be determined
Answers
The ideal gas law can be reorganized to arrive at: assuming that the volume and molecular weight of the gas remain constant:
P/T = constant indicates that the gas's pressure must rise with its temperature in order to maintain a constant ratio of pressure to temperature.
Consequently, if we heat the gas while maintaining its volume and molecular weight, its pressure will rise.
How does temperature work?In other words, it is a measure of how quickly the particles of a substance are moving. Temperature is a fundamental physics concept that can be measured in Kelvin (K), Fahrenheit (°F), and Celsius (°C) scales, among others.
On the Celsius scale, water boils at 100°C and freezes at 0°C at normal atmospheric pressure. On the Fahrenheit scale, water boils at 212°F and freezes at 32°F at normal atmospheric pressure. On the Kelvin scale, 0 K is the theoretical minimum temperature at which all particles have no kinetic energy.
To know more about Temperature:
brainly.com/question/23411503
#SPJ1
You have a solid object of unknown composition and mass. You determined that when this object absorbed 1.000 X 10^2J, its temperature increased by 2.0K. Calculate the objects heat capacity
Answers
Answer:
100 rbed KJ |0| +2k
Explanation:
How many ions are in 1
mole of potassium sulfate?
Answers
Answer:
We will need two potassium ions to balance the charge on the sulfate ion, so the proper chemical formula is K 2SO 4.
Explanation:
hope this helps
Using the measurements in the table, determine which unidentified metal has the lowest density?

Answers
Answer:
Metal C
Explanation:
Density = mass (g)/ volume (mL, which is the same thing as cm cubed)
Divide mass by volume for each metal, the metal with the lowest value (metal C) is your answer.
Answer:
IT IS
NOT. I REPEAT - IT IS NOT> D
Explanation:
i got it wrong :(
Which statement best describes the difference between a theory and a law? A. Scientific theories explain WHY a phenomenon occurs and a scientific law explains WHAT occurs. B. Scientific theories explain WHAT occurs and a scientific law explains WHY a phenomenon occurs. C. They both explain why something happens. D. They both tell us what happens.
Answers
Scientific theories explain why a phenomenon occurs and a scientific law explains what occurs. The correct option is A.
What is scientific theory?A scientific theory is an explanation for a phenomenon in the natural world or the universe that has undergone extensive testing and verification using approved procedures for observation, measurement, and result evaluation.
A scientific law often describes an observed phenomenon. It doesn't explain the phenomenon's existence or its origins.
A scientific theory is the explanation for a phenomenon. It is a myth that with enough study, theories can become laws.
Thus, the correct option is A.
For more details regarding scientific theory, visit:
https://brainly.com/question/17152046
#SPJ1
Lead of mass 0.75kg is heated from 21°c to its melting point and continues to be heated unit it has all melted. Calculate how much energy is supplied to the lead. [Melting point of lead 372.5°c specific latent heat of fusion of lead = 23000 Jkg 'k ']
Answers
Answer:
65.5J
Explanation:
ML=Q
ML=MC(change in temperature)
0.75 X 23000 =0.75 X 351 X C
C= 65.5J
The energy supplied to the lead to melt from 21°c to its melting point is 51521 Joules.
What is the specific heat capacity?Specific heat is the amount of heat energy supplied to change the temperature of one unit mass of a substance by 1 °C. The SI unit of the specific heat capacity of a substance is J/Kg.
The mathematical expression for the specific heat capacity can be written as:
Q = mCΔT Where C is the specific heat of the substance.
The specific heat capacity depends upon the starting temperature and is an intensive characteristic of the material.
Given, the melting point of the lead T₂ = 327.5° C
The initial temperature of the lead, T₁ = 21° C
The latent heat of the lead given, L = 230000 J/Kg K
The specific heat of the lead, C = 130 J/Kg K
The heat required to melt the lead from 12°C to 327.5 °C is :
Q = m× [C × (T₂ - T₁) + L ]
Q = 0.75 × [0.130 (327.5 - 21) + 23000]
Q = 51521 J
Learn more about specific heat, here:
brainly.com/question/11297584
#SPJ5
10. What is the molality of a solution
containing 288 g of calcium chloride
dissolved in 2.04 kg of water?
Answers
The choice of solution has a concentration of 1.144 mol/kg molality.
What exactly are molality and molarity?Molarity corresponds to the moles of solvent divided by the amount of solution in litres, whereas molality is equal with the moles of solvent divided by the quantity of solvent in kilogrammes.
Is one molarity the same as one molality?Since 1 mole of solute is present in 1 litre for the solution, which contains both the solute and the solvent, 1 molar aqueous solutions are more concentrated than one decays aqueous solutions.
To know more about molality visit:
https://brainly.com/question/26921570
#SPJ1
If d represents the density of a gas and k is a constant. The relationship between the rate of diffusion r, and d is ____?
Answers
The relationship between the rate of diffusion r, and d is r ∝ 1/√d.
The relationship between the rate of diffusion (r) and the density of a gas (d) can be explained using Graham's law of diffusion. According to this law, the rate of diffusion of a gas is inversely proportional to the square root of its density. Mathematically, it can be expressed as:
r ∝ 1/√d
where the symbol '∝' represents 'proportional to'. The constant of proportionality (k) can be introduced to this equation as:
r = k/√d
This equation shows that as the density of a gas increases, its rate of diffusion decreases. This is because denser gases have more molecules per unit volume and thus, they experience greater intermolecular collisions that hinder their movement. Therefore, it requires more energy for them to diffuse through a medium compared to less dense gases.
The relationship between the rate of diffusion and density is particularly important in understanding the behavior of gases in different environments. For instance, in a gas chromatography column, the rate of diffusion of a gas determines how quickly it moves through the column and separates from other components. Similarly, in the Earth's atmosphere, the rate of diffusion of greenhouse gases such as carbon dioxide affects their concentration and hence, their impact on climate change.
For more such questions on diffusion
https://brainly.com/question/29064792
#SPJ11
A secondary step in the process to produce ultra-pure silicon is to combine silicon tetrachloride with magnesium. How many grams of Si could be produced by reacting 2.00 kg of SiCl4 with excess Mg
Answers
The mass of silicon, Si produced from the reaction is 329.41 g
Balanced equationSiCl₄ + 2Mg —> 2MgCl₂ + Si
Molar mass of SiCl₄ = 28 + (35.5×4) = 170 g/mol
Mass of SiCl₄ from the balanced equation = 1 × 170 = 170 g
Molar mass of Si = 28 g/mol
Mass of Si from the balanced equation = 1 × 28 = 28 g
From the balanced equation above,
170 g of SiCl₄ reacted to produce 28 g of Si.
How to determine the mass of Si producedFrom the balanced equation above,
170 g of SiCl₄ reacted to produce 28 g of Si.
Therefore,
2 Kg (i.e 2000 g) of SiCl₄ will react to produce = (2000 × 28) / 170 = 329.41 g of Si
Thus, 329.41 g of Si were obtained from the reaction
Learn more about stoichiometry:
https://brainly.com/question/14735801
The container seems to have about 650 mL of copper(II) sulfate
solution in volume. Convert this volume to liters (L) of
solution.
Answers
The volume of copper (II) sulfate in a container is equivalent to 0.650L.
How to convert units of volume?Volume is the three-dimensional measure of space that comprises a length, a width and a height. It is measured in units of cubic centimeters (cm³) in metric, cubic inches or cubic feet in English measurement.
According to this question, a container seems to have about 650 mL of copper(II) sulfate solution in volume. The volume can be converted to litres as follows:
1 millilitre = 0.001 litre
650 millilitres = 0.650 litres
Therefore, 0.650L is the volume of copper II sulfate in litres.
Learn more about volume at: https://brainly.com/question/13666409
#SPJ1
In the hydrogenation of ethylene using a nickel catalyst, the initial concentration of ethylene is 1.65 mol⋅L−1 and its rate constant (k) is 0.0014 mol⋅L−1⋅s−1 . Determine the rate of reaction if it follows a zero-order reaction mechanism.
Answers
Answer:
.0014 M/s ( (mol*L^-1 / s) )
Explanation:
Since the rate law of a zero order reaction is Rate = k[A]^0, the rate is .0014 * (1.65)^0 = .0014
Drag conversion units onto the boxes in the equation to make conversions. Some boxes can be left empty. Click on a
unit to remove it from its position.
1 cmCu
9 g Cu
9.5 x 1021 atoms Cu
1 g Cu
1 kg
1000 g
1 cm
1 ml
1
1000 cm3
70.0 kg x
X
X
II
70.0 kg•L
Answers
We have that 70kg of Copper Cu is having a volume of \(V=7.76442liters\)
1 cmCu
=9 g Cu
9.5 x 10^21 atoms Cu
=1 g Cu
1 kg =1000 g
1 cm
=1 ml
1
=1000 cm3
70.0 kg x X _X II
70.0 kg•L
Generally We First determine the No of Atoms in 70kg of cu
No of Atoms of c\(u=\frac{weight}{atomic mass of cu}*n\)
Where
n=Avogadro's Constant
\(n= 6.02214076 x 10^{23}\)
Therefore
No of Atoms of cu\(=\frac{70*10^3}{63.5}*6.02214076 * 10^{23}\)
No of Atoms of cu=6.63858037x10^{26} atoms of Cu
Hence
We determine the the grams of copper derived from \(6.63858037x10^{26}\)atoms of Cu
No of grams of cu\(=\frac{6.63858037x10^{26}}{9.5 x 10^{21}}*1\)
No of grams of cu=69879.79337 g of Cu
Generally
No of grams of Cu=liters
Where
9 g of Cu= 1cm^3 Cu
Therefore
Volume of cu
\(V=(69879.79337)*\frac{1}{9}*\frac{1}{1000}\)
\(V=7.76442liters\)
In conclusion
70kg of Copper Cu is having a volume of \(V=7.76442liters\)
For more information on this visit
https://brainly.com/question/13677872
We are to drag the given conversion units onto the boxes in the equation in order to make conversions in the boxes that are left empty.
The dimensional analysis for the conversion ratio is given as follows
1cm^3 Cu = 9g Cu 9.5*10^21 atoms of Cu = 1 g Cu
1 kg = 1000 g 1 cm^3 = 1 mL
1 L = 1000 cm^3
Here is the box equation
box box box
70 g ×----- × ------ × ----- =
box box box
From the given information, the volume of the Cu sample in Liters is:
= 7.765 liters
From the parameters given:
the mass of the sample of Cu = 70 kgThe first thing we are to do is to calculate the number of atoms of Cu in 70 kg by using Avogadro's constant.
We know that the number of atoms of Cu is:
\(\mathbf{= \dfrac{mass \ weight \ of \ Cu}{atomic \ mass \ of \ cu}\times avogadro's \ constant}\)
where;
weight in Cu = \(\mathbf{70 * 10^{3}}\)the atomic mass of Cu = 63.5 Avogadro's constant = \(\mathbf{6.023 \times 10^{23} \ Cu \ atoms}\)the number of Cu atoms is:
\(\mathbf{= \dfrac{70*10^3}{63.5}\times 6.023 * 10^{23}}\)
\(\mathbf{= 6.6395 \times 10^{26} \ atoms \ of \ Cu}\)
However, from the given conversion ratios:
If \(\mathbf{9.5\times10^{2}}\) atoms of Cu = 1 gram of Cu
∴
atoms of Cu will be:
\(\mathbf{= \dfrac{6.6395 \times 10^{26} \ atoms \ of \ Cu \times 1 \ gram \ of \ Cu}{9.5*10^{21} \ atoms \ of \ Cu }}\)
\(\mathbf{= 0.69889 *10^{5} \ grams \ of \ Cu}\)
Since:
1cm³ Cu = 9g Cu and; 1 L = 1000 cm³∴
The correct setup of the sample in the box can be computed as follows:
\(\mathbf{= 0.69889 *10^{5} \ gram \ of \ Cu \ \times \dfrac{1 cm^3 \ of \ Cu }{9 \ g \ of \ Cu }\times \dfrac{1 \ Liter}{1000 \ cm^3}}\)
= 7.765 liters
Therefore, we can conclude that the Volume of Cu sample in liters is:
= 7.765 liters
Learn more about conversion ratios here:
https://brainly.com/question/15398863?referrer=searchResults
DH
QUESTION 1
[11]
Give reasons that might help to explain the following observations:
a)
A student was unsuccessful to prepare a Grignard reagent from 4-bromocyclohexanol.
b)
Benzenesulfonic acid does not undergo Friedel-Crafts alkylation.
c)
(2S, 3R)-2,3-Dibromobutane has a specific rotation, [a]o, of 0°.
d)
A student tries to prepare an ether via the Williamson ether synthesis from ethanol and
t-butyl bromide, but obtains an alkene instead.
e)
Doubling the concentration of a nucleophile has no effect on the rate of a substitution
reaction.
Answers
a. 4- bromocyclohexanol is an acidic proton
b. Benzenesulfonic acid deactivates the entire process
c. (2S, 3R)-2,3-Dibromobutane are racemic mixtures
d. Williamson synthesis cannot be used with tertiary alkyl halides
e. Nucleophiles are not involved in the rate determining step
The reasons that might help to explain the following observations are:a. Grignard reagents cannot be made if acidic functional groups are also present in the halogen compound and it is also destroyed by reaction with acidic hydrogen atoms of water, alcohols, phenols, or carboxylic acid groups.
4-bromocyclohexanol is an acidic proton compound
b. Benzenesulfonic acid does not undergo Friedel-Crafts alkylation because the carboxylic group causes deactivation, carboxylic group and the Lewis acid catalyst are also bonded in the process.
c. (2S, 3R)-2,3-Dibromobutane has a specific rotation, [a]o, of 0° are racemic mixtures having equal and opposite specific rotations and a racemic mixture has a specific rotation of zero (0°)
d. This is because the Williamson synthesis cannot be used with tertiary alkyl halides which in this case is t-butyl bromide because they undergo elimination reactions instead of participating in SN2 reactions and form alkenes instead of ethers.
e. Doubling the concentration of a nucleophile has no effect on the rate of a substitution reaction because the nucleophile is not involved in the rate-determining step of the SN-1 reaction.
Learn more about organic reactions here:
https://brainly.com/question/3529377
#SPJ1
For the reaction
4PH3(g)↽−−⇀6H2(g)+P4(g)
the equilibrium concentrations were found to be [PH3]=0.250 M, [H2]=0.580 M,
and [P4]=0.750 M.
What is the equilibrium constant for this reaction?
c=
Answers
The equilibrium constant for the reaction given that the equilibrium concentration of [PH₃] = 0.250 M, [H₂] = 0.580 M, and [P₄] = 0.750 M is 7.3
How do I determine the equilibrium constant?From the question given above, the following data were obtained:
Equation: 4PH₃(g) ⇌ 6H₂(g) + P₄(g)Concentration of PH₃, [PH₃] = 0.250 MConcentration of H₂, [H₂] = 0.580 MConcentration of P₄, [P₄] = 0.750 MEquilibrium constant (K) =?The equilibrium constant for the reaction can be obtained as shown below:
Equilibrium constant = [Product]ᵐ / [Reactant]ⁿ
Where
m and n are coefficients of products and reactants respectivelyEquilibrium constant = [H₂]⁶[P₄] / [PH₃]⁴
Equilibrium constant = [(0.580)⁶ × 0.750] / (0.250)⁴
Equilibrium constant = 7.3
Thus, the equilibrium constant for the reaction is 7.3
Learn more about equilibrium constant:
https://brainly.com/question/16589765
#SPJ1
A radioactive sample contains 3.00 g of an isotope with a half-life of 3.8 days.
How much of the isotope in grams will remain after 19.8 days?
Answers
Answer:So, about 0.093 g of the isotope will remain after 19.8 days.
Explanation:
The first step is to find the number of half-lives that have passed during 19.8 days:
Number of half-lives = time elapsed / half-life
Number of half-lives = 19.8 days / 3.8 days per half-life
Number of half-lives ≈ 5.21
This means that the initial amount of the isotope has been halved 5.21 times. The remaining fraction of the original amount can be calculated using the following formula:
Remaining fraction = (1/2)^(number of half-lives)
Substituting the values, we get:
Remaining fraction = (1/2)^5.21
Remaining fraction ≈ 0.031
Therefore, the amount of the isotope remaining after 19.8 days is:
Remaining amount = Remaining fraction x Initial amount
Remaining amount = 0.031 x 3.00 g
Remaining amount ≈ 0.093 g
So, about 0.093 g of the isotope will remain after 19.8 days.
The isotope in grams will remain after 19.8 days would be 0.081 grams.
The formula to calculate the left mass of a radioactive element can be deduced as -
\( \qquad\star\longrightarrow \underline{\boxed{\sf{m =m_{o} \times { \bigg(\dfrac{1}{2} \bigg)}^{ \dfrac{t}{T½}} }}} \\\)
Where-
\(\sf m_{o} \)is the initial mass of a radioactive elementT½ is the half life timet is the time periodm = Left mass of a radioactive element.According to the given specific parameters -
Initial mass,\(\sf m_{o} \) = 3 gHalf life time, T½= 3.8 days Time period, t =19.8 daysNow that we have all the required values, so we can plug them into the formula and solve for the left mass of a radioactive element-
\( \qquad \longrightarrow \sf \underline{m =m_{o} \times { \bigg(\dfrac{1}{2} \bigg)}^{ \dfrac{t}{T½} }} \\\)
\( \qquad\longrightarrow \sf m =3 \times { \bigg(\dfrac{1}{2} \bigg)}^{ \dfrac{19.8}{3.8} } \\\)
\(\qquad \longrightarrow \sf m =3 \times { \bigg(\dfrac{1}{2} \bigg)}^{ \dfrac{\cancel{19.8}}{\cancel{3.8}} } \\\)
\( \qquad\longrightarrow \sf m =3 \times { \bigg(\dfrac{1}{2} \bigg)}^{ 5.21052..... } \\\)
\( \qquad\longrightarrow \sf m =3 \times 0.02700... \\\)
\( \qquad\longrightarrow \sf m =0.081020....\;g \\\)
\( \qquad\longrightarrow \sf \underline{m =\boxed{\sf{0.081\;g}}} \\\)
Henceforth,about 0.081 g of the isotope in grams will remain after 19.8 days.