Why do macromolecules differ in the amount of energy.
Answers
Macromolecules differ in the amount of energy they contain because of the differences in their chemical structure.What are macromolecules?A macromolecule is a large molecule that is composed of thousands of atoms. They are formed by the joining of smaller molecules, called monomers, through a process known as polymerization.
The energy content of macromolecules is determined by the chemical bonds that hold them together. These bonds can be broken down to release energy that can be used by cells to fuel metabolic processes.The amount of energy that is released when these bonds are broken down differs among the different types of macromolecules.
For instance, lipids such as fats contain more energy per gram than carbohydrates such as sugars. This is because the chemical bonds in lipids are more complex and have a higher energy content than those in carbohydrates. Similarly, proteins have a higher energy content than nucleic acids because of their more complex structure.
Thus, macromolecules differ in the amount of energy they contain due to the differences in their chemical structure.
To know more about energy visit :-
https://brainly.com/question/13881533
#SPJ11
Related Questions
Which type of subatomic particle most directly determines the chemical reactivity of an atom? Protons Neutrons Electrons None of the above Answers b and Question 2 How many covalent bonds does carbon (atomic number 6, atomic mass 12) usually make in organic molecules?
Answers
1) Option 3. Electrons. The type of subatomic particle that most directly determines the chemical reactivity of an atom is electrons.
Electrons are negatively charged particles that occupy the outermost energy level, or valence shell, of an atom. The number of electrons in this valence shell, as well as the arrangement of these electrons, determines the chemical properties of an atom and its reactivity. For example, an atom that has a full valence shell, with 8 electrons in its outer energy level, is considered to be unreactive and unlikely to form chemical bonds. On the other hand, an atom with a partially filled valence shell, such as carbon, nitrogen, or oxygen, is likely to participate in chemical reactions and form bonds with other atoms.
2) carbon (atomic number 6, atomic mass 12) usually makes 4 covalent bonds in organic molecules
Carbon, with atomic number 6 and atomic mass 12, usually forms 4 covalent bonds in organic molecules. This is due to the presence of 4 valence electrons in the outermost shell of the carbon atom. In covalent bonding, atoms share electrons to form a bond and complete their outermost electron shell. Carbon can bond with other elements such as hydrogen, oxygen, nitrogen, etc. to form complex organic molecules such as carbohydrates, lipids, proteins, etc. The ability of carbon to form multiple covalent bonds with other atoms is what makes it the backbone of all living organisms and the central component of organic chemistry. The number of covalent bonds an atom can form is directly related to the number of valence electrons it has, which in turn determines its chemical reactivity.
Learn more about Subatomic particle here: brainly.com/question/29765133
#SPJ4
Which of the following is not a property of water?
1) Hydrogen bonding causes a high surface tension.
2) Hydrogen bonds exist only in the solid state.
3) Water can dissolve ionic and polar molecules.
4) Water's solid state is less dense than its liquid state.
Answers
Answer:
1
Explanation:
Hydrogen bonding causes a high surface tension
Hydrogen bonds existing only in the solid state refers to the false statement about water.
What is Water?This is a compound which consists of hydrogen and oxygen and is referred to as a universal solvent.
Its solid state is less dense than its liquid state and the hydrogen bonds exists in various states of matter thereby making option 2 the most appropriate choice.
Read more about Water here https://brainly.com/question/5060579
Two chemicals A and B are combined to form a chemical C. The rate, or velocity, of the reaction is proportional to the product of the instantaneous amounts of A and B not converted to chemical C. Initially, there are 40 grams of A and 50 grams of B, and for each gram of B, 2 grams of A is used. It is observed that 15 grams of C is formed in 6 minutes. How much (in grams) is formed in 12 minutes
Answers
In 12 minutes, 42.5 grams of C will be formed.
From the given information, we know that the rate of the reaction is proportional to the product of the amounts of A and B not yet converted to C. Let's use the variables x and y to represent the amounts of A and B, respectively, that have not yet been converted to C.
We are told that initially, there are 40 grams of A and 50 grams of B. We also know that for each gram of B, 2 grams of A are used. This means that after some time t, the amounts of A and B not yet converted to C are given by:
x = 40 - 2yt
y = 50 - yt
where t is measured in minutes.
We are given that 15 grams of C is formed in 6 minutes. We can use this information to find the value of the proportionality constant k.
The rate of the reaction is given by:
dC/dt = kxy
At t=0, x=40 and y=50, so the rate is:
dC/dt = k(40)(50) = 2000k
After 6 minutes, 15 grams of C have been formed, so:
dC/dt = 15/6
Setting these two expressions for dC/dt equal to each other and solving for k, we get:
2000k = 15/6
k = 0.000625
Now we can use the rate equation to find the amount of C formed after 12 minutes:
dC/dt = kxy
At t=12, x = 40 - 2y(12) = 40 - 24y
y = 50 - 12y
Substituting these expressions into the rate equation and integrating with respect to time from 0 to 12, we get:
C(12) - C(0) = ∫(0 to 12) k(40 - 24y)(50 - 12y) dy
Solving this integral, we get:
C(12) = 42.5 grams
Therefore, 42.5 grams of C are formed in 12 minutes.
To know more about Rate of Reaction:
https://brainly.com/question/24795637
#SPJ11
What is the relationship between melting/boiling point and intermolecular forces
Answers
What happens during a chemical reaction how do you know when a chemical reaction has occurred and how can you represent chemical reactions with equations??
Answers
We know that a chemical reaction has occurred by the appearance of a solid, evolution of a gas or change of color.
What is a chemical reaction?A chemical reaction refers to an interaction between reactants which leads to the formation of a product. A chemical reaction is represented using a chemical reaction equation.
We know that a chemical reaction has occurred when new substances appear in the system. This is signaled by the appearance of a solid, evolution of a gas or change of color.
Learn more about chemical reactions: https://brainly.com/question/6876669
if there are 10 low-energy conformational states per backbone unit, calculate the number of conformers per molecule
Answers
The number of conformers per molecule, given 10 low-energy conformational states per backbone unit, is 10 raised to the power of 'n'.
In the realm of molecular biology and chemistry, a molecule's conformation refers to its specific three-dimensional arrangement of atoms and bonds. Conformational states represent the various possible conformations that a molecule can adopt. The number of conformers per molecule depends on the number of available low-energy conformational states for each backbone unit.
If there are 10 low-energy conformational states per backbone unit, we can calculate the number of conformers per molecule by considering the total number of backbone units present. Let's assume a molecule consists of 'n' backbone units.
For each backbone unit, there are 10 possible low-energy conformational states. Thus, the total number of conformers for a single backbone unit is 10.
Considering the molecule has 'n' backbone units, the number of conformers per molecule can be obtained by raising the number of possible conformations for a single backbone unit (10) to the power of 'n'. Mathematically, this can be expressed as \(10^n.\)
Learn more about molecule
https://brainly.com/question/475709
#SPJ11
Kilograms represented by the mass defect for oxygen-16: 2.20 × 10 -28 kg what is the nuclear binding energy for oxygen-16? 3.0 × 108 j 6.60 × 10-20 j 1.98 × 10 -11 j 3.69 x 10-24 j
Answers
From the calculation, the binding energy of the oxygen atom is 1.98 * 10^11 J
What is the binding energy?The term binding energy has to do with the energy that must be supplied to the nucleus of an atom for the nucleus of the atoms to be bonded together.
We must note that;
E = mc^2
E = 2.20 × 10 -28 kg * ( 3 * 10^8m/s)^2 = 1.98 * 10^11 J
Learn more about binding energy:https://brainly.com/question/10095561
#SPJ4
Answer:
1.98 × 10 -11 J
Explanation:
edg
if 100.0 mL of liquid weighs 81.23g what is the density of the liquid
Answers
Answer:
812.3 kilogram/cubic meter
Explanation:
I used an online calculator.
Hope I helped!
35. a
When aqueous iron (III) chiondes added to aqueous potassium iodide a chemical con
ours and lodine is formed
Which statement is correct?
A todide sons are oxidised, they gain electrons in this reaction
lodide ions are oxidised, they lone electrons in this reaction
C trond) chionde is oxidised in this reaction
D Neither iodide ions nor iron (III) chlonde is ondised in this reachon

Answers
in liquid methanol, ch3oh, which intermolecular forces are present?
Answers
In liquid methanol (CH3OH), several intermolecular forces are present. Intermolecular forces are attractive forces that exist between molecules and play a crucial role in determining the physical properties of substances.
One of the primary intermolecular forces in liquid methanol is hydrogen bonding. Hydrogen bonding occurs when a hydrogen atom, bonded to a highly electronegative atom such as oxygen, nitrogen, or fluorine, is attracted to another electronegative atom in a neighboring molecule. In methanol, the hydrogen atom of the hydroxyl (-OH) group forms hydrogen bonds with the oxygen atom of adjacent methanol molecules. These hydrogen bonds are relatively strong and contribute to the high boiling point and viscosity of liquid methanol compared to nonpolar molecules of similar size.
Additionally, methanol molecules experience dipole-dipole interactions. Methanol is a polar molecule due to the difference in electronegativity between the oxygen and carbon atoms, leading to a partial positive charge on the carbon atom and a partial negative charge on the oxygen atom. These partial charges attract neighboring methanol molecules, resulting in dipole-dipole interactions.
Lastly, methanol also exhibits London dispersion forces, also known as van der Waals forces. These forces arise due to temporary fluctuations in electron distribution, resulting in temporary dipoles within molecules. These temporary dipoles induce dipoles in nearby molecules, leading to attractive forces. Although methanol is a polar molecule, it also experiences London dispersion forces, which are generally weaker than dipole-dipole interactions but still contribute to the overall intermolecular forces in the liquid.
To summarize, in liquid methanol (CH3OH), the intermolecular forces present include hydrogen bonding, dipole-dipole interactions, and London dispersion forces. These intermolecular forces collectively influence the physical properties of liquid methanol, such as its boiling point, viscosity, and solubility.
Learn more about Liquid Methanol :
https://brainly.com/question/13992085
#SPJ11
16. For the reaction below, A) calculate the total bond energy, using the equation: Reactant energy - product energy = total energy.
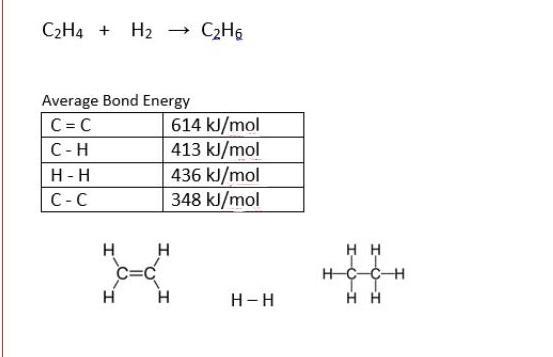
Answers
Answer:
C=C divided by the co effiecient 11 would make the denominator of the photosynthesis equal 11 over 5 making this problem a polywhirl which wouldnt matter in the sense of the quake in energy change so thus making the answer 728 kJ/mol
Explanation: back in my day
Part C
Next Emmerson pours dark blue–colored water on the soil around the well. The pictures show how his model looked over time. What do you observe?

Answers
Answer:
I obseve that the water is turnign a little green in the begining but when the water hit the gravel and dirt it became realy blue
Explanation:
Mark brainliest idc
Answer:
I observe that the water started off by turning green and the higher the water went the more blue it had gotten.
Explanation: lazy answer but if you want an answer here you go:)
Given this equation: N2 + 3 H2 → 2 NH3, how many moles of NH3 can be produced from 3.1 moles of H2?
Answers
First, we write down our reaction:
N2 + 3H2 → 2NH3
Don't forget to balance it.
We only use moles as units.
Procedure:
3 x 1 mole H2 ------------ 2 x 1 mole NH3
3.1 moles H2 ------------- x
x = 2.1 moles NH3 are produced
Answer: 2.1 moles NH3
comparison study on kerala and himachal pradesh on water and soil pls write water and soil separately, pls answer fast 20 points u will get
Answers
The main difference between the waters of Kerala and Himachal Pradesh is the presence of water lakes, and ponds in Kerala and the Himalayan Ranges and Rivers in Himachal Pradesh
The main difference between the soil of Kerala and Himachal Pradesh is that there is the presence of mountain soils in Himachal Pradesh, while Kerala has coastal allu-vium, mixed allu-vium, and acid saline, Kari, laterite, red, hill, black cotton, and forest soils.
What is a Comparision?This refers to the side-by-side view of two or more entities in order to find their differences and similarities.
Hence, we can see that Kerala and Himachal Pradesh are both in India and they are in different regions, which accounts for their differences in water and soil as listed above.
Read more about water and soil here:
https://brainly.com/question/20848502
#SPJ1
What evidence did Dwight "Rocky" Crandell find that indicated the map he made near Lake Tapps in Washington was a massive lahar from Mt. Rainier and not an old glacier? (Select all that apply)
- actively flowing lava
- whole logs mixed in with the rocks
- clay sized particles
- an abundance of snow and ice
Answers
The evidence that indicated that the map made near Lake Tapps in Washington was a massive lahar from Mt. Rainier and not an old glacier: Whole logs mixed in with the rocks, Clay-sized particles and An abundance of snow and ice
The following evidence indicated that the map made near Lake Tapps in Washington was a massive lahar from Mt. Rainier and not an old glacier:
Whole logs mixed in with the rocks: The presence of whole logs mixed in with the rocks suggests a rapid and powerful flow, characteristic of a lahar, rather than the slow movement associated with glaciers.
Clay-sized particles: The presence of clay-sized particles is often associated with lahars, as they can be easily transported by the flowing volcanic material.
An abundance of snow and ice: The presence of snow and ice is indicative of a recent event, as glaciers tend to accumulate and retain snow and ice over time. In the case of a lahar, the presence of snow and ice suggests a more recent deposition.
Learn more about Evidence, here:
https://brainly.com/question/31812026
#SPJ4
What would you call a solution that contains 300 g/l nacl at 25 c
Answers
The solution will be called as saturated solutions.
What are saturated solutions?A solution in which all of the solvent has been dissolved. Any additional solute will form crystals on the bottom of the container.
An example can include a solution that contains 300 g/l NaCl at 25 degree Celsius.
Thus, the solution will be called as saturated solution.
For more details regarding saturated solution, visit:
https://brainly.com/question/1851822
#SPJ1
What name is given to the following reaction?
sucrose + water → glucose + fructose
A) glucogenesis
B) hydrolysis
C) denaturation
D) dehydration reaction
Answers
The sucrose is a disaccharide molecule. The reaction of sucrose with water is known as hydrolysis reaction. The products formed are glucose and fructose. The correct option is B.
What is Hydrolysis?The reaction which involves the breaking down of carbohydrates into smaller units through water molecule is defined as the hydrolysis reaction. The sucrose molecule is composed of two monosaccharide units. The molecular formula of sucrose is C₁₂H₂₂O₁₁.
The hydrolysis of sucrose generates an equimolar mixture of fructose and glucose, commercially it is called the invert sugar. This inverted sugar is more sweeter than sucrose.
Thus the correct option is B.
To know more about hydrolysis, visit;
https://brainly.com/question/29100975
#SPJ1
2. How many mi hr is 30km/s?
Answers
The answer is 67 108.0888km/s.
cesium has one electron in its outer level. what will it most likely do in a reaction?
Answers
Answer:
Explanation:
Identify the chemical formula of ammonia.
Answers
Answer:
The chemical formula for ammonia is NH3
Explanation:
Nitrogen trihydride
7. Cecilia puts two magnetic toy trains very close to each other on a track. What will happen next, and
why.
Answers
explain using diagrams how potassium forms the compound potassium flouride when it reacts with flourine
Answers
Answer:
The answer to your question is given below.
Explanation:
Potassium (K) has 19 electrons with electronic configuration of 2, 8, 8, 1.
Fluorine (F) has 9 electrons with electronic configuration of 2, 7.
Fluorine needs 1 electron to complete it's octet configuration.
Hence, potassium (K), will lose 1 electron to fluorine (F) to form potassium ion (K+) with electronic configuration of 2, 8, 8. The fluorine atom (F) will receive the 1 electron from potassium to form the fluoride ion (F-) with electronic configuration of 2, 8.
**** Please see attached photo for further details.

Which number indicates the energy level of an atom's valence electrons?
O A. The period number
O B. The group number
O c. The atomic number
O D. The mass number
Answers
Answer:A, The Period Number
Explanation:
just took the test
Which statement is true about the potential energy diagram for an exothermic reaction? (5 points)

Answers
Answer:
Products have less potential energy than reactants.
Explanation:
Let's remember the concept of an exothermic reaction: a chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. Because the surroundings are gaining heat from the system, the temperature of the surroundings increases.
Now, let's see how looks a potential energy diagram for an exothermic reaction:
This represents that the products have less potential energy than reactants.

Explain in a three-paragraph essay the mechanics of how a battery works. How does the choice of metals used in a battery affect its performance? what specific metals work best?
Answers
A battery is a device that converts chemical energy into electrical energy through a process known as an electrochemical reaction.
How does a battery work ?When a battery is connected to a circuit, the electrochemical reaction causes a flow of electrons from the anode to the cathode, generating an electric current that can power a device.
The metal chosen for the anode must be capable of losing electrons easily, while the metal chosen for the cathode must be capable of accepting electrons. The choice of metals can also affect the voltage and capacity of the battery, as well as its overall efficiency.
In general, the metals used in a battery should have a large difference in their electronegativity values, which determines how easily an atom can attract electrons. Common metals used in batteries include zinc, lithium, nickel, and cadmium.
Find out more on batteries at https://brainly.com/question/16553902
#SPJ1
The primary way in which the amount of carbon dioxide in the atmosphere can be increased is by ____.
Answers
the burning of fossil fuels
the bond between adjacent amino acids is a(n) ________ bond.
Answers
The bond between adjacent amino acids is a covalent peptide bond. A protein is made up of a lengthy chain of amino acids that are connected by peptide bonds.
A water molecule is removed during a biological process that links the carboxyl group of one amino acid to the amino .Peptide bonds are the connected that hold the amino acids of a peptide together in a particular order. Two atoms share an electron pair equally in a covalent link. Peptide (amide) and disulfide links between amino acids, as well as C-C, C-O, and C-N bonds within amino acids, are examples of significant covalent bonds.
To learn more about peptide bond, click here.
https://brainly.com/question/28295128
#SPJ4
A Cell is B.00 un in diameter' and has a cell width of 60.0 nm thrck. If densty x (mass druided by volome) of the wall is the Same as thent of pure water (1000kym
−3
). What ts the mass (in my) of the cell wall cossuming cell is splowicul and the wall is thin sphericul slell?
Answers
The mass of the cell wall, assuming the cell is spherical and the wall is a thin spherical shell, is approximately 0.91 milligrams.
To calculate the mass of the cell wall, we first need to determine the volume of the wall.
The given diameter of the cell is 0.00 μm, which means the radius (r) of the cell is half of that, so r = 0.00/2 = 0.00 μm = 0.00 nm.Now, we need to find the volume of the cell wall, which can be approximated as a thin spherical shell. The volume of a thin spherical shell can be calculated using the formula:
V = 4/3 * π * (r_outer^3 - r_inner^3)
Since the cell is spherical, the inner radius of the shell is the same as the radius of the cell (r), and the outer radius of the shell is the sum of the radius of the cell (r) and the thickness of the wall (60.0 nm). Thus, the outer radius (r_outer) of the shell is:
r_outer = r + thickness = 0.00 + 60.0 = 60.0 nm
Substituting the values into the formula, we have:
V = 4/3 * π * (60.0^3 - 0.00^3)
= 4/3 * π * (216,000 nm^3)
= 288,000 π nm^3
Next, we need to calculate the mass of the cell wall using the density of pure water. The density (ρ) is given as 1000 kg/m^3, which is equivalent to 1000 kg/1,000,000,000 nm^3 since 1 m = 1,000,000,000 nm. Thus, the mass (m) of the cell wall is:
m = ρ * V
= 1000 kg/1,000,000,000 nm^3 * 288,000 π nm^3
= 0.000288 π kg
Now, we can calculate the mass of the cell wall by substituting the value of π (pi) as 3.14159:
m = 0.000288 * 3.14159 kg
= 0.000905 kg
≈ 0.91 mg
For more such questions on mass visit:
https://brainly.com/question/24191825
#SPJ8
why does the carbon monoxide generated in a gun barrel or in a backdraft ignite, whereas there is no such igniting in the muffler of a car?
Answers
Carbon monoxide is generated in a gun barrel or in a backdraft ignite, as there is less air. so combustion of carbon present there in limited amount of air produces carbon monoxide.
The gas carbon monoxide, which has the chemical formula CO, is toxic, combustible, tasteless, colourless, and somewhat less dense than air. One carbon atom and one oxygen atom bound together by three bonds make up carbon monoxide. The simplest carbon oxide is this one. The carbon monoxide ligand in coordination complexes is referred to as carbonyl. It is a crucial component in several industrial chemical processes.
When there is not enough oxygen or heat to make carbon dioxide during the partial combustion of carbon-containing substances, carbon monoxide is most frequently produced. There are a lot of biological and environmental factors that produce a lot of carbon monoxide as well. It is crucial for the creation of several chemicals, including as medicines, perfumes, and fuels. Carbon monoxide has an impact on a number of climate change-related processes after entering the atmosphere.
Learn more about Carbon monoxide:
https://brainly.com/question/29233528
#SPJ4
Complete question:
Carbon monoxide is the flammable gas that is partially responsible for the muzzle flash seen from a firearm. It is also one of the gases that can cause a backdraft to happen when firefighters open up poorly ventilated rooms. Automobiles produce carbon monoxide as a result of the negative oxygen balance of the fuel-air explosion that powers the engines. Why does the carbon monoxide generated in a gun barrel or in a backdraft ignite, whereas there is no such igniting in the muffler of a car? (CP)
What is a superconductor?
A. A conductor that operates at room temperature
B. A conductor that allows electricity to flow easily
C. A conductor that conducts electricity faster than common metals
D. A conductor that allows electricity to flow through nonmetal solids

Answers
Answer:
its B
Explanation:
a p e x
Answer:
the answer is b. allows electricity to flow easily