why does an atom with a high effective nuclear charge readily attract an electron from another atom to valence shell
Answers
its valence shell is because of the effective nuclear charge and its ability to attract the valence electrons. The explanation for this answer is given below.Effective Nuclear Charge (Zeff)Effective nuclear charge (Zeff) is the net positive charge experienced by valence electrons.
The valence electrons are attracted to the nucleus's positive charge, and the electron-electron repulsion counteracts this attraction. Thus, the effective nuclear charge (Zeff) determines how strongly the valence electrons are attracted to the nucleus.For instance, Boron (B) has an effective nuclear charge (Zeff) of +3, and the valence electrons are attracted to this effective nuclear charge.
Therefore, it can readily attract an electron from another atom to its valence shell due to its effective nuclear charge. Consequently, the electrons are drawn to the positive charge, and the valence electrons are more readily attracted to the positively charged nucleus. Hence, it results in a readily attracting electron from another atom to the valence shell.
TO know more about that valence visit:
https://brainly.com/question/31264554
#SPJ11
Related Questions
You want to compile a list of properties of a substance , but you don't have a way to measure mass it volume. What kinds of properties can you determine without knowing the amount of matter in the sample?
Answers
Explanation:
I Would Determine Mass,Volume And Weight
18) Match the appropriate benefit of dietary fiber intake to its associated disease. (Use each choice only once). Cardiovascular Disease A. Lowers Blood Cholesterol Hypertension B. Lowers Blood Pressure Type 2 Diabetes c. Decreases intestinal transit time and exposure to potential toxins D. Slows absorption of glucose Constipation Obesity E Slows rate of digestion, promotes satiety Colon Cancer F. Increases fecal bulk & promotes regularity
Answers
Appropriate benefit of dietary fiber intake to its associated disease.- c. Decreases intestinal transit time and exposure to potential toxins
Cardiovascular diseases (CVDs) are the most common cause of death worldwide, claiming the lives of an estimated 17.9 million persons each year. CVDs are a group of heart and blood disorders that include coronary artery disease, vascular disease, rheumatic fever, and others. Coronary heart disease could be cured, but treatment can help manage the symptoms and lower the risk of conditions such as heart attacks. Treatment may include changes in lifestyle, such as physical activity and quitting smoking. The most common symptom of heart disease is heart disease (CHD), which kills approximately 382,820 people every year. Every year, approximately 805,000 Americans suffer a heart attack.
To learn more about disease here:
https://brainly.com/question/8611708
#SPJ4
Which of these accurately describes a chemical reaction?
A. Products and reactants combine to form new reactants
B. Products and reactants combine to form new substances
C. Products combine to make reactants
D. Recatants combine to form products
Answers
Answer:
D
Explanation:
its just right
Answer:Reactants combine to form Products. Hope this helped! ;)
Explanation:
What trend does the atomic radius follow along a period on the periodic
table?
O A. The atomic radius gets smaller the farther right it appears on the
periodic table.
O B. The atomic radius stays constant across the periodic table.
O C. The atomic radius increases as the number of protons increases
across the table.
O D. The atomic radius gets larger the farther right it appears on the
periodic table.
Answers
How many grams of Ba(OH)2 would be made from 9.62 x 10^24
Answers
The mass of the solid is 2733 g
What is the moles?The mole is a useful unit of measurement in chemistry because it allows scientists to easily compare the amounts of different substances in chemical reactions. By knowing the molar amounts of the reactants and products in a chemical reaction, scientists can calculate important information such as the mass of products formed, the amount of energy released or absorbed, and other key chemical properties.
We know that;
1 mole of the compound would contain 6.02 * 10^23 molecules
x moles of the compound will contain 9.62 x 10^24 molecules
x= 15.98 moles
Then;
Mass = 15.98 moles * 171 g/mol
= 2733 g
Learn more about mass of solid:https://brainly.com/question/18064917
#SPJ1
What is the volume of 6.78 mol of hydrogen gas at STP?
Answers
Answer:
151.87dm³
Explanation:
Given parameters:
Volume of hydrogen gas = 6.78mole
Unknown:
Volume of hydrogen gas formed at STP = ?
Solution:
To solve this problem;
1 mole of a gas at STP = 22.4dm³ So;
6.78 mole of gas = 6.78 x 22.4 = 151.87dm³
What is the rate constant of a first-order reaction that takes 490 secondsseconds for the reactant concentration to drop to half of its initial value
Answers
The rate constant of a first-order reaction that takes 555 seconds for the reactant concentration to drop to half of its initial value is 1.2 x 10^-3 sec^-1.
A first-order reaction refers to a chemical reaction in which the rate of reaction is proportional to the concentration of the reactant. In other words, doubling the reactant concentration doubles the reaction rate.
The time taken that is taken for original population of radioactive atoms to decay to half of initial value is called the half-life. The half-life of a first-order reaction is a constant that is related to the rate constant (k) for the reaction given by: t1/2 = 0.693/k. Radioactive decay reactions are first-order reactions.
According to the provided information, t1/2 = 555 seconds
Hence,
555 = 0.693/k
k = 0.693/555 = 0.0012486 = 1.2 x 10^-3 sec^-1
Learn more about First-order reaction:
brainly.com/question/518682
#SPJ4
The valences of metal x,y and z are 1,2 and 3 respectively. What are the formulae of their;a) hydroxides, b) sulphates, c) hydrogen, d) carbonates, e) nitrates, f) phosphates
Answers
Answer:
See answer below
Explanation:
AS we know that the valence for those metals X, Y, and Z are 1, 2 and 3, we can determine the formula of each compound.
1. Hydroxides.
An hydroxide is formed when an oxyde of a metal reacts with water. When this happens, the general molecular formula is:
Meₐ(OH)ₙ
Where:
a: valence or charge of the hydroxide (Which is -1)
n: valence of the metal.
Following this, the formula for X, Y and Z would be:
XOH
Y(OH)₂
Z(OH)₃
2. Sulphates
Sulphates follow a similar rule of hydroxide in the general molecular formula, but instead of having a charge of -1, it has a charge of -2 so:
Mₐ(SO₄)ₙ
So, following the rule:
X₂SO₄
Y₂(SO₄)₂ ------> YSO₄
Z₂(SO₄)₃
3. Hydrogens
Following the same rule as the previous, hydrogens works with a charge of -1, so:
MₐHₙ
Then:
XH
YH₂
ZH₃
4. Carbonates.
This follows the same rule as sulphates, with the same charge so:
Mₐ(CO₃)ₙ
Then:
X₂CO₃
YCO₃
Z₂(CO₃)₃
5. Nitrates
Follow the same rule as the hydroxides, with the same charge of -1.
Mₐ(NO₃)ₙ
Then:
XNO₃
Y(NO₃)₂
Z(NO₃)₂
6. Phosphates
In the case of phosphates, these have a charge of -3 so:
Mₐ(PO₄)ₙ
Then:
X₃PO₄
Y₃(PO₄)₂
Z₃(PO₄)₃ ----> ZPO₄
Hope this helps
does gravity affect the orbit of the international space
Answers
Answer:
yes
Explanation:gergrgregbfbwerbebw
complete the reactions to show the synthesis of the following compound from cyclohexene as the starting material.
Answers
Learn the concentration and beginning rate in order to determine the correct number of significant digits. The starting rate of reaction is 98.0 m/s at a specific concentration of h2 and i2.
What, using an example, is concentration?
1) Percent Concentration:
The amount of solute that completely dissolves in 100 g of solvent. We know that there are 20 g of solute in 100 g of solution if the solution's concentration is 20 percent. Example: The solution is made by combining 10 g of salt with 70 g of water. Find the solution's concentration using mass percent.
What would you say about concentration?
The amount of solute contained in a specific amount of solution is the substance's concentration. Typically, concentrations are described in terms of molarity, which is the number of
To know more about cyclohexane visit:
https://brainly.com/question/6854548
#SPJ4
what is the ph of a solution obtained by dissolving two extra-strength aspirin tablets, containing 470 mg of acetylsalicylic acid each, in 270 ml of water?
Answers
The ph of a solution obtained by dissolving two extra-strength aspirin tablets of mass 470 mg of acetylsalicylic acid is 2.58.
What is mass?Mass refers to the amount of matter in the physical body. It is also a measure of body inertia, resistance to acceleration when a net force is applied.An object's mass also determines the strength of gravity on other objects. The SI unit of mass is Kg. Mass (symbol m) is a dimensionless quantity that describes the amount of matter in a particle or object. The standard unit of mass in the international system is "SI". Kilograms (kg). The terms mass and weight are often used interchangeably, but they have very different meanings. The mass is the same wherever one goes in space. Weight, on the other hand, varies from place to place.Each tablet has a mass of 470 mg. The mass of 2 tablets is 2 × 470 mg = 940 mg = 0.940 g
To learn more about mass from the given link :
https://brainly.com/question/19694949
#SPJ4
Baed on the thermodynamic propertie provided for water, determine the energy change when the temperature of 0. 250 kg of water decreaed from 121 °C to 51. 5 °C
Answers
Based on the thermodynamic properties provided for water, the energy change when the temperature of 0.650 kg of water decreased from 101 °c to 51.0 °c is 1,609.66 kJ
What are thermodynamic properties of water?
Thermal characteristics of water include its density, freezing point, boiling point, latent heat of evaporation, melting point, and critical temperature, among others.
Here we calculate the boiling point of water is 100°C. So at 101°C, the water is steam. The specific heat first from 101 to 100 calculated as
E = mCΔT, where c for steam is 1.996 kJ/kg·°C
E₁ = (0.65 kg)(1.996 kJ/kg·°C)(101 - 100°C) = 1.2974 kJ
The latent heat when steam turns to liquid. The heat of vaporization of water is 2260 kJ/kg.
E₂ = mHvap = (0.65 kg)(2260 kJ/kg) = 1469 kJ
Thus solving the energy to bring down the temperature to 51°C. The specific heat of liquid water is 4.187 kJ/kg·°C.
E₃ = (0.65 kg)(4.187 kJ/kg·°C)(100 - 51°C) = 139.36 kJ
Total energy = 1.2974 kJ+1469 kJ+139.36 kJ = 1,609.66 kJ
Therefore , the energy change when the temperature of 0.650 kg of water decreased from 101 °c to 51.0 °c is 1,609.66 kJ
To know more about thermodynamic properties from the given link
https://brainly.com/question/24969033
#SPJ4
how does simple molecules covalent bonds have low melting points and diamond(macromolecules) have high melting point?
Answers
Answer:
Simple covalent molecules have low melting points because their weak intermolecular forces cause the bonds to break easily, leading to a low temperature required to change the solid to a liquid. In contrast, diamonds, which are made up of a large network of strong covalent bonds, require a high amount of energy to break these bonds and cause the transition from solid to liquid, resulting in a high melting point.
Hope this helps! Enjoy Learning<3
What would be the vital capacity be for a man that has a tidal volume of 500mL, a residual volume of 1100 mL, an expiratory reserve of 1100 mL and an inspiratory reserve of 3000 mL
Answers
The vital capacity will be 4600ml.
What is vital capacity?The highest amount of air a person can inhale following their maximal exhalation is known as their vital capacity. It is equivalent to the total of the inspiratory, tidal, and expiratory reserve volumes. It roughly corresponds to Forced Vital Capacity. A wet or conventional spirometer can assess a person's vital capacity.
Normal people have a 3 to 5-liter vital capacity.
It enables simultaneous inhalation of the greatest possible volume of clean air and exhalation of the greatest possible volume of stale air. So, by increasing gaseous exchange between the body's various tissues, it improves the amount of energy available for bodily function.
VC = TV₊IRV₊ERV
where,
VC = Vital capacity
TV = Tidal volume
IRV = inspiratory reserve volume
ERV = expiratory reserve volume
VC = 500 ₊ 3000 ₊ 1100
VC = 4600ml
Therefore, the vital capacity will be 4600ml.
To know more about vital capacity refer to: https://brainly.com/question/14877276
#SPJ4
How many molecules of water are there in 1. 222 grams of water
Answers
1.22 grams of water has 5.0 x10^22 H2O water molecules.
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds. There is no guarantee that the term will include ions that meet this criterion, depending on the context.
Calculate the number of moles of water = mass / molar mass of water
Moles = 1.2 g/ 18 g/mol = 0.083 mol H2O
1 mole of any substance = 6.02 x 10^23 H2O molecules (Avogadro's number)
Resolution:
Molecules = 0.083 moles (6.02 x 10^23 H2O molecules/mole)
= 0.5 x 10^23 or 5.0 x10^22 H2O molecules
answer:
Number of molecules = 5.0 x 10^22 H2O molecules
Learn more about Molecules
brainly.com/question/14130817
#SPJ4
In which of the following substances is chlorine in the lowest oxidation state?
a. Cl2
b. KCl
c. KClO
d. KClO4
Answers
The substance with chlorine in the lowest oxidation state is b. KCl, where chlorine has an oxidation state of -1.
The oxidation state of an element indicates the charge it would have in a compound or ion if electrons were completely transferred.
a. In Cl₂, each chlorine atom shares electrons equally, resulting in a neutral chlorine molecule. The oxidation state of each chlorine atom in Cl₂ is 0.
b. In KCl, potassium (K) is a Group 1 element with an oxidation state of +1. Chlorine (Cl) has an oxidation state of -1 to balance the charge. This is the lowest oxidation state of chlorine among the given options.
c. In KClO, potassium (K) has an oxidation state of +1, and oxygen (O) usually has an oxidation state of -2. We can assign chlorine (Cl) an oxidation state of +1 to balance the charges. Therefore, chlorine in KClO has an oxidation state of +1.
d. In KClO₄, potassium (K) has an oxidation state of +1, and oxygen (O) usually has an oxidation state of -2. To balance the charges, chlorine (Cl) must have an oxidation state of +7. Therefore, chlorine in KClO₄ has an oxidation state of +7.
Based on the given options, the substance with chlorine in the lowest oxidation state is b. KCl, where chlorine has an oxidation state of -1.
Learn more about the oxidation state here:
https://brainly.com/question/31315172
#SPJ11
how can knowledge of percent composition help you as a consumer ? how can you promote responsible consumerism ?
Answers
Answer:
it is a nice question....my mind tells me that the first is it use me as a good vibes and can use to anything the second i will do my best too absorbe it.
Explanation:
Hope this help...
1. Using PbS + 3PbO -> 3Pb + SO2a. How many moles of Pb will form from 17 moles of PbO (excess PbS)?b. How many moles of PbO are needed to make 27.4 moles of SO2 (excess PbS)c. Give 17.6 moles of PbS and 36 moles of PbO, which is the limiting reactant (show mathematical proof). It’s
Answers
Answer:
a. 25.5 moles of Pb will be formed.
b. 54.8 moles of PbO are needed.
c. PbS is the limiting reactant.
Explanation:
1st) It is necessary to write the balanced chemical reaction:
\(PbS+2PbO\rightarrow3Pb+SO_2\)From the balanced chemical reaction we know that 1 mole of PbS reacts with 2 moles of PbO to produce three moles of Pb and 1 mole of SO2.
2nd) Using the stoichiometry of the reaction and a mathematical rule of three, we can calculate the moles of Pb that will be produced from 17 moles of PbO and excess of PbS:
\(\begin{gathered} 2molesPbO-3molesPb \\ 17molesPbO-x=\frac{17molesPbO*3molesPb}{2molesPbO} \\ x=25.5molesPb \end{gathered}\)So, 25.5 moles of Pb will be formed.
3rd) Using the stoichiometry of the reaction and a mathematical rule of three, we can calculate the moles of PbO that are needed to make 27.4 moles of SO2 in excess of PbS:
\(\begin{gathered} 1molSO_2-2molPbO \\ 27.4molSO_2-x=\frac{27.4molSO_2*2molPbO}{1molSO_2} \\ x=54.8molPbO \end{gathered}\)So, 54.8 moles of PbO are needed.
4th) Using the stoichiometry of the reaction we can calculate the limiting reactant:
- Calculation from 17.6 moles of PbS:
\(\begin{gathered} 1molPbS-2molPbO \\ 17.6molPbS-x=\frac{17.6molPbS*2molPbO}{1molPbS} \\ x=35.2molPbO \end{gathered}\)From the stoichiometry of the reaction 1 mol of PbS reacts with 2 moles of PbO, so the 17.6 moles of PbS will need 35.2 moles of PbO to react properly, but we have 36g of PbO, so PbO will be the excess reactant and PbS the limiting reactant.
- Calculation from 36 moles of PbO:
We can do this calculation to confirm the previous one:
\(\begin{gathered} 2molPbO-1molPbS \\ 36molPbO-x=\frac{36molPbO*1molPbS}{2molPbO} \\ x=18molPbS \end{gathered}\)In this case, we can see that 36 moles of PbO will need 18 moles of PbS to react properly, but we only have 17.6 moles of PbS. Here we confirm that PbS is the limiting reactant.
Solve for me please. I don’t understand this

Answers
1. 3.50 moles of MgCl2 are known.
Unknown: HCl moles needed.
According to the equilibrium equation, 2 moles of HCl are converted into 1 mole of MgCl2.
Therefore, to make 3.50 moles of MgCl2, we need:
(7.00 mol HCl) = (3.50 mol MgCl2) (2 mol HCl / 1 mol MgCl2)
Hence, 3.50 moles of MgCl2 can be prepared from 7.00 moles of HCl.
2. The Pb content is known at 50 g. milligram content is unknown.
According to the balanced equation, 1 mole of Mg gives 1 mole of Pb.
To determine the moles of lead:
The mass of Pb / molar mass of Pb is equal to moles of Pb.
The molar mass of Pb is 207.2 g/mol.
Moles of Pb = 50 g Pb / 207.2 g/mol Pb
From the balanced equation, the stoichiometry is 1:1 between Mg and Pb.
Hence, the moles of Mg required will be equal to the moles of Pb.
Grams of Mg required = Moles of Mg × Molar mass of Mg
3. 2.0 moles of Fe2O3 are known.
Unknown: Required O2 grams
According to the balanced equation, 3 moles of oxygen are converted into 1 mole of Fe2O3.
Therefore, to make 2.0 moles of Fe2O3, the following would be required:
6.0 mol O2 (2.0 mol Fe2O3) is equal to (3 mol O2) / 1 mol Fe2O3.
grams of oxygen required = moles of oxygen + molar mass
4. 25.0 g of Fe are known.
Unknown: FeCl3 production in grams
According to the balanced equation, 2 moles of Fe are converted into 2 moles of FeCl3.
To determine the moles of iron:
equals the mass of Fe divided by its molar mass.
The molar mass of Fe is 55.8 g/mol.
Fe moles are equal to 25.0 g Fe and 55.8 g/mol Fe.
The balanced equation shows that the stoichiometry between Fe and FeCl3 is 2:2.
As a result, the amount of FeCl3 formed will be equal to the amount of Fe.
moles of FeCl3 formed grams equal to molar mass of FeCl3
Learn more about balanced equation, here:
https://brainly.com/question/31242898
#SPJ1
The atomic masses of 35^Cl (75.53 percent) and 37^Cl (24.47 percent) are 34.968 and 36.956 amu, respectively. Calculate the average atomic mass of chlorine. The percentages in parentheses denote the relative abundances
Answers
An element can have multiple isotopes. Isotopes correspond to variations of the same element with respect to the number of neutrons in its nucleus. the number they give us, 35 and 37 correspond to the mass number of chlorine. The percentage will be how abundant the element is.
To find the average atomic mass we must multiply the mass of the isotope by its respective percentage of abundance and add these two results.
So, the average atomic mass of Cl will be:
\(AtomicMassCl=34.968amu\times75.53\%+36.956amu\times24.47\%\)\(\begin{gathered} AtomicMassCl=26.411amu+9.043amu \\ AtomicMassCl=35.454amu \end{gathered}\)Answer: the average atomic mass of chlorine is 35.454 amu
PLS ANSWER!! ASAP!! 10 POINTS!!
Which of the following properties of gold does not explain why this metal is often used in jewelry?
A. Gold is a good conductor of electricity.
B. Gold is shiny.
C. Gold is very malleable.
D. Gold is a solid.
Answers
Answer: A is the correct answer
Explanation: It conducts more electricity than almost all other metals ans gems in jewelry
what is the bond order for a second-period diatomic particle containing five electrons in antibonding molecular orbitals and eight electrons in bonding molecular orbitals?
Answers
The bond order for a second-period diatomic particle containing five electrons in antibonding molecular orbitals and eight electrons in bonding molecular orbitals is 1.5
Bond order is defined as the number of electrons in bonding molecular orbitals minus the number of electrons in antibonding molecular orbitals divided by two. As a result, we may determine the bond order of this diatomic particle by the formula: Bond order = (number of bonding electrons - number of antibonding electrons) / 2
Bond order = (8 - 5) / 2
Bond order = 1.5.
This diatomic molecule, according to the bond order, is a stable molecule since the bond order is greater than 1, indicating that it is a double bond. The molecule has an overall bond strength that is greater than a single bond, but not as strong as a triple bond. So therefore he bond order for a second-period diatomic particle containing five electrons in antibonding molecular orbitals and eight electrons in bonding molecular orbitals is 1.5
Learn more about bond order at:
https://brainly.com/question/30641030
#SPJ11
Which of the following summaries expresses the main points of the passage best?
I believe gravity is the most important aspect of our universe. Without it we would all be floating off
into the universe. There wouldn't be any orbits; instead, all planetary bodies would simply float
around, running into each other when they crossed paths and just wandering forever.
There is a gravitational force between all objects in the universe. Gravitational force is what keeps all
components of our solar system in orbit around the Sun, as well as moons in orbit around planets. The
force of gravity affects Earth's tides and holds us on Earth's surface. The force of gravity between
objects depends on their masses and the distance between them.
Gravity is hard to understand and scientists have little to no understanding of how it works. We know
that gravity is out there, but the specifics are often lost on us. Plants, animals, and humans are all able
to grow tall due to the pull on Earth from the Sun. Without the Sun we would all just stretch out along
Earth's surface
None of the above
Answers
Answer:B
Explanation:
The summary which expresses the main points of the Gravitational force best is the second one.
What is gravitational force?Gravitational force is a attraction force which is present between two objects and represented as:
F = gm₁m₂ / r², where
g = gravitational constant
m₁ = mass of one object
m₂ = mass of another object
r = distance between two objects
Because of gravity all objects will have a accurate position and particular order.
Hence second paragraph expresses best.
To know more about gravitational force, visit the below link:
https://brainly.com/question/19050897
which of the following forces stabilize protein 3-dimensional structure? choice 1 of 6:ionic interactions choice 2 of 6:h-bonding choice 3 of 6:van der waals forces choice 4 of 6:metal ions choice 5 of 6:disulfide bonds choice 6 of 6:all of the above
Answers
All of the above forces (ionic interactions, H-bonding, van der Waals forces, metal ions, and disulfide bonds) play a role in stabilizing the 3-dimensional structure of proteins. Thus, the correct answer is Choice 6 of 6: all of the above.
Ionic interactions occur between positively and negatively charged amino acid residues, which helps to maintain the overall charge balance of the protein. H-bonding involves the sharing of electrons between atoms, and helps to hold the protein's secondary and tertiary structures in place. Van der Waals forces are relatively weak interactions between atoms, but they can contribute to the stability of the protein by helping to hold the atoms in place. Metal ions can also play a role in stabilizing the protein by binding to specific amino acid residues. Disulfide bonds form between cysteine residues and help to hold the protein's tertiary structure in place.
Learn more about protein 3-dimensional here: brainly.com/question/5684610
#SPJ4
WHAT IS THE CHEMICAL FORMULA FOR inorganic benzene
Answers
Answer: B3N3H6
Explanation:
I am pretty sure the formula for benzene is B3N3H6. If I am wrong, I am sorry, I learned this a long time ago.
when 0.367 mol of a weak acid, hx, is dissolved in 2.00 l of aqueous solution, the ph of the resultant solution is 2.60. calculate ka for hx. report your answer rounded to two significant figures using e- notation.
Answers
when 0.367 mol of a weak acid, hx, is dissolved in 2.00 l of aqueous solution, the ph of the resultant solution is 2.60. ka for hx is 3.405 × \(10^{-5}\)
Number of moles = 0.367
Volume of solution = 2l
concentration = 0.367/2 = 0.1835 mol/L
ph = 2.60
we know
ph = - log [H+]
2.51 × \(10^{-2.60}\)M = [H+]
The acid HX dissociate as
HX → H+ + X-
The acid dissociation constant Ka, for the dissociation reaction is
Ka = [H+][X-]/[HX] ; at equilibrium, [H+] = [X-]
Ka = 3.405 × \(10^{-5}\)
A solution in which water serves as the solvent is called an aqueous solution. The most common way to represent it in chemical equations is to add (aq) to the appropriate chemical formula. For instance, the formula for a solution of table salt, or sodium chloride (NaCl), in water is Na+(aq) + Cl The word aqueous, which derives from the word aqua, means that something is connected to, resembles, or is dissolved in water. Water is a common solvent in chemistry because it is an excellent solvent and abundant in nature. Since water is frequently used as the experiment's solvent, unless otherwise stated, the term "solution" refers to an aqueous solution.
Learn more about Aqueous solution here:
https://brainly.com/question/14097392
#SPJ4
Draw a carboxylic acid that results from the hydrolysis of glyceryl tributyrate. Include only one carboxylic acid product in your answer
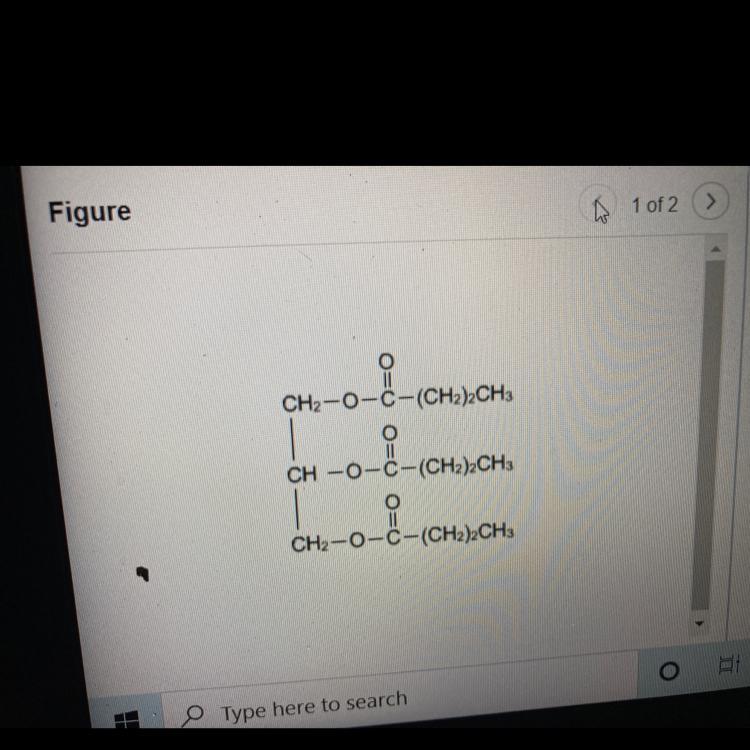
Answers
Glyceryl tributyrate will react with 3 mol of water and form a glycerol and 3 mol of carboxylic acid.

identify the limiting reactant when 7.28 grams of magnesium oxide reacts with 4.50 grams of aluminum to make magnesium and aluminum oxide? i need a typed answer a link wont work
Answers
Answer:
the limiting reactant is aluminum
Explanation:
Marcus is going hiking. He packs some homemade snacks to take with him. He puts raisins, nuts, chocolate pieces, and dried fruit in a bag. What is the best description of this homemade snack?
Answers
Explanation:
He puts raisins, nuts, chocolate pieces, and dried fruit in a bag. This would be considered a mixture of a snack.
The best description for Marcuses homemade snack is a mixture.
What is the volume of a flask that contains 0.730 moles of Nitrogen at a pressure of 698 mmHg and a temperature of 35.4°C?
Answers
Answer:
V = 20.1 L
Explanation:
Given data:
Volume of flask = ?
Number of moles of nitrogen gas = 0.730 mol
Pressure = 698 mmHg (698/760 = 0.92 atm)
Temperature = 35.4°C
Solution:
The given problem will be solve by using general gas equation,
PV = nRT
P= Pressure
V = volume
n = number of moles
R = general gas constant = 0.0821 atm.L/ mol.K
T = temperature in kelvin
Now we will convert the temperature.
35.4+273 = 308.4 K
by putting values,
0.92 atm× V = 0.730 mol ×0.0821 atm.L/ mol.K × 308.4 K
V = 18.48 atm.L / 0.92 atm
V = 20.1 L